Synthesis and Characterization of Novel Polythiophenes Containing Pyrene Chromophores: Thermal, Optical and Electrochemical Properties
Abstract
:1. Introduction
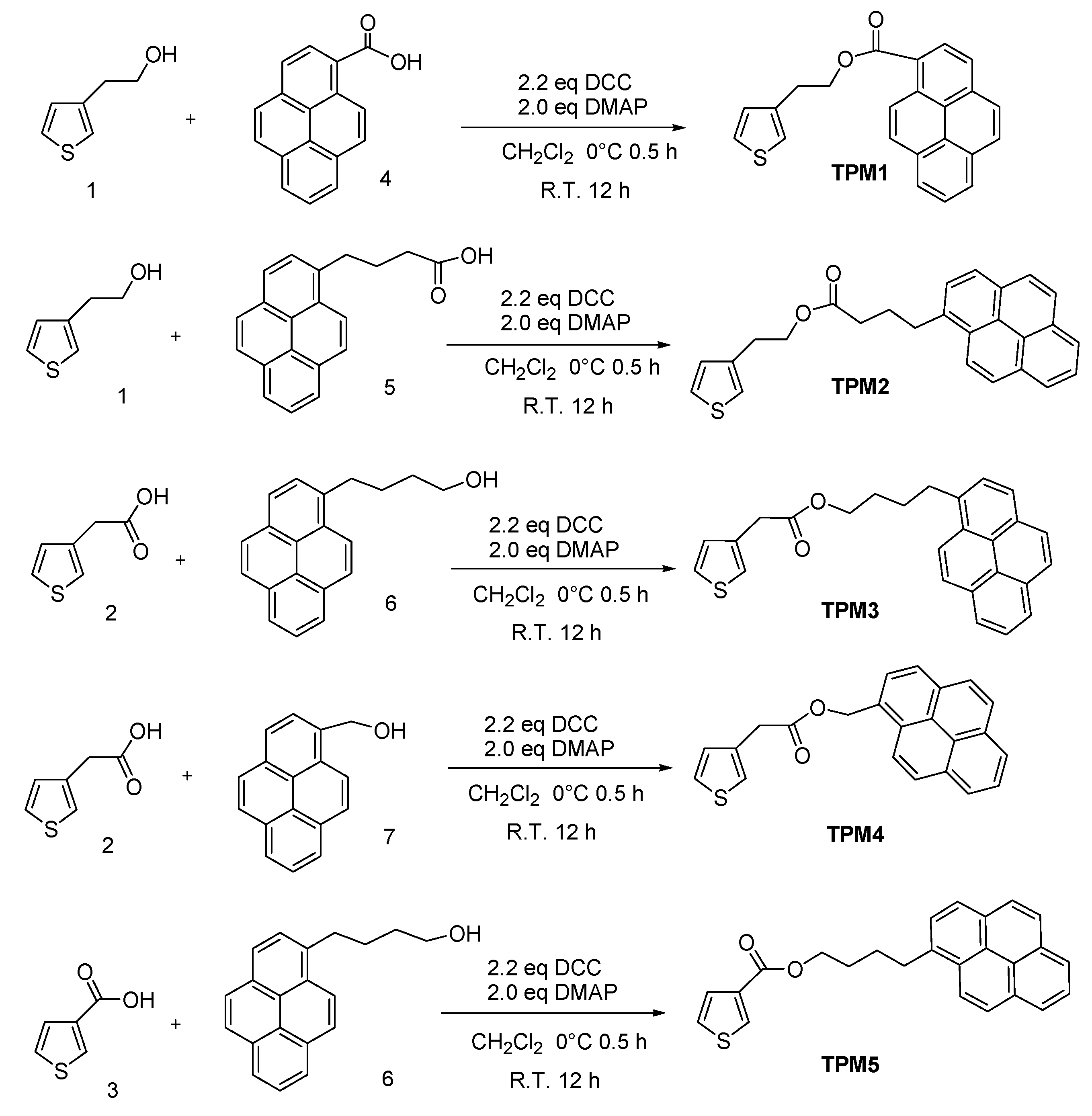
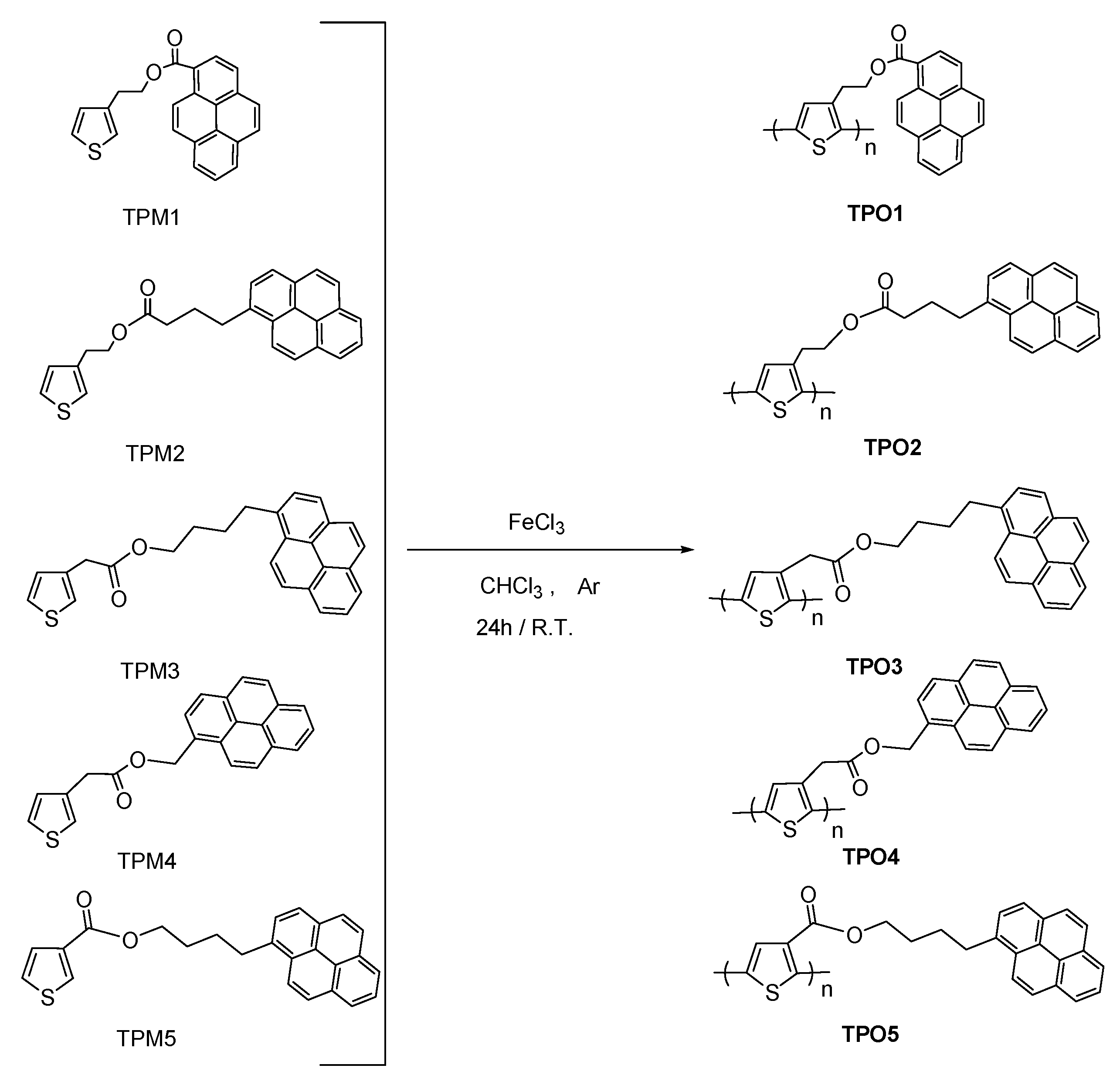
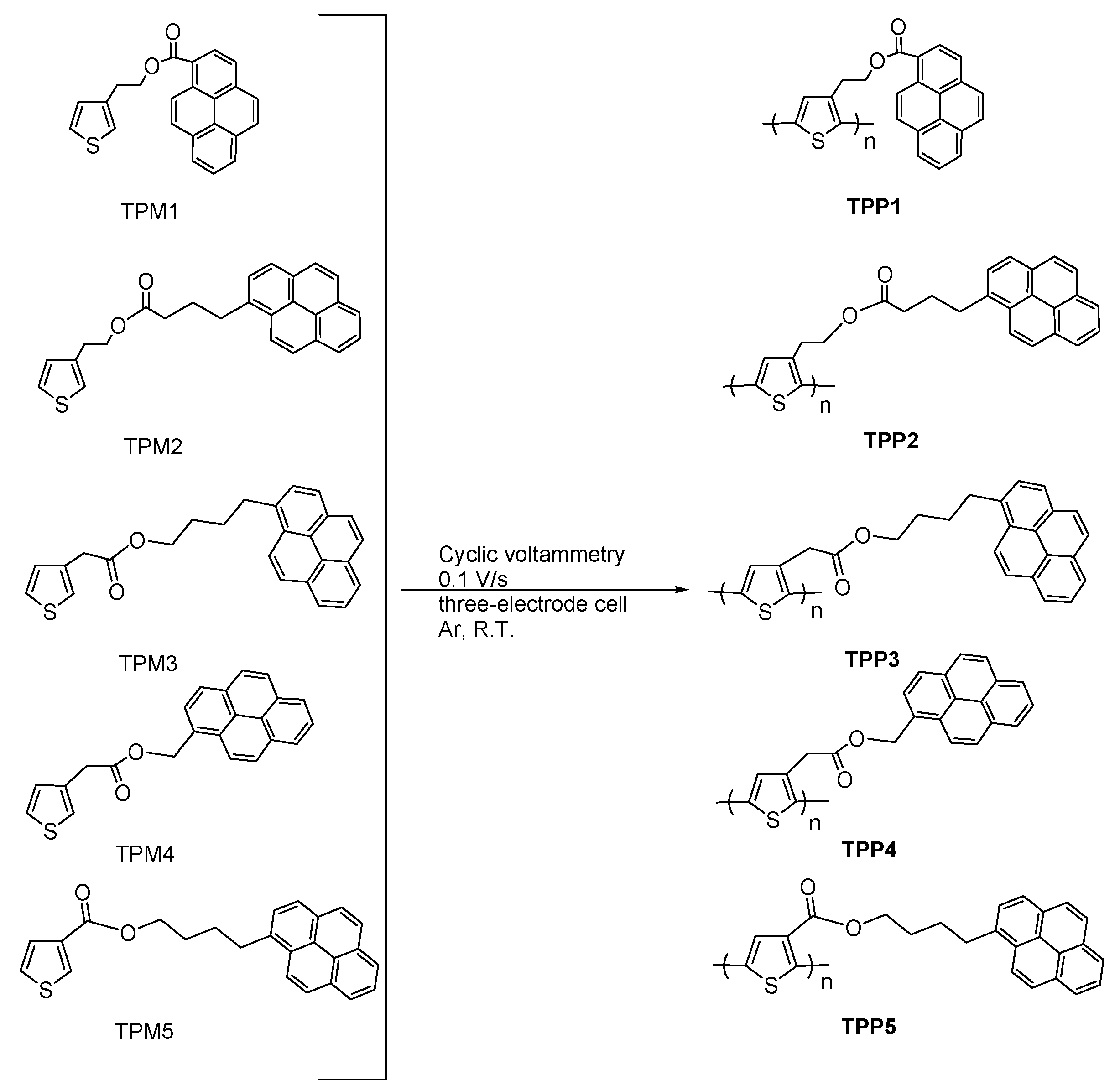
2. Results and Discussion
2.1. Spectroscopical Characterization
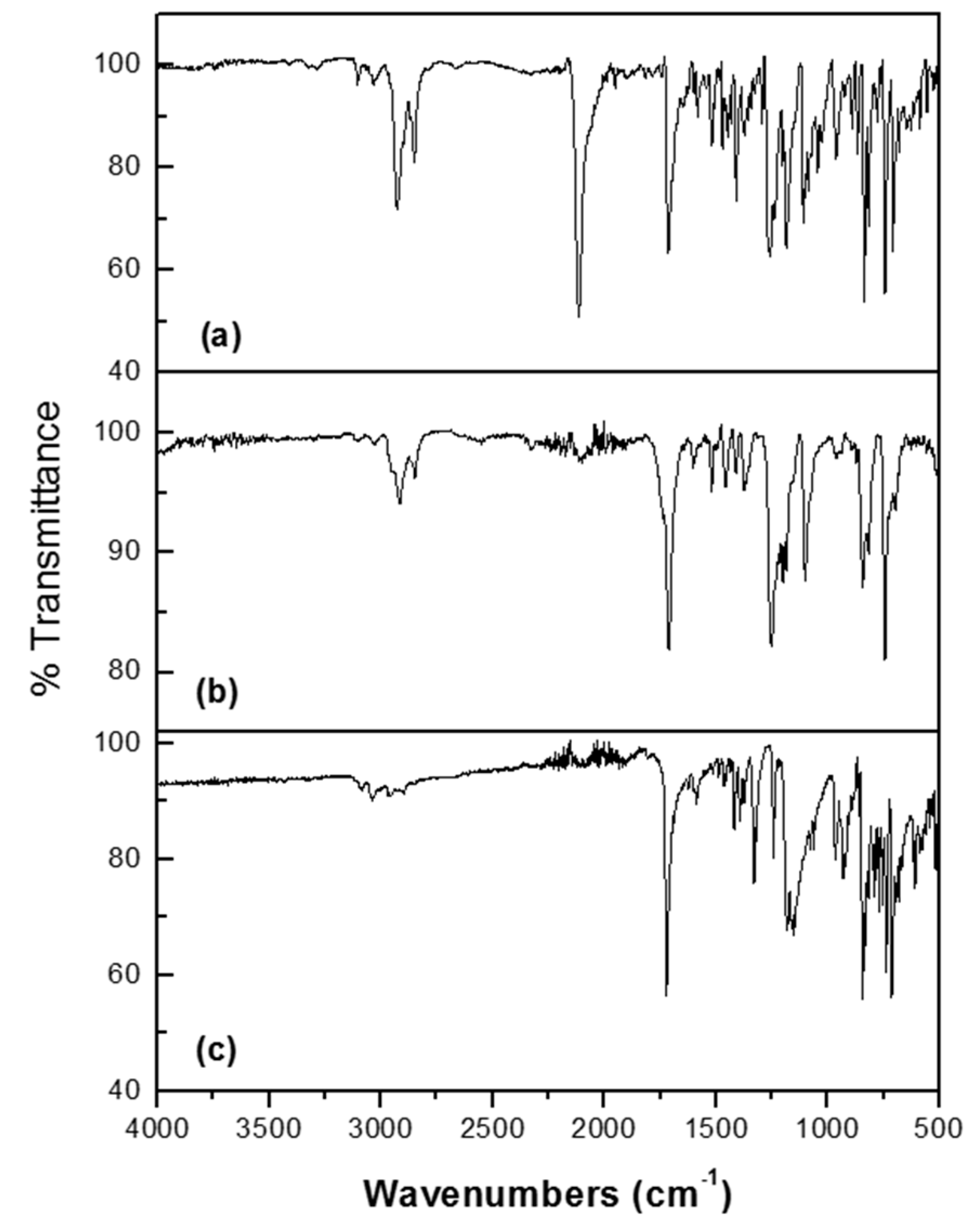
2.2. Thermal Properties
| Compound | m.p. (°C) | Compound | m.p. (°C)/Tg (°C) | T10 (°C) | Degradation (°C) | % Wt Remaining |
|---|---|---|---|---|---|---|
| TPM1 | 117.6 | TPO1 | >250/38.0 | 292.6 | 310–348 | 3.33 |
| TPM2 | 66 | TPO2 | >250/36.0 | 299.3 | 310–330 | 12.16 |
| TPM3 | 83.3 | TPO3 | >250/39.1 | 272.7 | 277–347 | 12.14 |
| TPM4 | 67.7 | TPO4 | >250/36.1 | 255.9 | 260–315 | 38.71 |
| TPM5 | 96 | TPO5 | >250/37.9 | 273.2 | 313–374 | 4.659 |

2.3. Optical Properties of the Monomers
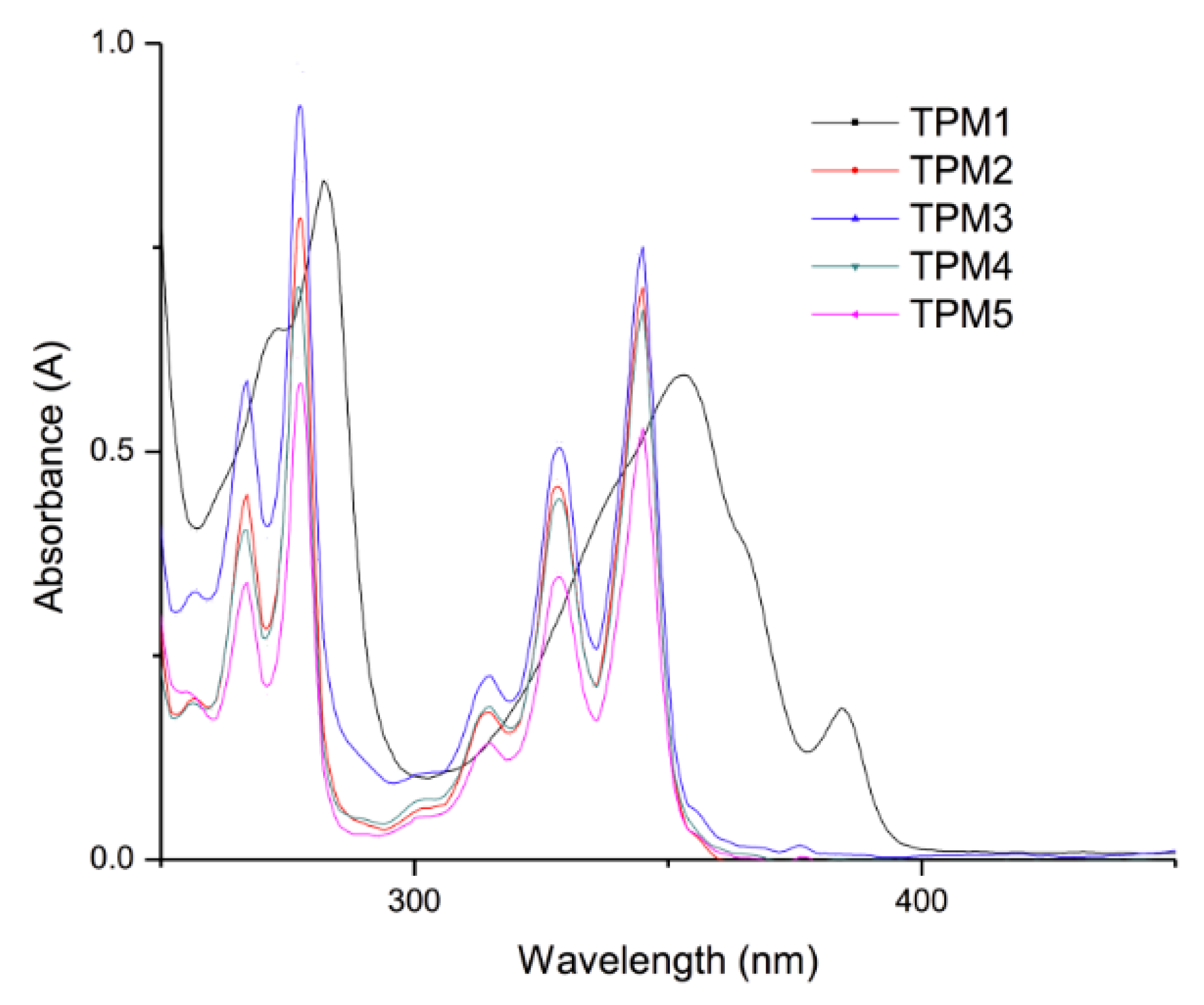
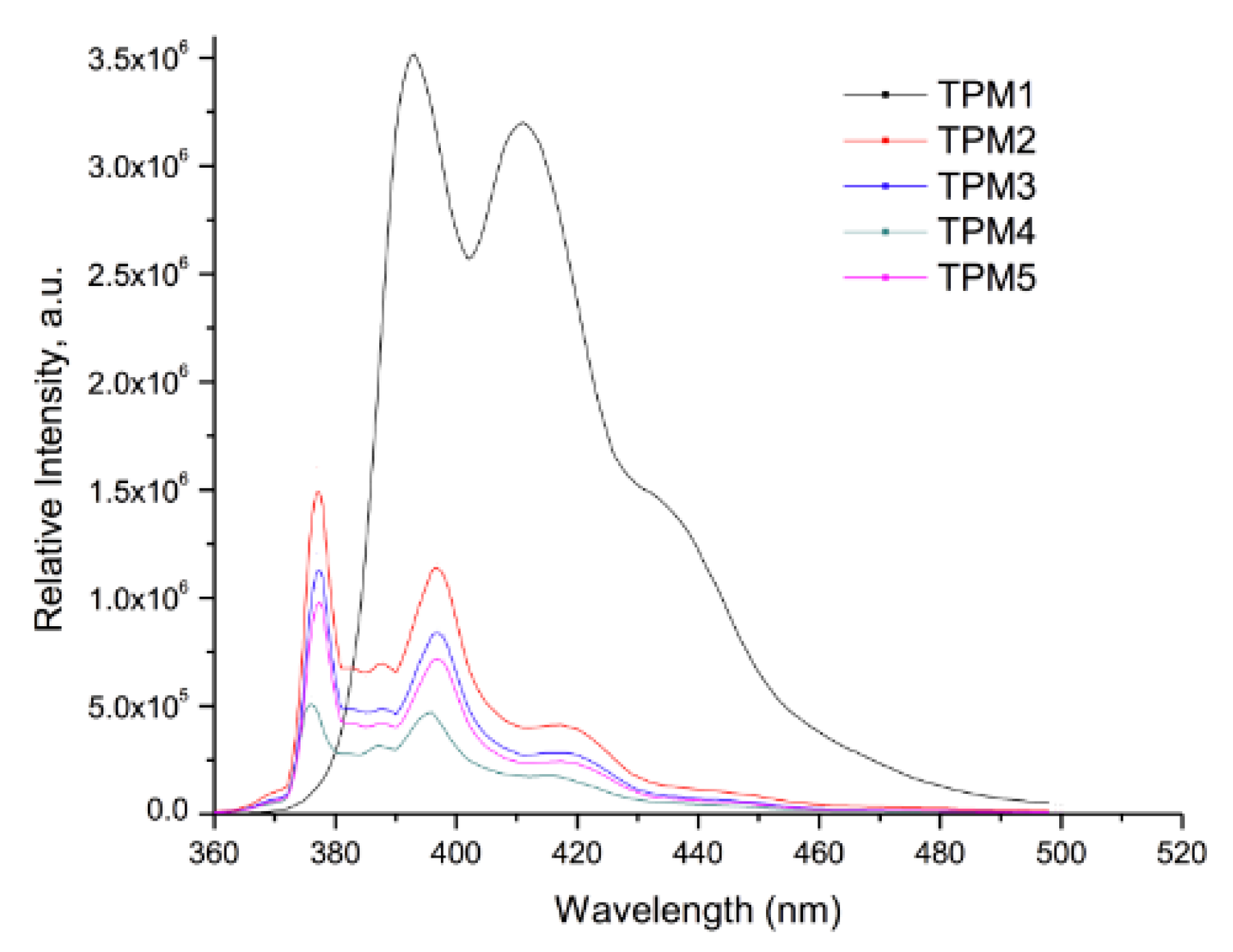
| Compound | Absorption (λ nm) | Cut off (nm) | Emission (nm) | Cut off (nm) |
|---|---|---|---|---|
| TPM1 | 353 | 450 | 391–440 a | 500 |
| TPM2 | 345 | 450 | 377–416 a | 500 |
| TPM3 | 345 | 450 | 377–416 a | 500 |
| TPM4 | 345 | 450 | 377–416 a | 500 |
| TPM5 | 345 | 450 | 377–416 a | 500 |
2.4. Optical Properties of the Polymers

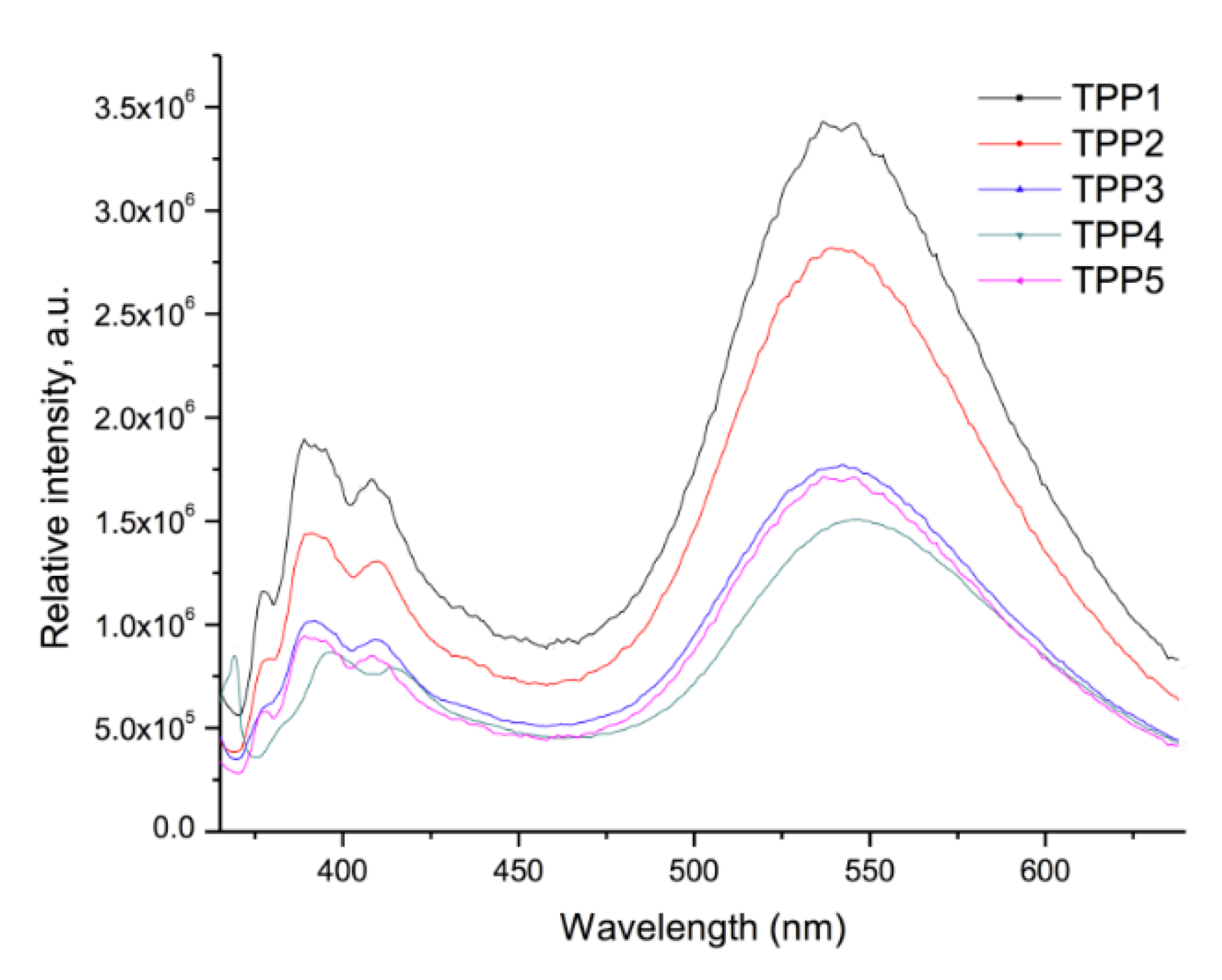
| Compound | Absorption (λ nm) | Cut off (nm) | Emission (λ nm) λexc = 360 nm | Cut off (nm) |
|---|---|---|---|---|
| TPP1 | 275 b–356 a | 450 | 388 c, 409 c, 545 d | 640 |
| TPP2 | 273 b–345 a | 450 | 391 c, 410 c, 539 d | 640 |
| TPP3 | 273 b–345 a | 450 | 389 c, 409 c, 543 d | 640 |
| TPP4 | 274 b–345 a | 450 | 397 c, 413 c, 543 d | 640 |
| TPP5 | 275 b–357 a | 450 | 390 c, 409 c, 544 d | 640 |
