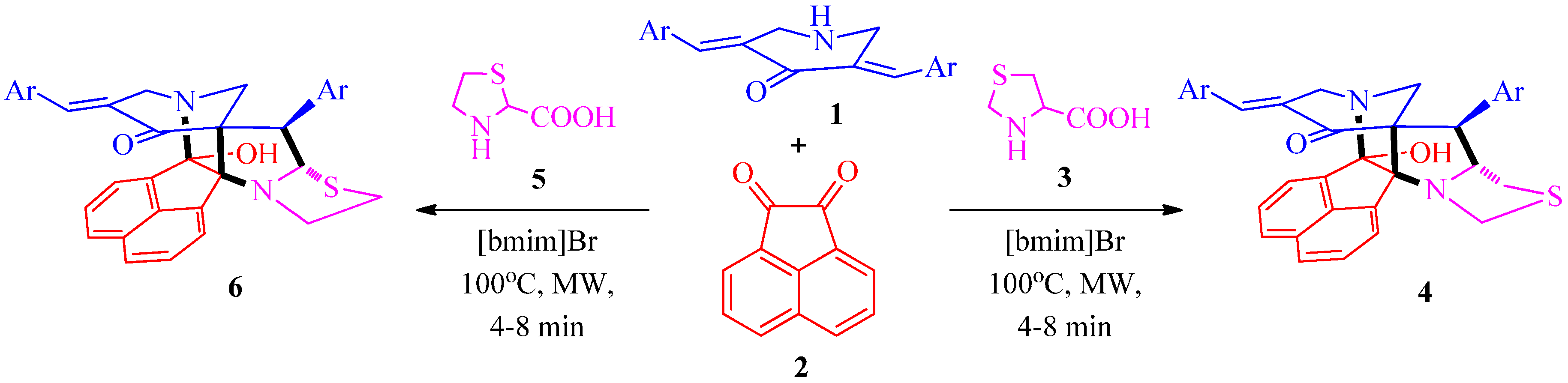
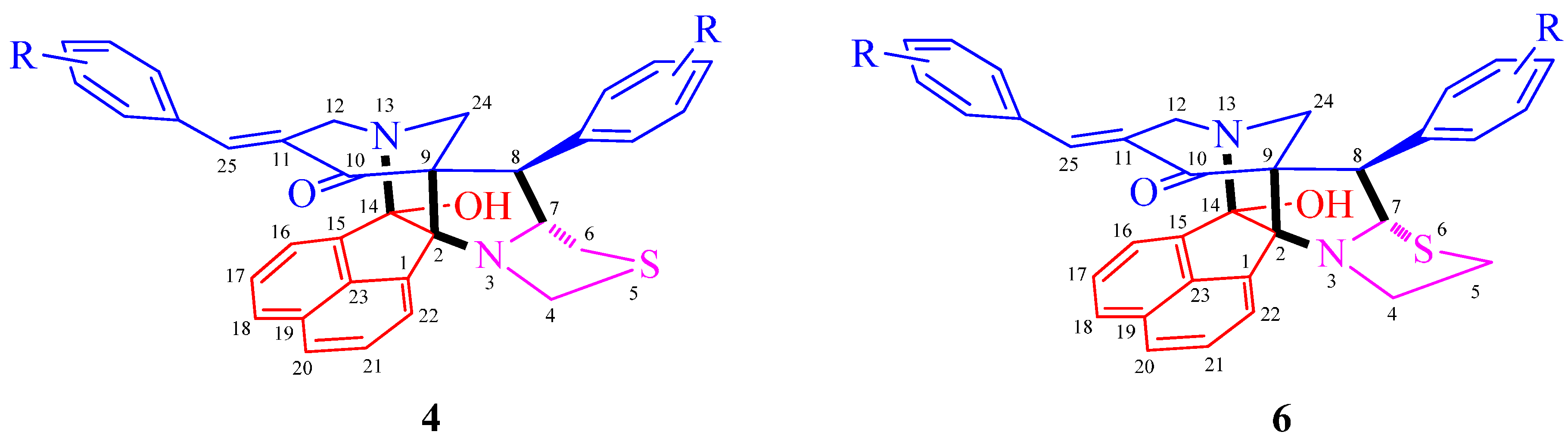
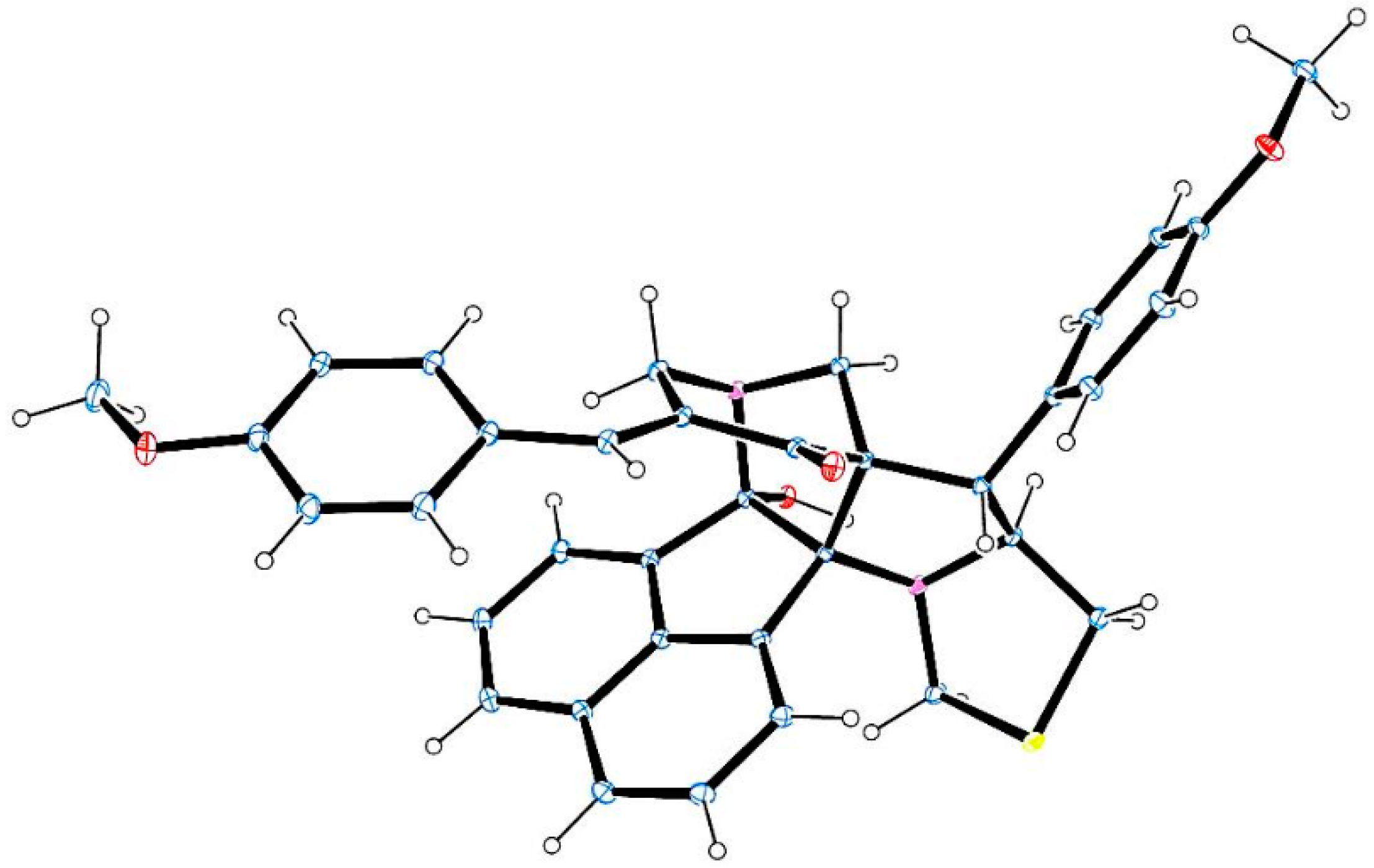
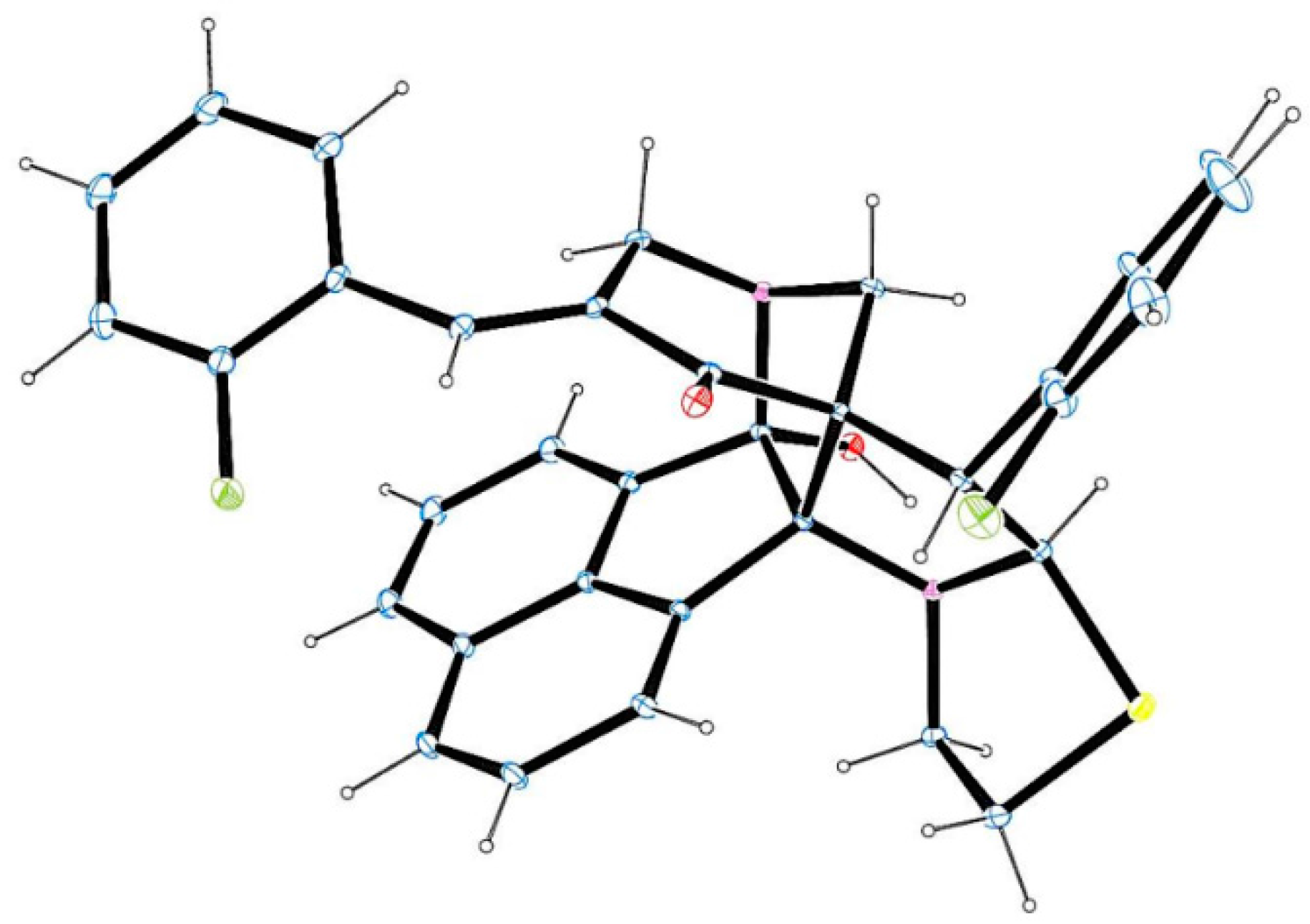
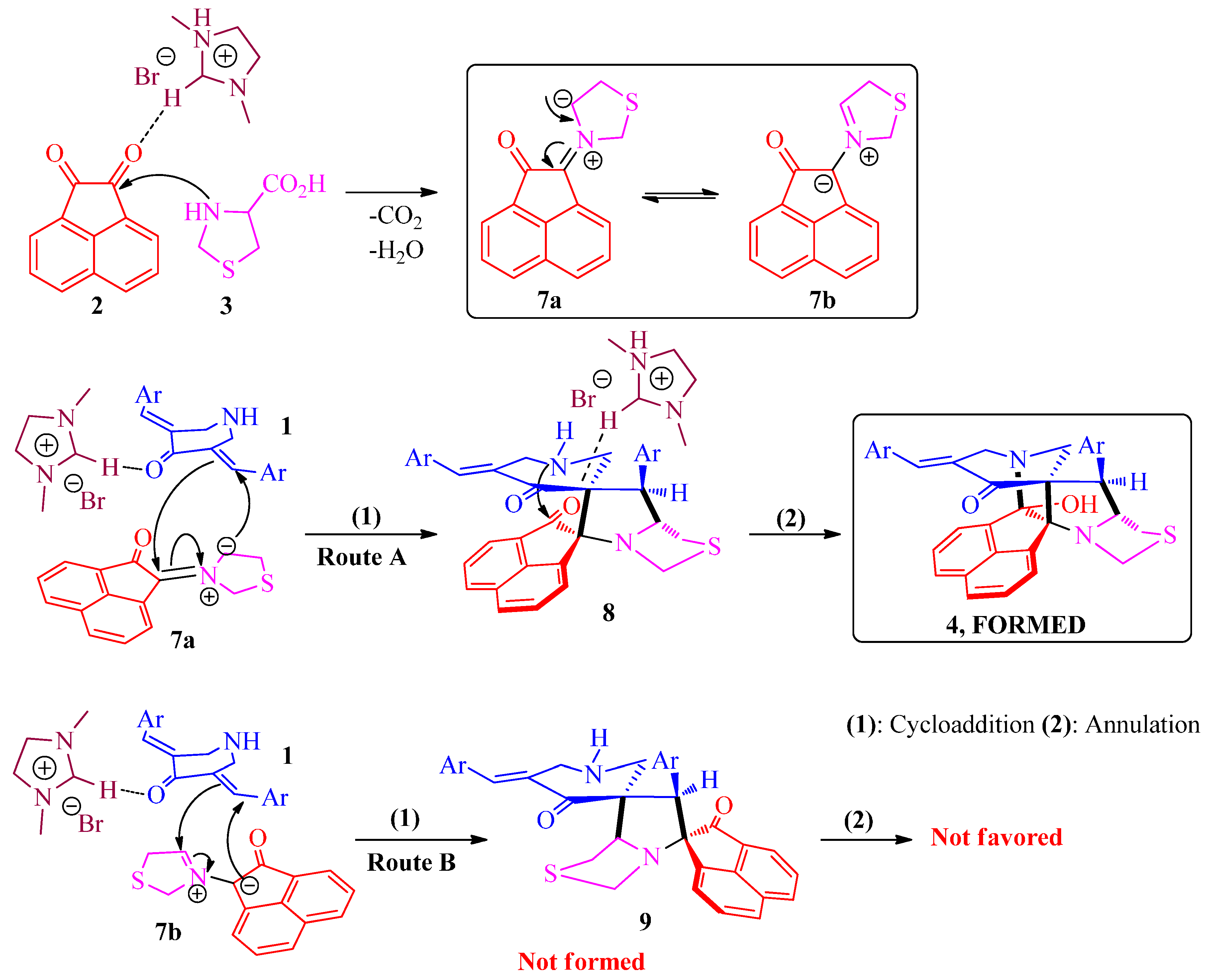
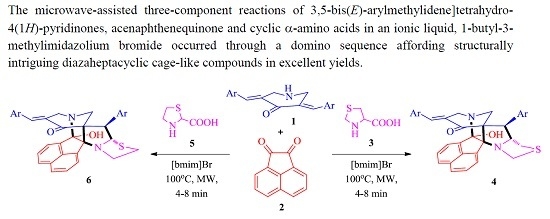
3.2. General Procedure for the Synthesis of Diazaheptacyclic Cage Compounds 4a–n or 6a–m
