The Anti-Atherosclerotic Effect of Naringin Is Associated with Reduced Expressions of Cell Adhesion Molecules and Chemokines through NF-κB Pathway
Abstract
:1. Introduction
2. Results
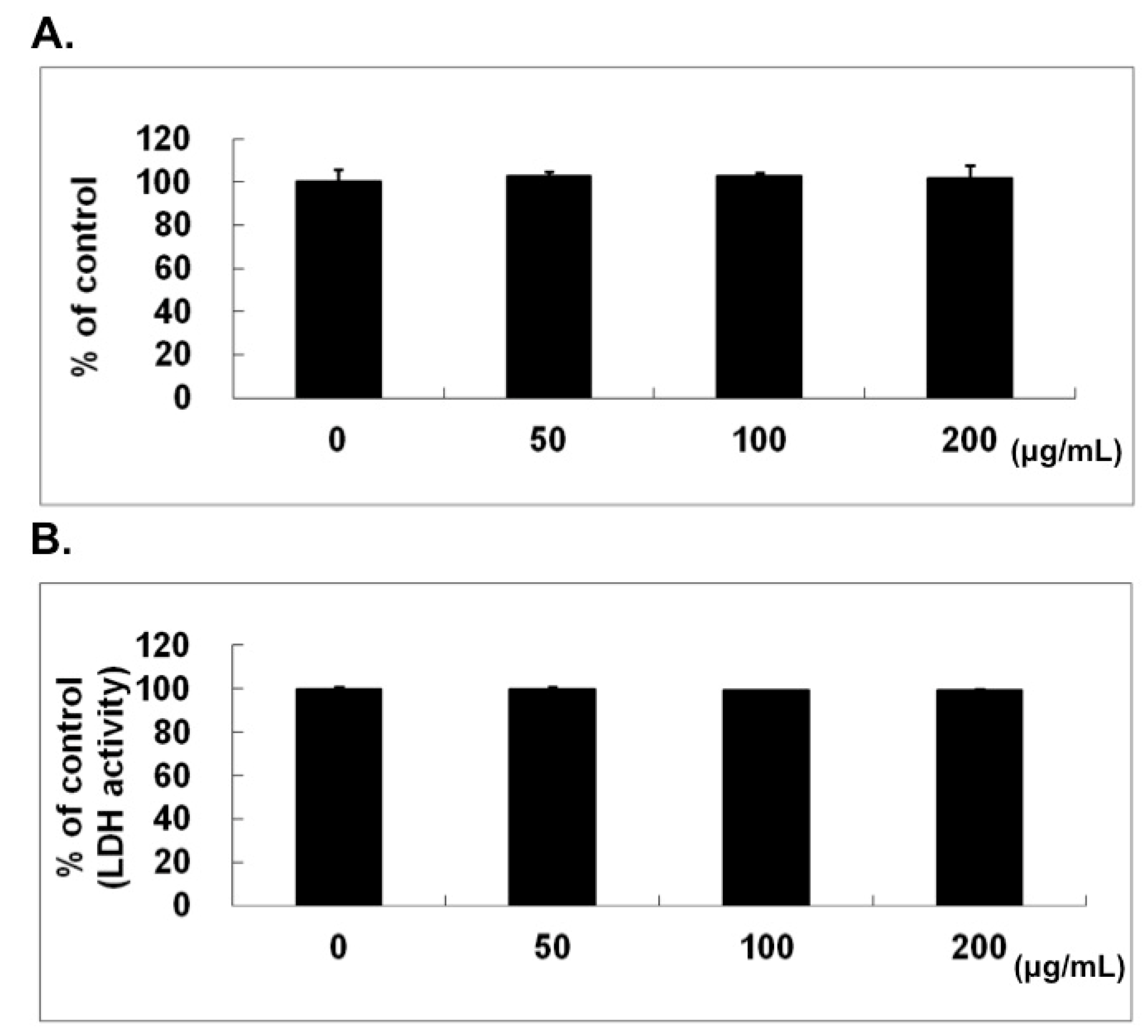
2.1. No Cytotoxic Effect of Naringin on HUVECs
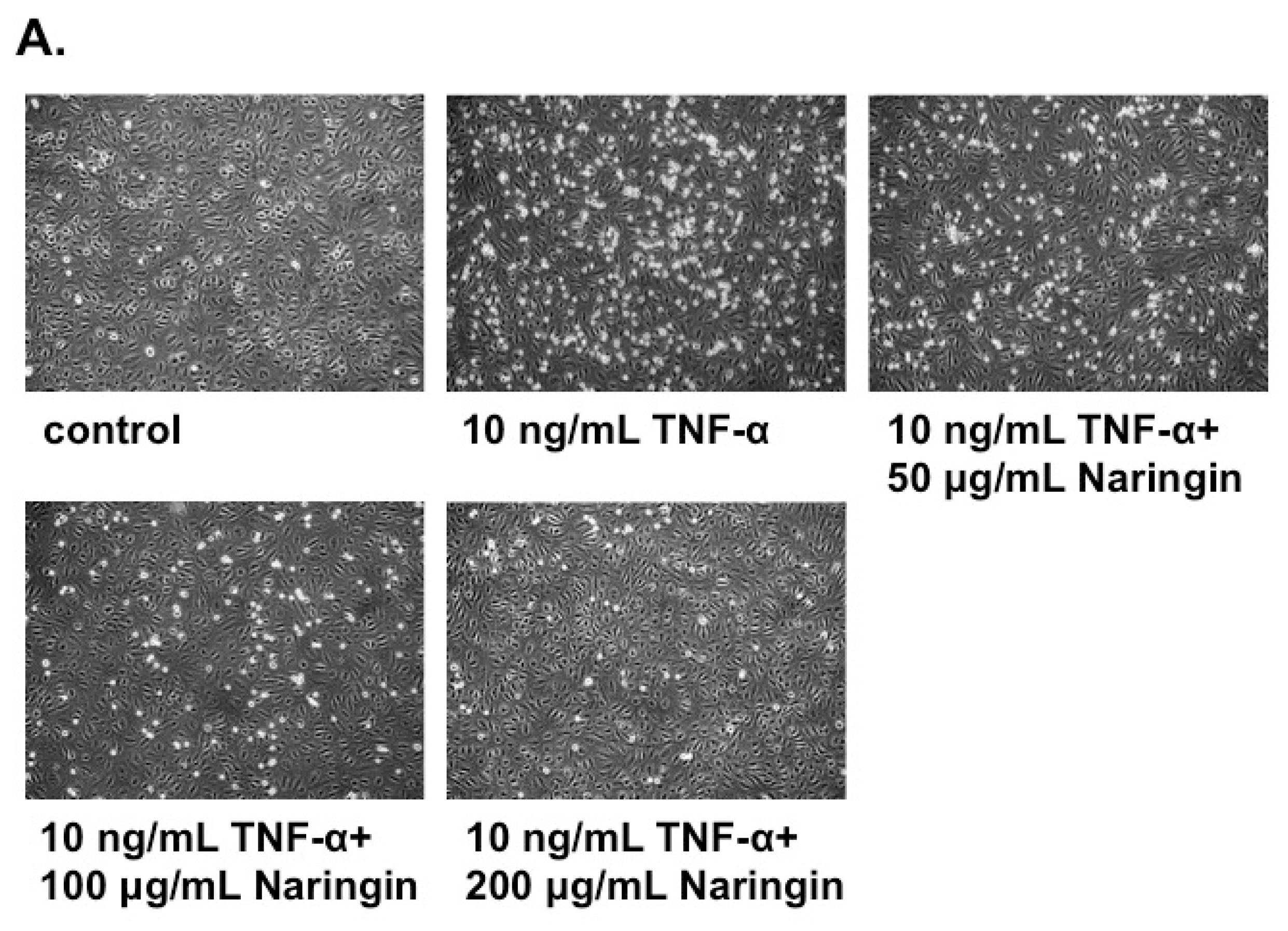
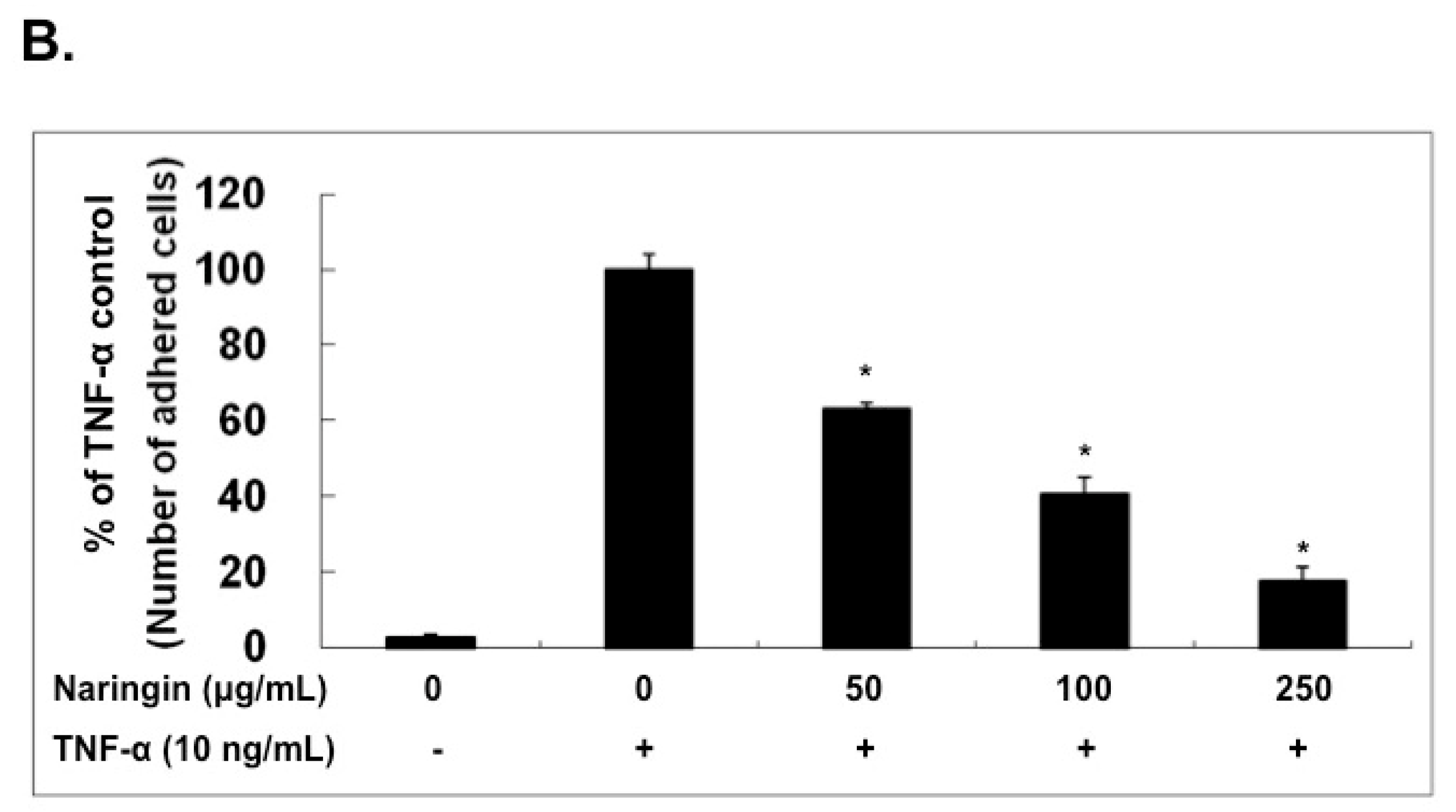
2.2. Naringin Inhibited the Adhesion of Monocytes to TNF-α-Stimulated HUVECs
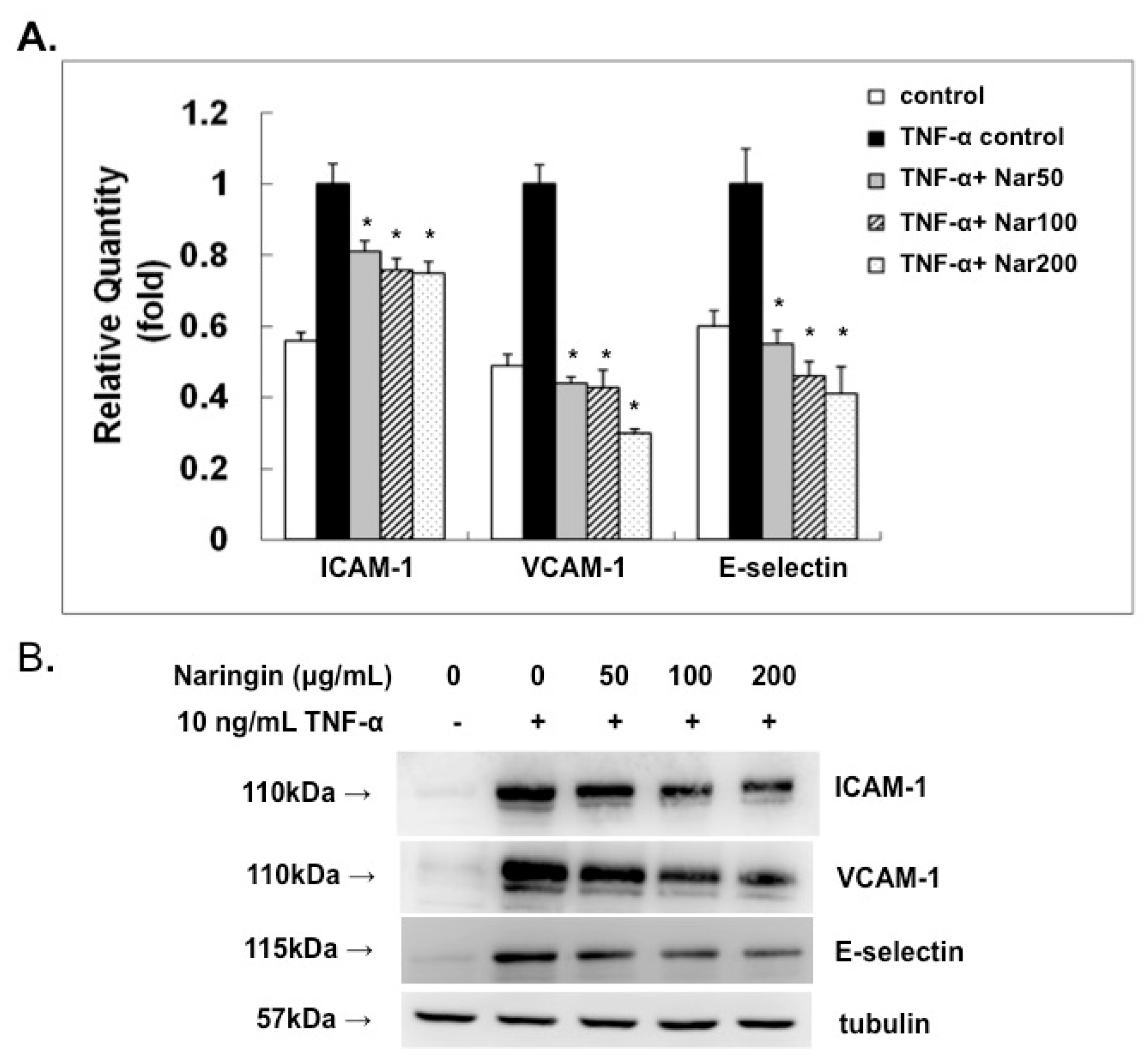
2.3. Effect of Naringin on mRNA Expression and Protein Levels of ICAM-1, VCAM-1, and E-Selectin

2.4. Effect of Naringin on the Expression of Fractalkine, MCP-1, and RANTES in TNF-α-Stimulated HUVECs
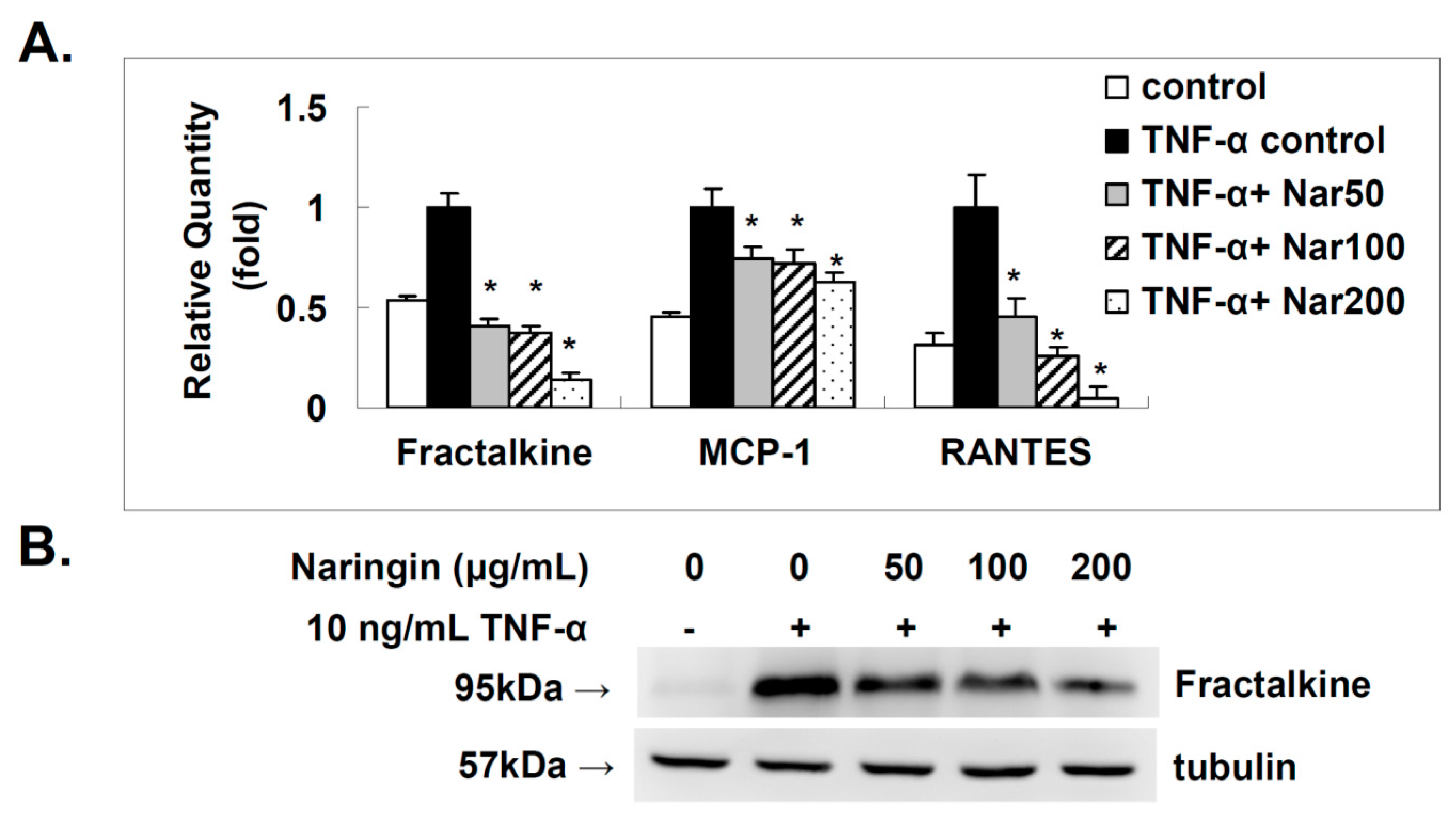
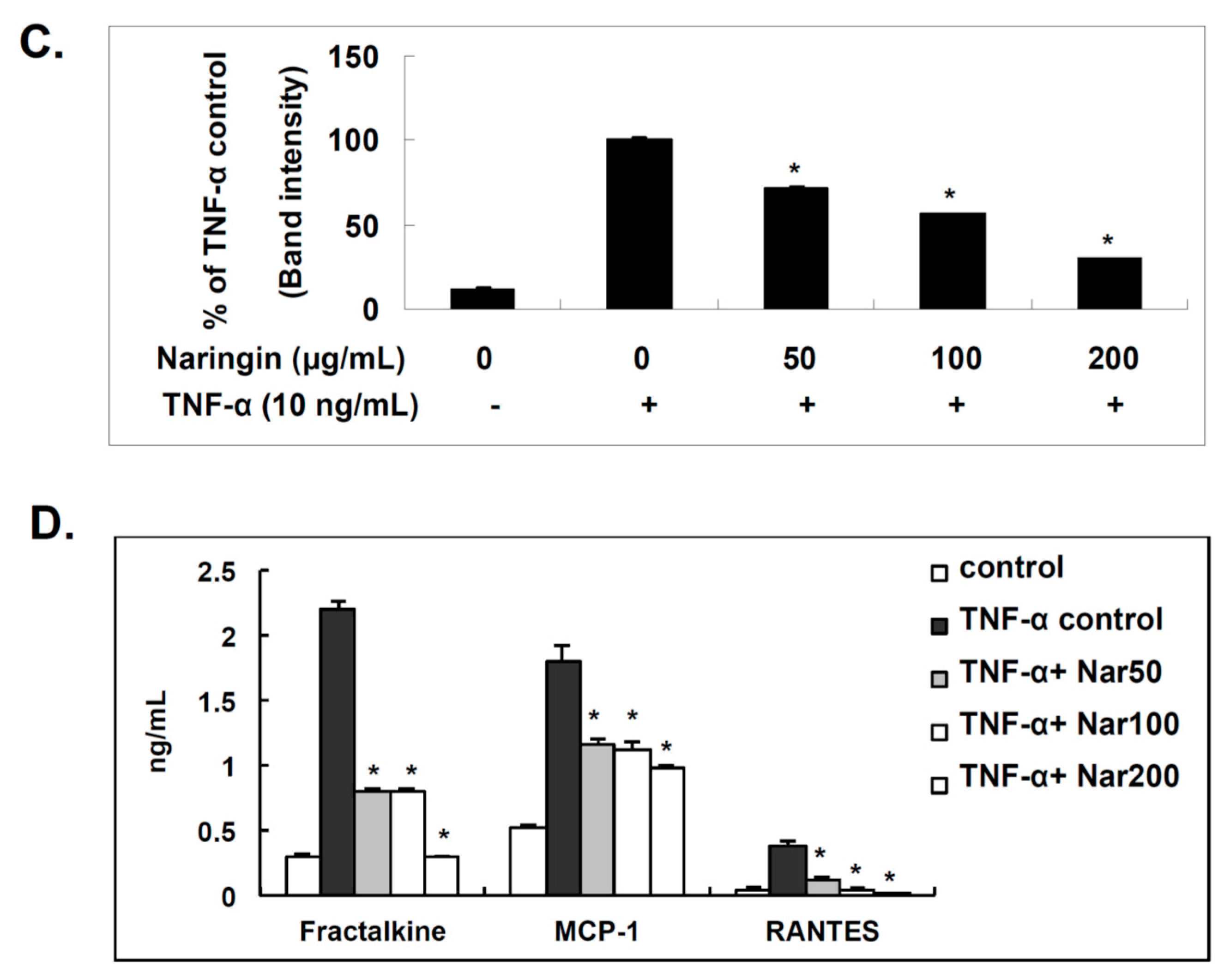
2.5. Naringin Inhibited Nuclear Translocation of NF-κB Stimulated by TNF-α
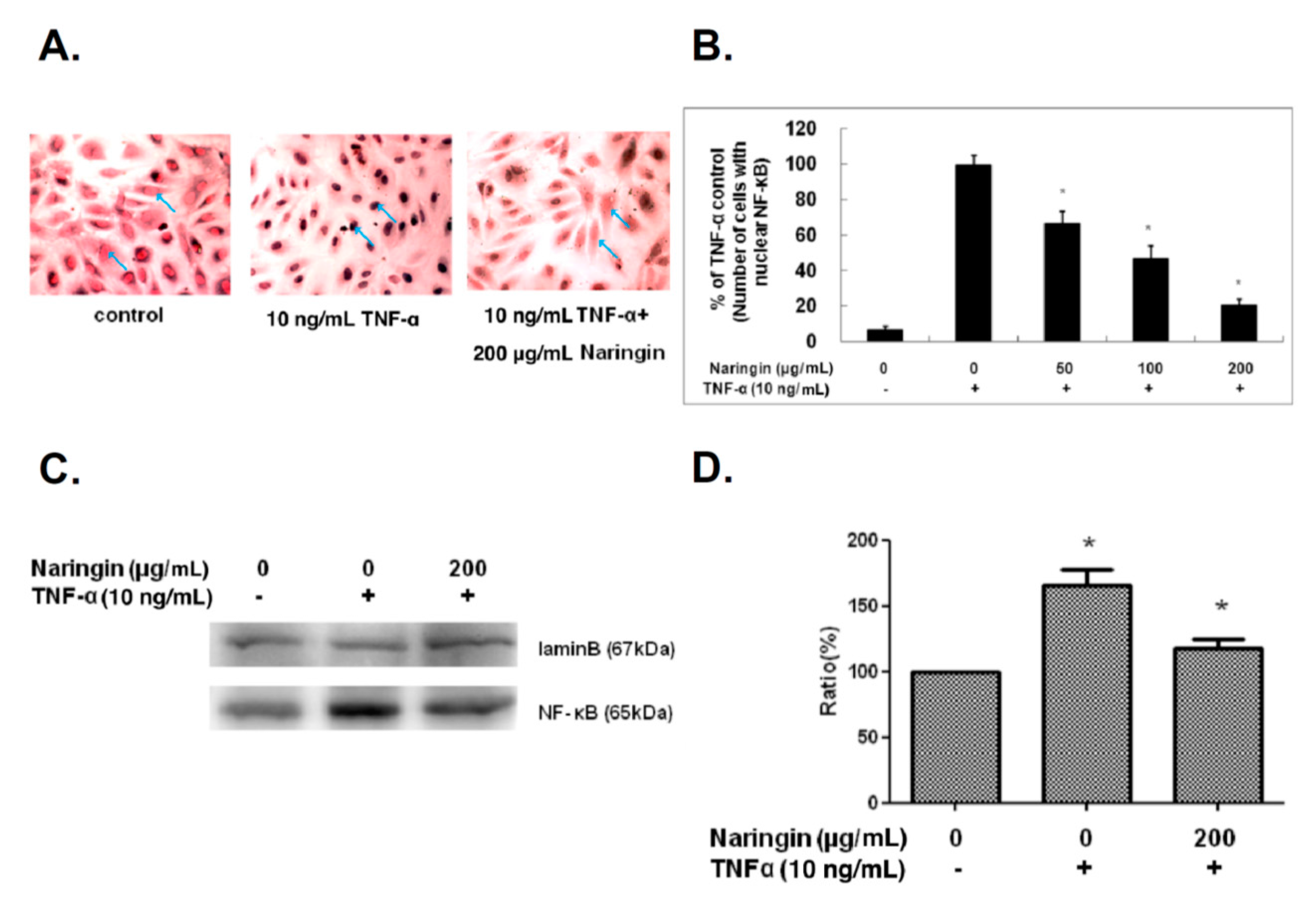
2.6. Inhibitory Effect of Naringin on the Activation of IKK/NF-κB Pathway Stimulated by TNF-α
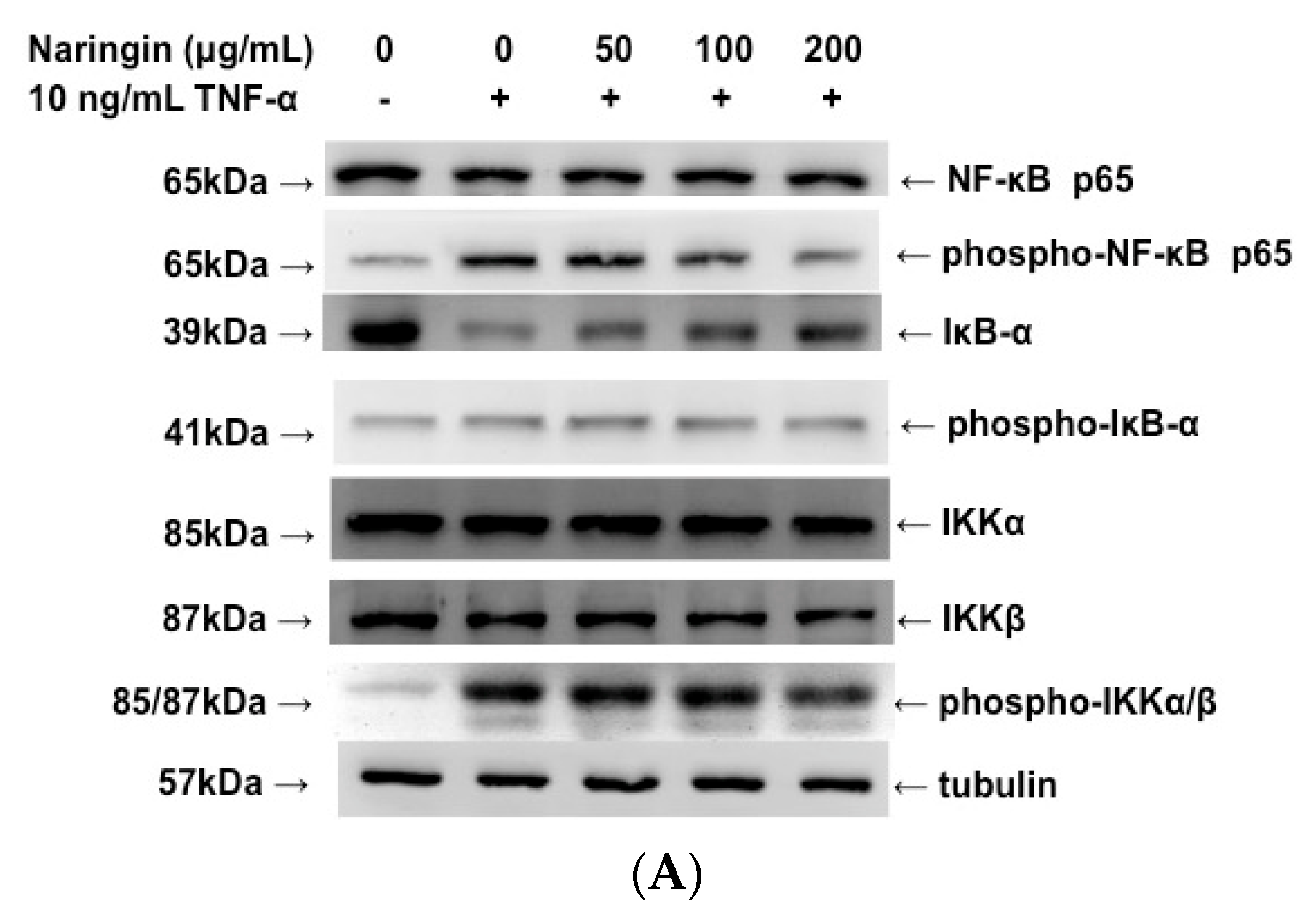
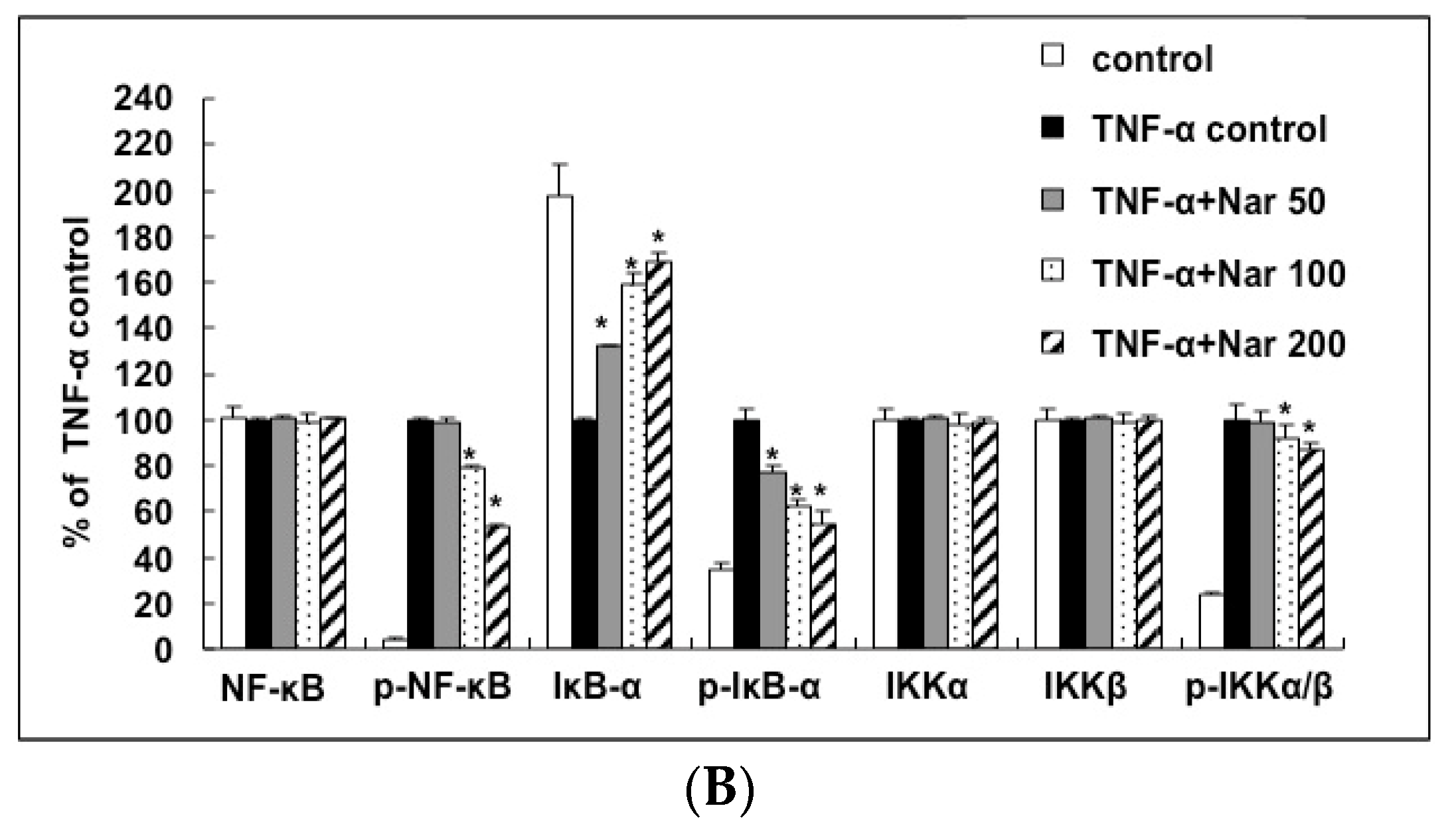
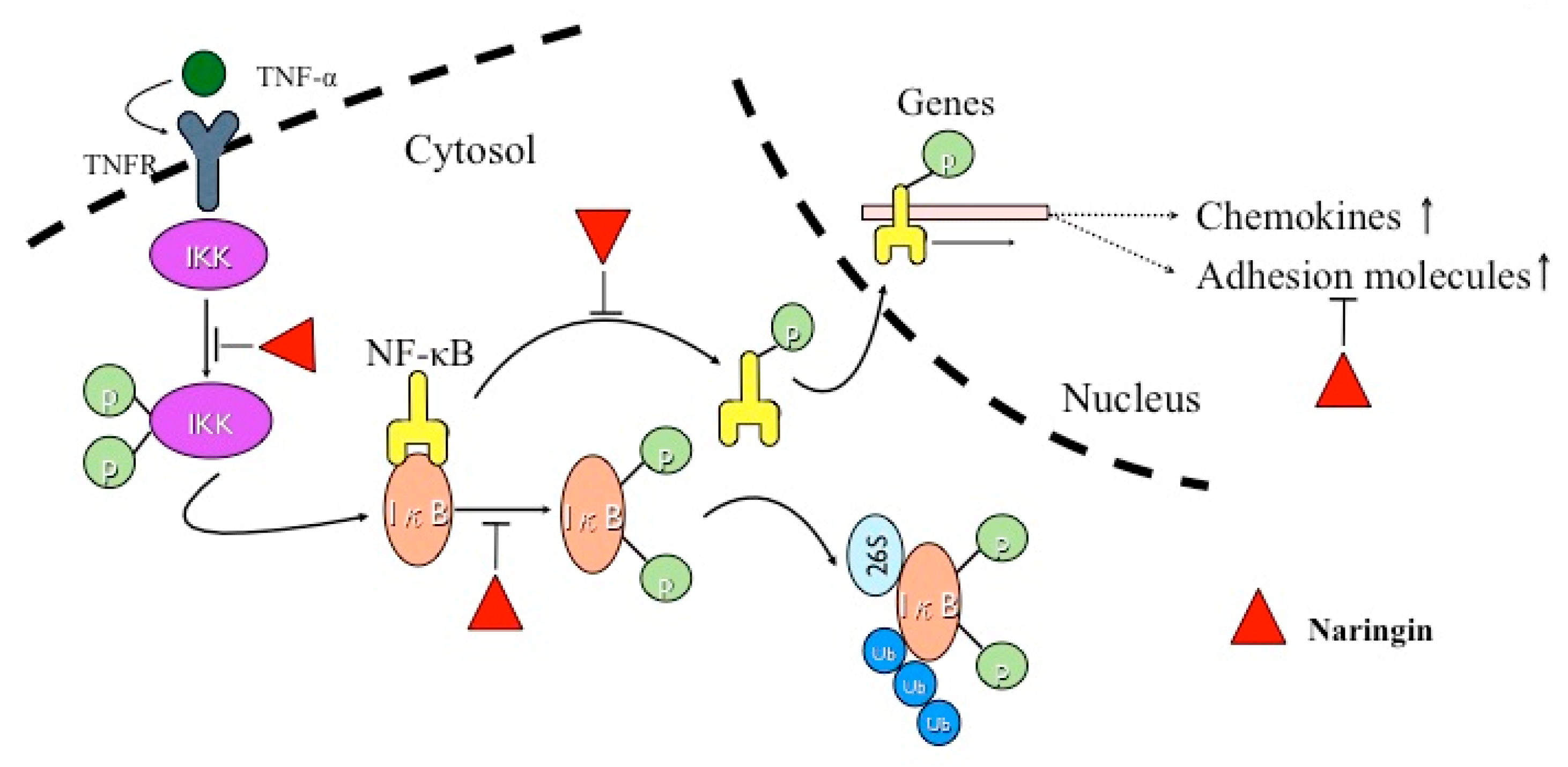
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. MTT Assay
4.3. LDH Assay
4.4. Monocyte Adhesion Assay
4.5. RNA Isolation and Real-Time Polymerase Chain Reaction
4.6. Protein Extraction and Western Blot Analysis
4.7. Fractalkine, MCP-1, and RANTES ELISA
4.8. Immunocytochemical Staining of NF-κB in HUVECs
4.9. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Murray, C.J.; Lopez, A.D. Mortality by cause for eight regions of the world: Global burden of disease study. Lancet 1997, 349, 1269–1276. [Google Scholar] [CrossRef]
- Ross, R. Cell biology of atherosclerosis. Annu. Rev. Physiol. 1995, 57, 791–804. [Google Scholar] [CrossRef] [PubMed]