2.5. Electrochemical Properties of Monomers and Polymers

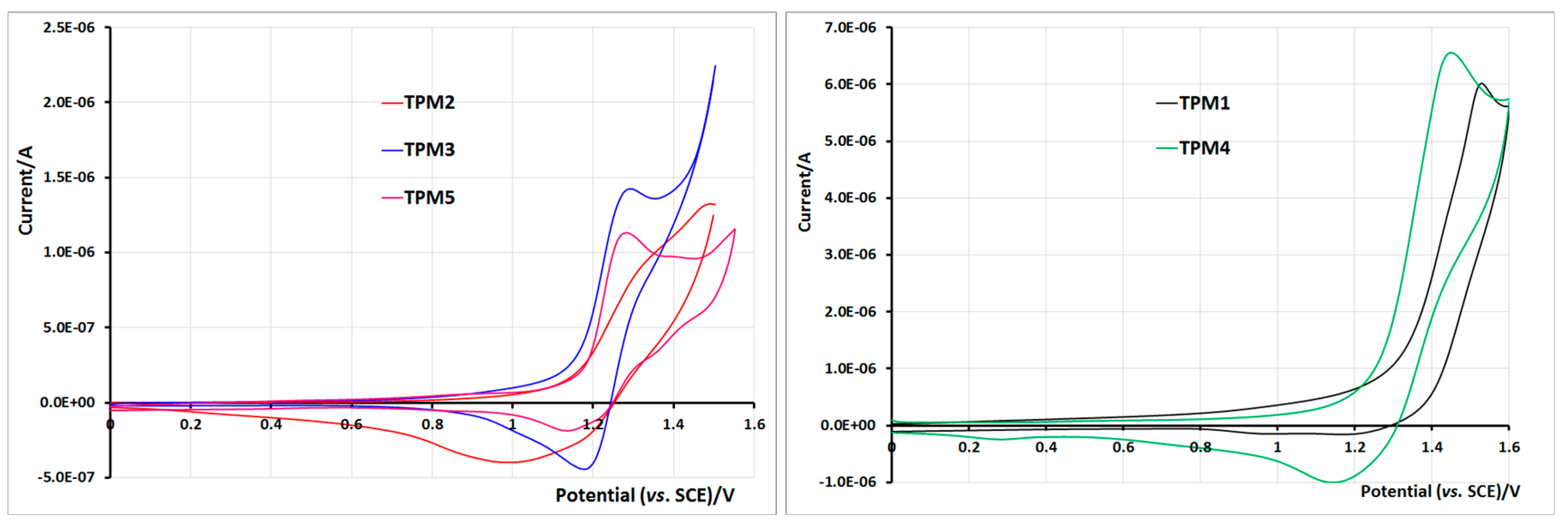
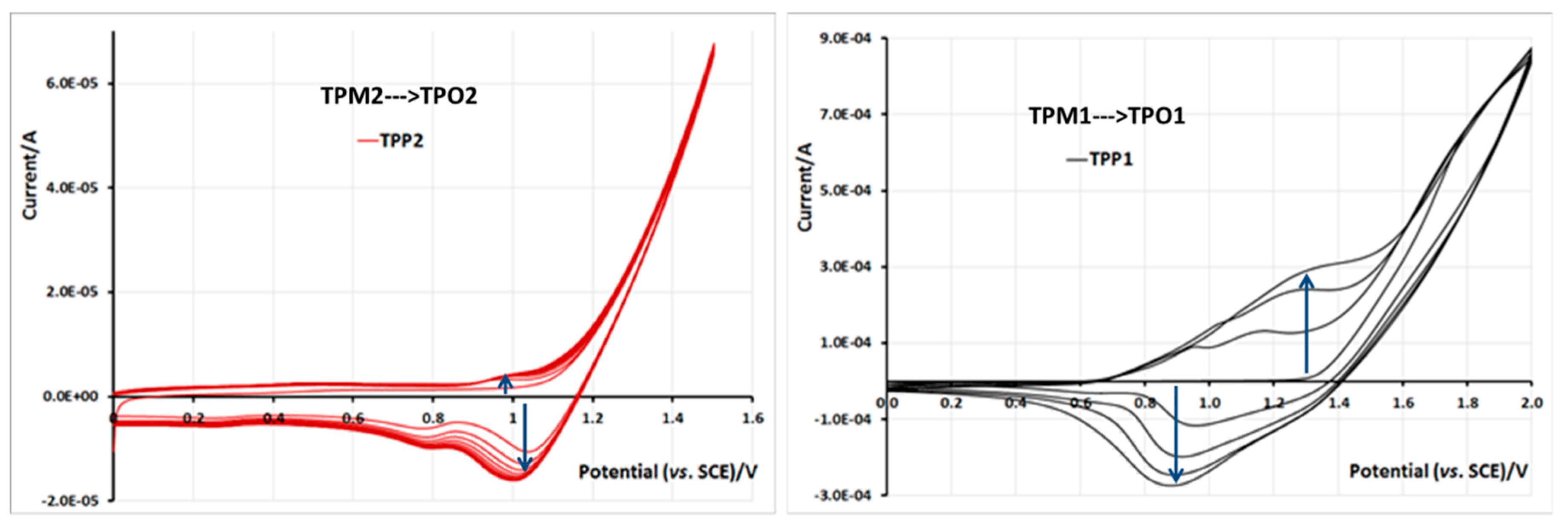
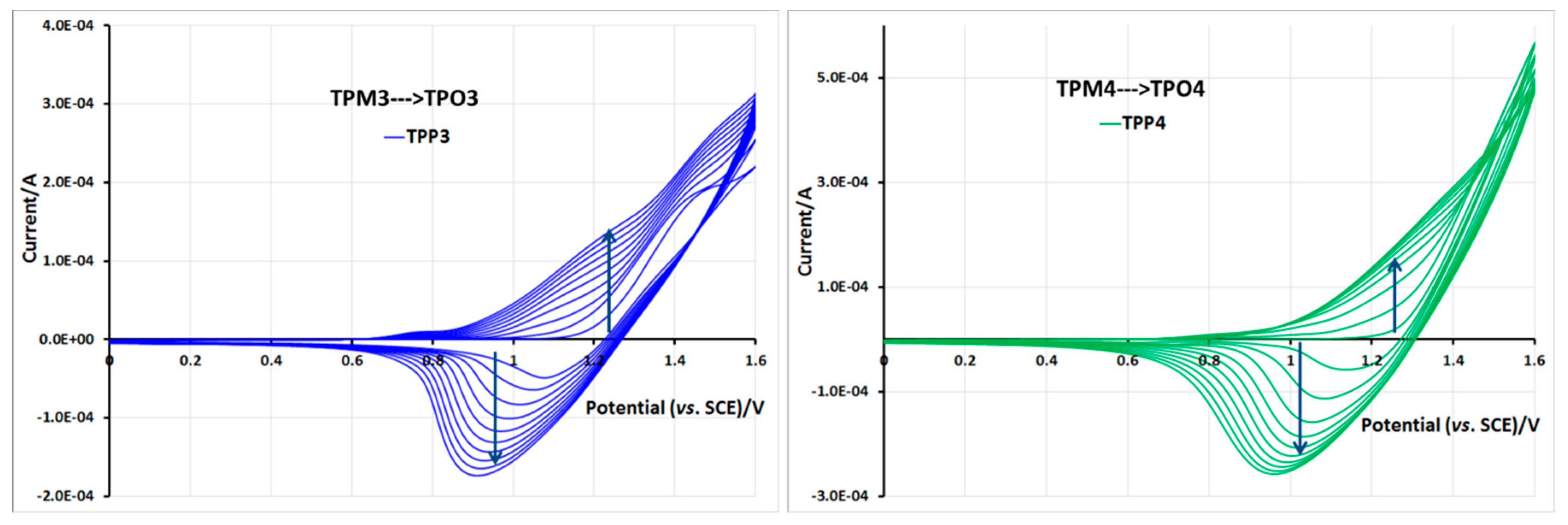
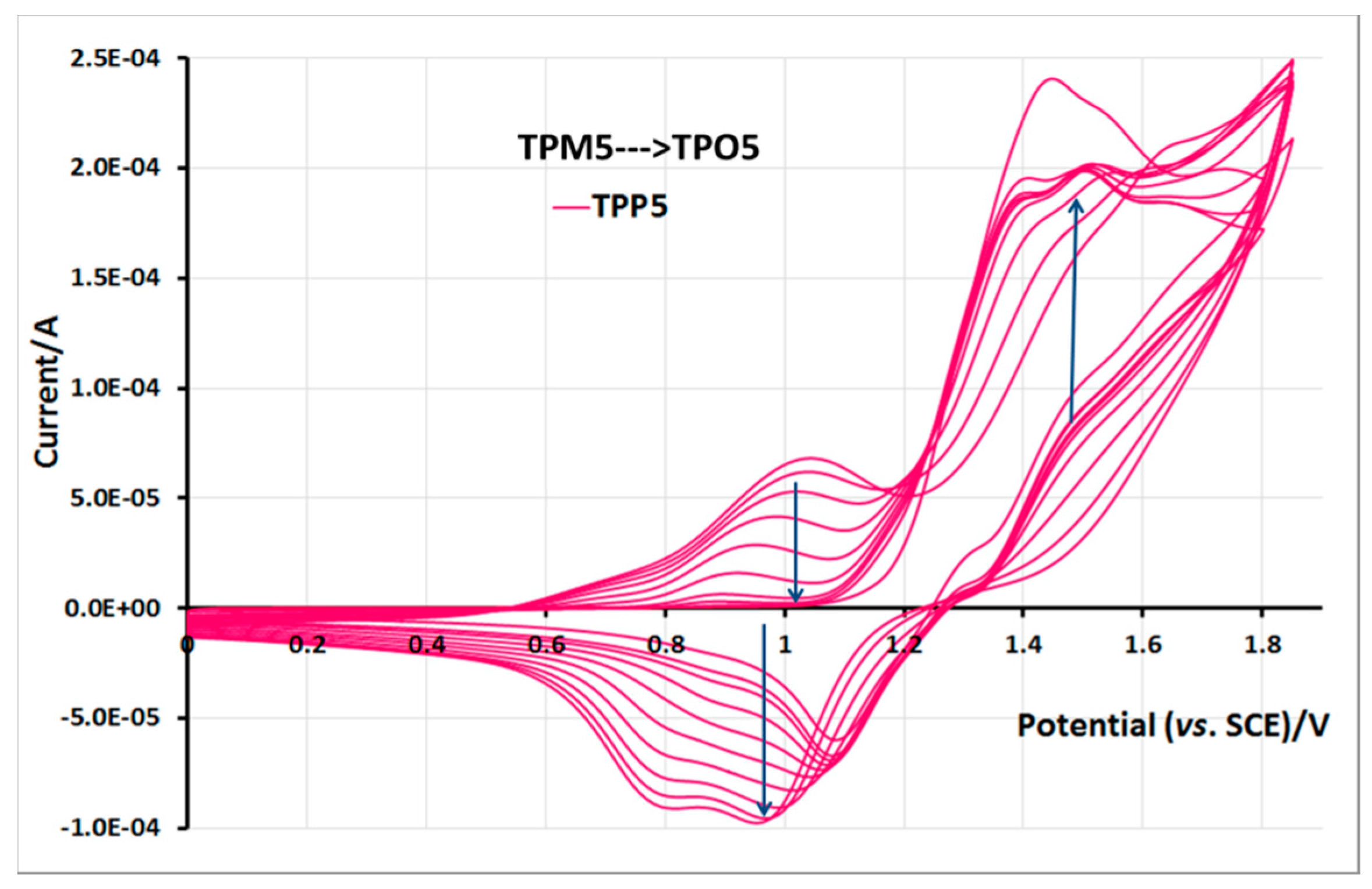
3. Experimental Section
3.1. Apparatus
3.2. Chemicals
3.3. Electrochemical Polymerization
3.4. Synthesis of the Monomers
General Synthesis
3.5. Synthesis of the Oligomers and the Polymers
3.5.1. Chemical Polymerization
3.5.2 Synthesis of TPO1–5
3.6. Thermal Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Javadi, A.; Najjar, W.; Bahadori, S.; Vatanpour, V.; Malek, A.; Abouzari-Lotf, E.; Shockravi, A. High refractive index and low-birefringence polyamides containing thiazole and naphthalene units. RSC Adv. 2015, 5, 91670–91682. [Google Scholar] [CrossRef]
- Fukuzaki, N.; Higashihara, T.; Ando, S.; Ueda, M. Synthesis and Characterization of Highly Refractive Polyimides Derived from Thiophene-Containing Aromatic Diamines and Aromatic Dianhydrides. Macromolecules 2010, 43, 1836–1843. [Google Scholar] [CrossRef]
- Javadi, A.; Shockravi, A.; Rafieimanesh, A.; Malek, A.; Ando, S. Synthesis and structure-property relationships of novel thiazole-containing poly(amide imide)s with high refractive indices and low birefringences. Polym. Int. 2015, 64, 486–495. [Google Scholar] [CrossRef]
- Liu, J.G.; Nakamura, Y.; Terraza, C.A.; Shibasaki, Y.; Ando, S.; Ueda, M. Highly Refractive Polyimides Derived from 2,8-Bis(p-aminophenylenesulfanyl)dibenzothiophene and Aromatic Dianhydrides. Macromol. Chem. Phys. 2008, 209, 195–203. [Google Scholar] [CrossRef]
- Javadi, A.; Shockravi, A.; Koohgard, M.; Malek, A.; Shourkaei, F.A.; Ando, S. Nitro-substituted polyamides: A new class of transparent and highly refractive materials. Eur. Polym. J. 2015, 66, 328–341. [Google Scholar] [CrossRef]