An equimolar mixture of 3,5-bis[(E)-arylmethylidene]tetrahydro-4(1H)-pyridinone 1, acenaphthenequinone 2 and thiaproline 3 or 5 in 100 mg of [bmim]Br was irradiated in a CEM microwave synthesizer at 100 °C for 4–8 min. After completion of the reaction (TLC), ethyl acetate (10 mL) was added and the reaction mixture stirred for 15 min. The ethyl acetate layer was then separated, washed with water (50 mL) and the solvent evaporated under reduced pressure. The resultant precipitate was dried in vacuum and subjected to crystallization from petroleum ether–ethyl acetate mixture (2:8) to obtain pure 4 or 6. The ionic liquid [bmim]Br after extraction of the product was dried under vacuum and reused for subsequent reactions.
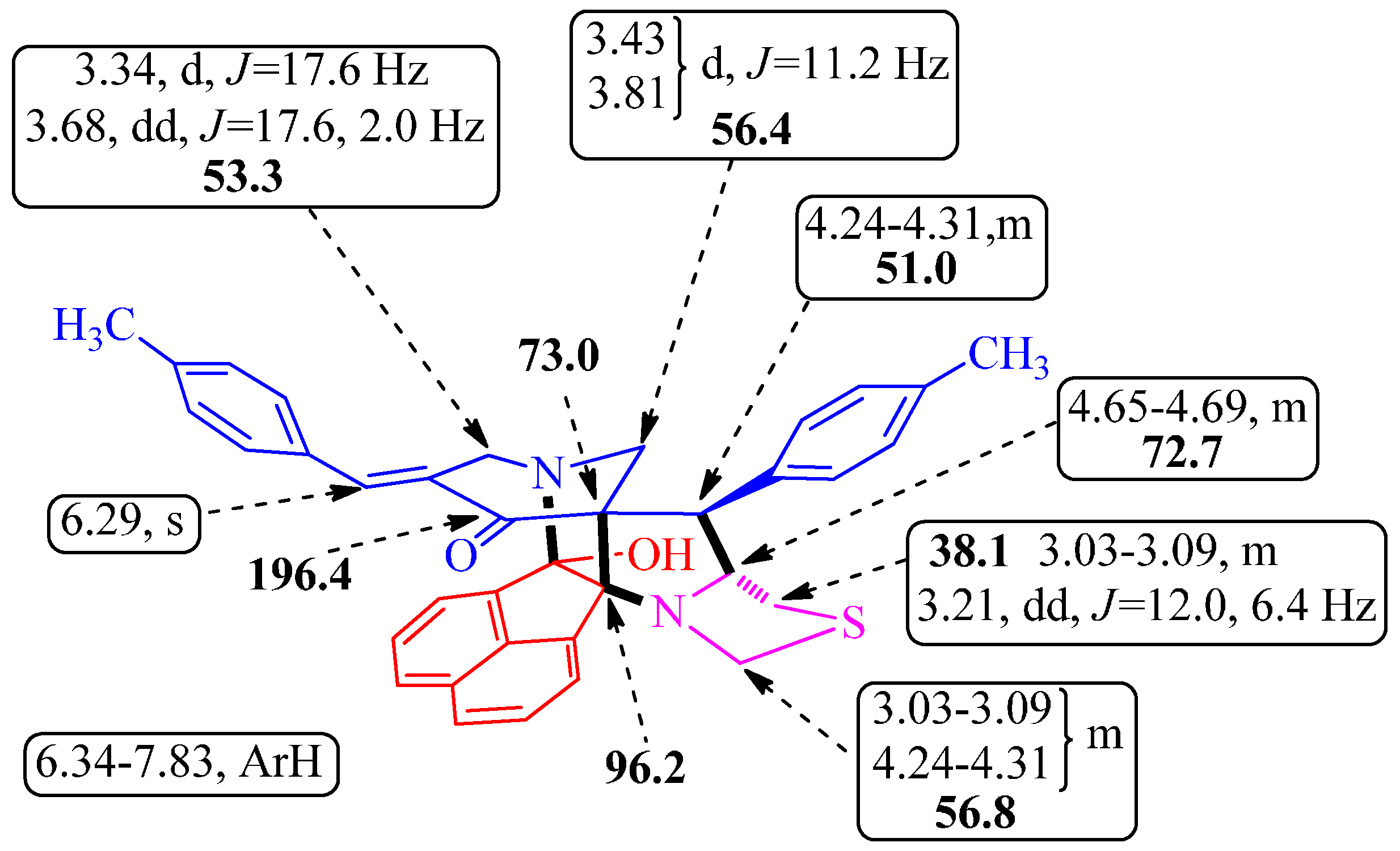
14-Hydroxy-8-(phenyl)-11-[(E)-phenylmethylidene]-5-thia-3,13-diazaheptacyclo-[13.7.1.19,13.02,9.02,14.03,7.019,23]tetracosa-1(22),15(23),16,18,20-pentaen-10-one (4a) White solid, 92% (0.175 g), m.p. 140–142 °C, IR (KBr) υmax 3420, 1721, 1685, 1602 cm−1; 1H-NMR (300 MHz, CDCl3): δH 3.03–3.12 (m, 2H, H-4a and H-6a), 3.22 (dd, J = 12.0, 