- Blankenberg, S.; Barbaux, S.; Tiret, L. Adhesion molecules and atherosclerosis. Atherosclerosis 2003, 170, 191–203. [Google Scholar] [CrossRef]
- Gustafson, B.; Hammarstedt, A.; Andersson, C.X.; Smith, U. Inflamed adipose tissue: A culprit underlying the metabolic syndrome and atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 2276–2283. [Google Scholar] [CrossRef] [PubMed]
- Springer, T.A. Traffic signals for lymphocyte recirculation and leukocyte emigration: The multistep paradigm. Cell 1994, 76, 301–314. [Google Scholar] [CrossRef]
- Baynes, J.W. Role of oxidative stress in development of complications in diabetes. Diabetes 1991, 40, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Hartge, M.M.; Kintscher, U.; Unger, T. Endothelial dysfunction and its role in diabetic vascular disease. Endocrinol. Metab. Clin. N. Am. 2006, 35, 551–560. [Google Scholar] [CrossRef] [PubMed]
- Wolff, S.P.; Jiang, Z.Y.; Hunt, J.V. Protein glycation and oxidative stress in diabetes mellitus and ageing. Free Radic. Biol. Med. 1991, 10, 339–352. [Google Scholar] [CrossRef]
- De Martin, R.; Hoeth, M.; Hofer-Warbinek, R.; Schmid, J.A. The transcription factor NF-kappa B and the regulation of vascular cell function. Arterioscler. Thromb. Vasc. Biol. 2000, 20, E83–E88. [Google Scholar] [CrossRef] [PubMed]
- Nijveldt, R.J.; van Nood, E.; van Hoorn, D.E.; Boelens, P.G.; van Norren, K.; van Leeuwen, P.A. Flavonoids: A review of probable mechanisms of action and potential applications. Am. J. Clin. Nutr. 2001, 74, 418–425. [Google Scholar] [PubMed]