- Chan, H.S.O.; Ng, S.C. Synthesis, characterization and applications of thiophene-based functional polymers. Prog. Polym. Sci. 1998, 23, 1167–1231. [Google Scholar] [CrossRef]
- Zotti, G.; Marin, R.A.; Gallazzi, M.C. Electrochemical Polymerization of Mixed Alkyl-Alkoxybithiophenes and -terthiophenes. Substitution-Driven Polymerization from Thiophene Hexamers to Long-Chain Polymers. Chem. Mater. 1997, 9, 2945–2950. [Google Scholar] [CrossRef]
- Hosseini, S.H.; Entezami, A. Chemical and electrochemical synthesis of homopolymer and copolymers of 3-methoxyethoxythiophene with aniline, thiophene and pyrrole for studies of their gas and vapour sensing. Polym. Adv. Technol. 2001, 12, 524–534. [Google Scholar] [CrossRef]
- Roux, C.; Leclerc, M. Rod-to-coil transition in alkoxy-substituted polythiophenes. Macromolecules 1992, 25, 2141–2144. [Google Scholar] [CrossRef]
- Lévesque, I.; Leclerc, M. Ionochromic and Thermochromic Phenomena in a Regioregular Polythiophene Derivative Bearing Oligo(oxyethylene) Side Chains. Chem. Mater. 1996, 8, 2843–2849. [Google Scholar] [CrossRef]
- Levésque, I.; Bazinet, P.; Roovers, J. Optical Properties and Dual Electrical and Ionic Conductivity in Poly(3-methylhexa(oxyethylene)oxy-4-methylthiophene). Macromolecules 2000, 33, 2952–2957. [Google Scholar] [CrossRef]
- Marsella, M.J.; Swager, T.M. Designing Conducting Polymer-Based Sensors: Selective Ionochromic Response in Crown Ether Containing Polythiophenes. J. Am. Chem. Soc. 1993, 115, 12214–12215. [Google Scholar] [CrossRef]
- Lévesque, I.; Leclerc, M. Novel Dual Photochromism in Polythiophene Derivatives. Macromolecules 1997, 30, 4347–4352. [Google Scholar] [CrossRef]