6.3 Hz, 1H, H-6b), 3.35 (d, J = 17.4 Hz, 1H, H-12a), 3.44 (d, J = 11.4 Hz, 1H, H-24a), 3.68 (dd, J = 17.4, 1.8 Hz, 1H, H-12b), 3.81 (d, J = 11.4 Hz, 1H, H-24b), 4.27–4.35 (m, 2H, H-4b and H-8), 4.67–4.73 (m, 1H, H-7), 6.27 (s, 1H, H-25), 6.39–6.42 (m, 2H, ArH), 7.06–7.11 (m, 3H, ArH), 7.25–7.38 (m, 4H, ArH), 7.50–7.60 (m, 5H, ArH), 7.74 (d, J = 8.1 Hz, 1H, ArH), 7.83 (d, J = 6.9 Hz, 1H, ArH); 13C-NMR (75 MHz, CDCl3): δc 38.1, 51.4, 53.4, 56.4, 57.0, 72.7, 73.2, 96.3, 104.1, 121.6, 125.2, 126.2, 126.8, 127.8, 128.0, 128.2, 128.4, 128.8, 129.1, 129.6, 130.0, 131.2, 133.2, 134.2, 134.5, 136.2, 136.9, 137.0, 138.5, 196.7. Anal. calcd for C34H28N2O2S: C, 77.24; H, 5.34; N, 5.30. Found: C, 77.39; H, 5.23; N, 5.38%.