- Grassi, D.; Desideri, G.; Necozione, S.; Lippi, C.; Casale, R.; Properzi, G.; Blumberg, J.B.; Ferri, C. Blood pressure is reduced and insulin sensitivity increased in glucose-intolerant, hypertensive subjects after 15 days of consuming high-polyphenol dark chocolate. J. Nutr. 2008, 138, 1671–1676. [Google Scholar] [PubMed]
- Grassi, D.; Lippi, C.; Necozione, S.; Desideri, G.; Ferri, C. Short-term administration of dark chocolate is followed by a significant increase in insulin sensitivity and a decrease in blood pressure in healthy persons. Am. J. Clin. Nutr. 2005, 81, 611–614. [Google Scholar] [PubMed]
- Stocker, R.; O’Halloran, R.A. Dealcoholized red wine decreases atherosclerosis in apolipoprotein E gene-deficient mice independently of inhibition of lipid peroxidation in the artery wall. Am. J. Clin. Nutr. 2004, 79, 123–130. [Google Scholar] [PubMed]
- Hertog, M.G.; Feskens, E.J.; Hollman, P.C.; Katan, M.B.; Kromhout, D. Dietary antioxidant flavonoids and risk of coronary heart disease: The zutphen elderly study. Lancet 1993, 342, 1007–1011. [Google Scholar] [CrossRef]
- Hertog, M.G.; Kromhout, D.; Aravanis, C.; Blackburn, H.; Buzina, R.; Fidanza, F.; Giampaoli, S.; Jansen, A.; Menotti, A.; Nedeljkovic, S.; et al. Flavonoid intake and long-term risk of coronary heart disease and cancer in the seven countries study. Arch. Intern. Med. 1995, 155, 381–386. [Google Scholar] [CrossRef] [PubMed]
- Knekt, P.; Jarvinen, R.; Reunanen, A.; Maatela, J. Flavonoid intake and coronary mortality in finland: A cohort study. BMJ 1996, 312, 478–481. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Jeong, T.S.; Choi, Y.K.; Hyun, B.H.; Oh, G.T.; Kim, E.H.; Kim, J.R.; Han, J.I.; Bok, S.H. Anti-atherogenic effect of citrus flavonoids, naringin and naringenin, associated with hepatic ACAT and aortic VCAM-1 and MCP-1 in high cholesterol-fed rabbits. Biochem. Biophys. Res. Commun. 2001, 284, 681–688. [Google Scholar] [CrossRef] [PubMed]