- Yoshino, K.; Nakajima, S.; Onada, M.; Sugimoto, R. Electrical and optical properties of poly(3-alkylthiophene). Synth. Met. 1989, 28, 349–357. [Google Scholar] [CrossRef]
- Kumpumbu-Kalemba, L.; Leclerc, M. Electrochemical characterization of monolayers of a biotinylated polythiophene: Towards the development of polymeric biosensors. Chem. Commun. 2000, 1847–1848. [Google Scholar] [CrossRef]
- Raymond, F.; di Césare, N.; Belletéte, M.; Durocher, G.; Leclerc, M. Molecular Design of a Thermochromic Polythiophene Derivative. Adv. Mater. 1998, 10, 599–602. [Google Scholar] [CrossRef]
- Zhou, E.; He, C.; Tan, Z.; Yang, C.; Li, Y. Synthesis and properties of polythiophenes with conjugated side-chains containing carbon–carbon double and triple bonds. J. Polym. Sci. A 2006, 44, 4916–4922. [Google Scholar] [CrossRef]
- Perepichka, I.F.; Perepichka, D.F.; Meng, H.; Wudl, F. Light-Emitting Polythiophenes. Adv. Mater. 2005, 17, 2281–2305. [Google Scholar] [CrossRef]
- Tanaka, F.; Kawai, T.; Kojima, S.; Yoshino, K. Electrical and optical properties of poly(3-alkoxythiophene) and their application for gas sensor. Synth. Met. 1999, 102, 1358–1359. [Google Scholar] [CrossRef]
- Rella, R.; Siciliano, P.; Quaranta, F.; Primo, T.; Valli, L.; Schenetti, L.; Mucci, A.; Iarossi, D. Gas sensing measurements and analysis of the optical properties of poly[3-(butylthio)thiophene] Langmuir-Blodgett films. Sens. Actuators 2000, 68, 203–209. [Google Scholar] [CrossRef]
- Rella, R.; Siciliano, P.; Quaranta, F.; Primo, T.; Valli, L.; Schenetti, L. Poly[3-(butylthio)thiophene] Langmuir-Blodgett films as selective solid state chemiresistors for nitrogen dioxide. Colloids Surf. A Physicochem. Eng. Aspects 2002, 198–200, 829–833. [Google Scholar] [CrossRef]