14-Hydroxy-8-(2-methylphenyl)-11-[(E)-(2-methylphenyl)methylidene]-5-thia-3,13-diazaheptacyclo[13.7.1.19,13.02,9.02,14.03,7.019,23]tetracosa-1(22),15(23),16,18,20-pentaen-10-one (4b) Pale yellow solid, 90% (0.165 g), m.p. 127–129 °C, IR (KBr) υmax 3422, 1725, 1680, 1598 cm−1; 1H-NMR (300 MHz, CDCl3): δH 1.56 (s, 3H, CH3), 2.82 (s, 3H, CH3), 2.98 (d, J = 12.3 Hz, 1H, H-6a), 3.14 (dd, J = 12.3, 6.3 Hz, 1H, H-6b), 3.32–3.44 (m, 3H, H-4a, H-12a and H-24a), 3.72 (d, J = 17.4 Hz, 1H, H-12b), 3.83 (d, J = 11.4 Hz, 1H, H-24b), 4.47–4.65 (m, 3H, H-4b and H-8 and H-7), 6.03 (d, J = 7.5 Hz, 1H, ArH), 6.53 (s, 1H, H-25), 6.84–7.04 (m, 3H, ArH), 7.18–7.45 (m, 5H, ArH), 7.57–7.69 (m, 3H, ArH), 7.78 (d, J = 8.1 Hz, 1H, ArH), 7.89 (d, J = 6.9 Hz, 1H, ArH); 13C-NMR (75 MHz, CDCl3): δc 19.6, 21.2, 37.2, 46.3, 52.8, 56.6, 57.4, 73.9, 74.9, 96.6, 103.8, 121.8, 125.1, 125.4, 126.1, 126.3, 126.8, 126.9, 127.7, 128.2, 128.4, 128.5, 128.9, 130.1, 131.3, 131.8, 132.7, 133.4, 134.4, 135.4, 135.8, 137.1, 137.5, 138.7, 139.3, 196.2. Anal. calcd for C36H32N2O2S: C, 77.67; H, 5.79; N, 5.03. Found: C, 77.80; H, 5.70; N, 5.12%.
14-Hydroxy-8-(2-methoxylphenyl)-11-[(E)-(2-methoxylphenyl)methylidene]-5-thia-3,13-diazaheptacyclo[13.7.1.19,13.02,9.02,14.03,7.019,23]tetracosa-1(22),15(23),16,18,20-pentaen-10-one (4c) Pale yellow solid, 85% (0.149 g), m.p. 166–168 °C, IR (KBr) υmax 3426, 1722, 1683, 1600 cm−1; 1H-NMR (300 MHz, CDCl3): δH 3.03 (d, J = 12.0 Hz, 1H, H-6a), 3.12 (dd, J = 12.0, 6.3 Hz, 1H, H-6b), 3.28–3.40 (m, 3H, H-4a, H-12a and H-24a), 3.56 (s, 3H, OCH3), 3.68 (dd, J = 17.7, 2.1 Hz, 1H, H-12b), 3.81 (d, J = 11.1 Hz, 1H, H-24b), 3.96 (s, 3H, OCH3), 4.59–4.83 (m, 3H, H-4b and H-8 and H-7), 5.99 (dd, J = 7.8, 1.2 Hz, 1H, ArH), 6.47 (s, 1H, H-25), 6.57–6.63 (m, 2H, ArH), 6.93–7.11 (m, 3H, ArH), 7.22–7.37 (m, 2H, ArH), 7.48 (dd, J = 8.1, 1.8 Hz, 1H, ArH), 7.55–7.60 (m, 3H, ArH), 7.74 (d, J = 8.1 Hz, 1H, ArH), 7.90 (d, J = 6.9 Hz, 1H, ArH); 13C-NMR (75 MHz, CDCl3): δc 37.6, 47.1, 53.1, 55.2, 56.2, 56.3, 57.6, 71.8, 73.8, 96.0, 103.5, 110.3, 112.0, 119.8, 121.1, 121.4, 123.4, 125.2, 125.5, 126.0, 126.6, 127.8, 128.3, 129.1, 129.9, 130.4, 131.3, 131.8, 131.9, 133.0, 134.5, 137.1, 138.7, 157.7, 158.9, 196.5. Anal. calcd for C36H32N2O4S: C, 73.45; H, 5.48; N, 4.76. Found: C, 73.63; H, 5.39; N, 4.89%.