- Choe, S.C.; Kim, H.S.; Jeong, T.S.; Bok, S.H.; Park, Y.B. Naringin has an antiatherogenic effect with the inhibition of intercellular adhesion molecule-1 in hypercholesterolemic rabbits. J. Cardiovasc. Pharmacol. 2001, 38, 947–955. [Google Scholar] [CrossRef] [PubMed]
- Tacke, F.; Alvarez, D.; Kaplan, T.J.; Jakubzick, C.; Spanbroek, R.; Llodra, J.; Garin, A.; Liu, J.; Mack, M.; van Rooijen, N.; et al. Monocyte subsets differentially employ CCR2, CCR5, and CX3CR1 to accumulate within atherosclerotic plaques. J. Clin. Investig. 2007, 117, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Dawson, T.C.; Kuziel, W.A.; Osahar, T.A.; Maeda, N. Absence of CC chemokine receptor-2 reduces atherosclerosis in apolipoprotein e-deficient mice. Atherosclerosis 1999, 143, 205–211. [Google Scholar] [CrossRef]
- Gosling, J.; Slaymaker, S.; Gu, L.; Tseng, S.; Zlot, C.H.; Young, S.G.; Rollins, B.J.; Charo, I.F. MCP-1 deficiency reduces susceptibility to atherosclerosis in mice that overexpress human apolipoprotein B. J. Clin. Investig. 1999, 103, 773–778. [Google Scholar] [CrossRef] [PubMed]
- Lesnik, P.; Haskell, C.A.; Charo, I.F. Decreased atherosclerosis in CX3CR1–/– mice reveals a role for fractalkine in atherogenesis. J. Clin. Investig. 2003, 111, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, C.K.; Fuchs, E. It’s got you covered. NF-kappab in the epidermis. J. Cell Biol. 2000, 149, 999–1004. [Google Scholar] [CrossRef] [PubMed]