- Rivers, T.J.; Hudson, T.W.; Schmidt, C. Synthesis of a Novel, Biodegradable Electrically Conducting Polymer for Biomedical Applications. Adv. Funct. Mater. 2002, 12, 33–37. [Google Scholar] [CrossRef]
- Mousavi, Z.; Bobacka, J.; Ivaska, A. Potentiometric Ag+ Sensors Based on Conducting Polymers: A Comparison between Poly(3,4-ethylenedioxythiophene) and Polypyrrole Doped with Sulfonated Calixarenes. Electroanalysis 2005, 17, 1609–1615. [Google Scholar] [CrossRef]
- Tang, Y.; He, F.; Yu, M.; Feng, F.; An, L.; Sun, H.; Wang, S.; Li, Y.; Zhu, D. A Reversible and Highly Selective Fluorescent Sensor for Mercury(II) Using Poly(thiophene)s that Contain Thymine Moieties. Macromol. Rapid Commun. 2006, 27, 389–392. [Google Scholar] [CrossRef]
- Pandey, P.C.; Upadhyay, S.; Singh, G.; Prakash, R.; Srivastava, R.C.; Seth, P.K. A New Solid-State pH Sensor and its Application in the Construction of All Solid-State Urea Biosensor. Electroanalysis 2000, 12, 517–521. [Google Scholar] [CrossRef]
- Singhal, R.; Takashima, K.; Kaneto, K.; Samanta, S.B.; Annapoorni, S.; Malhotra, B.D. Langmuir-Blodgett films of poly (3-dodecyl thiophene) for application to glucose biosensor. Sens. Actuators 2002, 86, 42–48. [Google Scholar] [CrossRef]
- Singhal, R.; Chaubey, A.; Kaneto, K.; Takashima, W.; Malhotra, B.D. Poly-3-hexyl thiophene Langmuir-Blodgett films for application to glucose biosensor. Biotechnol. Bioeng. 2004, 85, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Béra-Abérem, M.; Ho, H.A.; Leclerc, M. Functional Polythiophenes as Optical Chemo and Biosensors. Tetrahedron 2004, 60, 11169–11173. [Google Scholar] [CrossRef]
- Almeida, S.; Rivera, E.; Reyna-Gonzalez, J.M.; Huerta, G.; Tapia, F.; Aguilar-Martínez, M. Synthesis and characterization of novel polythiophenes bearing oligo(ethylene glycol) spacers and crown ethers. Synth. Met. 2009, 159, 1215–1223. [Google Scholar] [CrossRef]
- Tapia, F.; Reyna-González, J.M.; Huerta, G.; Almeida, S.; Rivera, E. Synthesis and characterization of novel polythiophenes bearing oligo(ethylene glycol) segments and azobenzene units. Polym. Bull. 2010, 64, 581–594. [Google Scholar] [CrossRef]
- Aguilar-Ortiz, E.; Zaragoza-Galán, G.; Rein, R.; Solladié, N.; Aguilar-Martinez, M.; Macías-Ruvalcaba, N.; Rivera, E. Preparation and characterization of novel polythiophenes bearing oligo(ethylene glycol) spacers and porphyrin units: Optical and electrochemical properties. Synth. Met. 2012, 162, 1000–1009. [Google Scholar] [CrossRef]
- Winnik, F.M. Photophysics of preassociated pyrenes in aqueous polymer solutions and in other organized media. Chem. Rev. 1993, 93, 587–614. [Google Scholar] [CrossRef]
- Rivera, E.; Belletête, M.; Zhu, X.X.; Durocher, G.; Giasson, R. Novel polyacetylenes containing pendant 1-pyrenyl groups: Synthesis, characterization, and thermal and optical properties. Polymer 2002, 43. [Google Scholar] [CrossRef]
- Rivera, E.; Wang, R.; Zhu, X.X.; Zargarian, D.; Giasson, R. Preparation of cis-poly(1-ethynylpyrene) using (1-Me-indenyl)(PPh3)Ni–C≡C–Ph/methylaluminoxane as catalyst. J. Mol. Catal. 2003, 204, 325–332. [Google Scholar] [CrossRef]
- Belletête, M.; Rivera, E.; Giasson, R.; Zhu, X.X.; Durocher, G. UV-Vis and fluorescence study of polyacetylenes with pendant 1-pyrenyl groups: A comparative investigation of cis-and trans-poly (1-ethynyl-pyrene). Synth. Met. 2004, 14, 37–42. [Google Scholar] [CrossRef]