- Zandi, E.; Karin, M. Bridging the gap: Composition, regulation, and physiological function of the IκB kinase complex. Mol. Cell. Biol. 1999, 19, 4547–4551. [Google Scholar] [CrossRef] [PubMed]
- Nathan, C. Points of control in inflammation. Nature 2002, 420, 846–852. [Google Scholar] [CrossRef] [PubMed]
- Ross, R. Atherosclerosis—An inflammatory disease. N. Engl. J. Med. 1999, 340, 115–126. [Google Scholar] [CrossRef]
- Kim, S.W.; Kim, C.E.; Kim, M.H. Flavonoids inhibit high glucose-induced up-regulation of ICAM-1 via the p38 MAPK pathway in human vein endothelial cells. Biochem. Biophys. Res. Commun. 2011, 415, 602–607. [Google Scholar] [CrossRef] [PubMed]
- Avogaro, A.; de Kreutzenberg, S.V. Mechanisms of endothelial dysfunction in obesity. Clin. Chim. Acta 2005, 360, 9–26. [Google Scholar] [CrossRef] [PubMed]
- Kandhare, A.D.; Raygude, K.S.; Ghosh, P.; Ghule, A.E.; Bodhankar, S.L. Neuroprotective effect of naringin by modulation of endogenous biomarkers in streptozotocin induced painful diabetic neuropathy. Fitoterapia 2 2012, 83, 650–659. [Google Scholar] [CrossRef] [PubMed]
- Chanet, A.; Milenkovic, D.; Deval, C.; Potier, M.; Constans, J.; Mazur, A.; Bennetau-Pelissero, C.; Morand, C.; Berard, A.M. Naringin, the major grapefruit flavonoid, specifically affects atherosclerosis development in diet-induced hypercholesterolemia in mice. J. Nutr. Biochem. 2012, 23, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.J.; Kim, D.I.; Kim, W.J.; Moon, S.K. Naringin inhibits matrix metalloproteinase-9 expression and AKT phosphorylation in tumor necrosis factor-α-induced vascular smooth muscle cells. Mol. Nutr. Food Res. 2009, 53, 1582–1591. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.J.; Moon, G.S.; Choi, W.S.; Kim, W.J.; Moon, S.K. Naringin-induced p21WAF1-mediated G(1)-phase cell cycle arrest via activation of the Ras/Raf/ERK signaling pathway in vascular smooth muscle cells. Food Chem. Toxicol. 2008, 46, 3800–3807. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Xu, H.; Peng, J.; Wang, C.; Jin, Y.; Liu, K.; Sun, H.; Qin, J. Potent anti-inflammatory effect of dioscin mediated by suppression of TNF-α-induced VCAM-1, ICAM-1and EL expression via the NF-κB pathway. Biochimie 2015, 110, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.; Nallasamy, P.; Liu, D.; Shah, H.; Li, J.Z.; Chitrakar, R.; Si, H.; McCormick, J.; Zhu, H.; Zhen, W.; et al. Luteolin protects against vascular inflammation in mice and TNF-α-induced monocyte adhesion to endothelial cells via suppressing IΚBα/NF-κB signaling pathway. J. Nutr. Biochem. 2015, 26, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Zhang, X.; Zheng, H.; Hu, D.; Zhang, Y.; Guan, Q.; Liu, L.; Ding, Q.; Li, Y. Clematichinenoside inhibits VCAM-1 and ICAM-1 expression in TNF-α-treated endothelial cells via NADPH oxidase-dependent IκB kinase/NF-κB pathway. Free Radic. Biol. Med. 2015, 78, 190–201. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Guo, Z.P.; Jiao, X.Y.; Jia, R.Z.; Zhang, Y.H.; Li, J.Y.; Huang, X.L.; Liu, H.J. Peoniflorin suppresses tumor necrosis factor-α induced chemokine production in human dermal microvascular endothelial cells by blocking nuclear factor-κB and ERK pathway. Arch. Dermatol. Res. 2011, 303, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.C.; Chu, C.F.; Wang, C.N.; Wu, H.T.; Bi, K.W.; Pang, J.H.; Huang, S.T. The anti-atherosclerotic effect of tanshinone IIA is associated with the inhibition of TNF-α-induced VCAM-1, ICAM-1 and