- Rivera, E.; Aguilar-Martínez, M.; Terán, G.; Flores, R.F.; Bautista-Martínez, J.A. Thermal, optical, electrochemical properties and conductivity of trans-and cis-poly (1-ethynylpyrene): A comparative investigation. Polymer 2005, 46, 4789–4798. [Google Scholar] [CrossRef]
- Morales-Saavedra, O.; Rivera, E. Linear and nonlinear optical properties of trans-and cis-poly (1-ethynylpyrene) based sonogel hybrid materials. Polymer 2006, 47, 5330–5337. [Google Scholar] [CrossRef]
- Morales-Espinoza, E.G.; Aguilar-Ortíz, E.; Vázquez-Arce, A.; Vázquez-Torres, H.; Rodriguez-Alba, E.; Rivera, E. Synthesis and characterization of novel luminescent polythiophenes containing pyrene units and oligo (ethylene glycol) spacers: Thermal and optical properties. Synth. Met. 2015, 199, 223–231. [Google Scholar] [CrossRef]
- Rodriguez-Alba, E.; Ortíz-Palacios, J.; Morales-Espinoza, E.G.; Vonlanthen, M.; Valderrama, B.X.; Rivera, E. Synthesis, characterization and optical properties of novel oligothiophenes bearing pyrene units attached via well defined oligo(ethylene glycol) spacers. Synth. Met. 2015, 206, 92–105. [Google Scholar] [CrossRef]
- Farcas, A.; Jarroux, N.; Ghosh, I.; Guégan, P.; Nau, W.M.; Harabagiu, V. Polyrotaxanes of Pyrene-Triazole Conjugated Azomethine and α-Cyclodextrin with High Fluorescence Properties. Macromol. Chem. Phys. 2009, 210, 1440–1449. [Google Scholar] [CrossRef]
- Müllen, K.; Wegner, G. Electronic Materials: The Oligomeric Approach; Wiley-VCH: Weinheim, Germany, 1998. [Google Scholar]
- Fichou, D. Handbook of Oligo and Polythiophenes; Wiley-VCH: Weinheim, Germany, 1999. [Google Scholar]
- Otsubo, T.; Aso, Y.; Takimiya, K. Functional oligothiophenes as advanced molecular electronic materials. J. Mater. Chem. 2002, 12, 2565–2575. [Google Scholar] [CrossRef]