CX3CL1 expression. Phytomedicine 2014, 21, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Bourcier, T.; Sukhova, G.; Libby, P. The nuclear factor κ-B signaling pathway participates in dysregulation of vascular smooth muscle cells in vitro and in human atherosclerosis. J. Biol. Chem. 1997, 272, 15817–15824. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Castranova, V.; Shi, X. New insights into the role of nuclear factor-κB in cell growth regulation. Am. J. Pathol. 2001, 159, 387–397. [Google Scholar] [CrossRef]
- Silverman, N.; Maniatis, T. Nf-kappab signaling pathways in mammalian and insect innate immunity. Genes Dev. 2001, 15, 2321–2342. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Zhou, Y.; Shen, P. NF-κB and its regulation on the immune system. Cell. Mol. Immunol. 2004, 1, 343–350. [Google Scholar] [PubMed]
- Marcu, K.B.; Otero, M.; Olivotto, E.; Borzi, R.M.; Goldring, M.B. NF-κB signaling: Multiple angles to target OA. Curr. Drug Targets 2010, 11, 599–613. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.C.; Sundaram, C.; Reuter, S.; Aggarwal, B.B. Inhibiting NF-κB activation by small molecules as a therapeutic strategy. Biochim. Biophys. Acta 2010, 1799, 775–787. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wang, C.; Peng, J.; Liang, J.; Jin, Y.; Liu, Q.; Meng, Q.; Liu, K.; Sun, H. Naringin inhibits TNF-α induced oxidative stress and inflammatory response in HUVECs via Nox4/NF-κB and PI3K/Akt pathways. Curr. Pharm. Biotechnol. 2014, 15, 1173–1182. [Google Scholar] [CrossRef] [PubMed]
- Chtourou, Y.; Aouey, B.; Kebieche, M.; Fetoui, H. Protective role of naringin against cisplatin induced oxidative stress, inflammatory response and apoptosis in rat striatum via suppressing ROS-mediated NF-κB and P53 signaling pathways. Chem. Biol. Interact. 2015, 239, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Lin, W.; Liang, C.; Zhang, D.; Yang, F.; Zhang, Y.; Zhang, X.; Feng, J.; Chen, C. Naringin rescued the TNF-α-induced inhibition of osteogenesis of bone marrow-derived mesenchymal stem cells by depressing the activation of NF-кB signaling pathway. Immunol. Res. 2015, 62, 357–367. [Google Scholar] [CrossRef] [PubMed]
- Chomczynski, P.; Sacchi, N. Single-step method of rna isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 1987, 162, 156–159. [Google Scholar] [CrossRef]
- Sample Availability: Not available.
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsueh, T.-P.; Sheen, J.-M.; Pang, J.-H.S.; Bi, K.-W.; Huang, C.-C.; Wu, H.-T.; Huang, S.-T. The Anti-Atherosclerotic Effect of Naringin Is Associated with Reduced Expressions of Cell Adhesion Molecules and Chemokines through NF-κB Pathway. Molecules 2016, 21, 195. https://doi.org/10.3390/molecules21020195
Hsueh T-P, Sheen J-M, Pang J-HS, Bi K-W, Huang C-C, Wu H-T, Huang S-T. The Anti-Atherosclerotic Effect of Naringin Is Associated with Reduced Expressions of Cell Adhesion Molecules and Chemokines through NF-κB Pathway. Molecules. 2016; 21(2):195. https://doi.org/10.3390/molecules21020195
Chicago/Turabian StyleHsueh, Tun-Pin, Jer-Ming Sheen, Jong-Hwei S. Pang, Kuo-Wei Bi, Chao-Chun Huang, Hsiao-Ting Wu, and Sheng-Teng Huang. 2016. "The Anti-Atherosclerotic Effect of Naringin Is Associated with Reduced Expressions of Cell Adhesion Molecules and Chemokines through NF-κB Pathway" Molecules 21, no. 2: 195. https://doi.org/10.3390/molecules21020195