- Acharya, A.; Mishra, R.; Roy, G.S. Characterization of CdSe/Polythiophene nanocomposite by TGA/DTA, XRD, UV-VIS Spectroscopy, SEM-EDXA and FTIR. Armen. J. Phys. 2010, 3, 195–202. [Google Scholar]
- Bachman, J.C.; Kavian, R.; Graham, D.J.; Young Kim, D.; Noda, S.; Nocera, D.G.; Shao-Horn, Y.; Woo Lee, S. Electrochemical polymerization of pyrene derivatives on functionalized carbon nanotubes for pseudocapacitive electrodes. Nat. Commun. 2015, 6. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Martínez, M.; Bautista Martínez, J.A.; Rivera, E. Thermal, Optical, Electrochemical Properties and conductivity of Pyrene Monomers. Des. Mon. Polym. 2008, 11, 173–178. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy, 3rd ed.; Springer: New York, NY, USA, 2006. [Google Scholar]
- Sample Availability: Not available.
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valderrama-García, B.X.; Rodríguez-Alba, E.; Morales-Espinoza, E.G.; Moineau Chane-Ching, K.; Rivera, E. Synthesis and Characterization of Novel Polythiophenes Containing Pyrene Chromophores: Thermal, Optical and Electrochemical Properties. Molecules 2016, 21, 172. https://doi.org/10.3390/molecules21020172
Valderrama-García BX, Rodríguez-Alba E, Morales-Espinoza EG, Moineau Chane-Ching K, Rivera E. Synthesis and Characterization of Novel Polythiophenes Containing Pyrene Chromophores: Thermal, Optical and Electrochemical Properties. Molecules. 2016; 21(2):172. https://doi.org/10.3390/molecules21020172
Chicago/Turabian StyleValderrama-García, Bianca X., Efraín Rodríguez-Alba, Eric G. Morales-Espinoza, Kathleen Moineau Chane-Ching, and Ernesto Rivera. 2016. "Synthesis and Characterization of Novel Polythiophenes Containing Pyrene Chromophores: Thermal, Optical and Electrochemical Properties" Molecules 21, no. 2: 172. https://doi.org/10.3390/molecules21020172