8-(2-Bromophenyl)-11-[(E)-(2-bromophenyl)methylidene]-14-hydroxy-5-thia-3,13-diazaheptacyclo[13.7.1.19,13.02,9.02,14.03,7.019,23]tetracosa-1(22),15(23),16,18,20-pentaen-10-one (4d) White solid, 91% (0.144 g), m.p. 184–186 °C, IR (KBr) υmax 3424, 1725, 1680, 1595 cm−1; 1H-NMR (300 MHz, CDCl3): δH 3.11 (dd, J = 12.3, 6.3 Hz, 1H, H-6b), 3.20 (d, J = 12.3 Hz, 1H, H-6a), 3.36–3.42 (m, 3H, H-4a, H-12a and H-24a), 3.68 (dd, J = 17.7, 3.0 Hz, 1H, H-12b), 3.82 (d, J = 11.4 Hz, 1H, H-24b), 4.37–5.07 (m, 3H, H-4b and H-8 and H-7), 5.82–5.87 (m, 1H, ArH), 6.51 (s, 1H, H-25), 6.93–7.00 (m, 2H, ArH), 7.12–7.18 (m, 1H, ArH), 7.27–7.50 (m, 4H, ArH), 7.57–7.76 (m, 4H, ArH), 7.82 (d, J = 8.1 Hz, 1H, ArH), 7.96 (d, J = 6.9 Hz, 1H, ArH); 13C-NMR (75 MHz, CDCl3): δc 36.6, 49.3, 52.4, 56.3, 57.2, 74.0, 74.7, 96.3, 103.5, 121.6, 124.2, 125.4, 126.5, 126.9, 127.4, 127.7, 127.9, 128.2, 128.3, 128.7, 129.3, 129.5, 130.0, 131.5, 132.9, 133.7, 133.9, 134.6, 134.7, 135.3, 137.0, 137.1, 138.5, 195.3. Anal. calcd for C34H26Br2N2O2S: C, 59.49; H, 3.82; N, 4.08. Found: C, 59.64; H, 3.70; N, 4.21%.
8-(2-Chlorophenyl)-11-[(E)-(2-chlorophenyl)methylidene]-14-hydroxy-5-thia-3,13-diazaheptacyclo[13.7.1.19,13.02,9.02,14.03,7.019,23]tetracosa-1(22),15(23),16,18,20-pentaen-10-one (4e) White solid, 90% (0.156 g), m.p. 146–148 °C, IR (KBr) υmax 3418, 1720, 1681, 1601 cm−1; 1H-NMR (300 MHz, CDCl3): δH 3.12–3.14 (m, 2H, H-6a and H-6b), 3.34–3.42 (m, 3H, H-4a, H-12a and H-24a), 3.69 (dd, J = 17.7, 2.7 Hz, 1H, H-12b), 3.81 (d, J = 11.4 Hz, 1H, H-24b), 4.39–5.06 (m, 3H, H-4b and H-8 and H-7), 5.98 (d, J = 7.5 Hz, 1H, ArH), 6.51 (s, 1H, H-25), 6.90–7.13 (m, 3H, ArH), 7.19–7.29 (m, 2H, ArH), 7.37–7.62 (m, 5H, ArH), 7.69 (d, J = 8.1 Hz, 1H, ArH), 7.80 (d, J = 8.4 Hz, 1H, ArH), 7.95 (d, J = 6.9 Hz, 1H, ArH); 13C-NMR (75 MHz, CDCl3): δc 36.9, 46.5, 52.7, 56.3, 57.4, 74.0, 74.4, 96.3, 103.6, 121.6, 125.3, 126.2, 126.5, 127.3, 127.4, 128.0, 128.3, 128.7, 129.0, 129.7, 130.0, 131.2, 131.4, 132.7, 132.9, 133.7, 134.2, 134.4, 135.3, 136.6, 137.0, 138.4, 195.5. Anal. calcd for C34H26Cl2N2O2S: C, 68.34; H, 4.39; N, 4.69. Found: C, 68.50; H, 4.48; N, 4.52%.
8-(2-Fluorophenyl)-11-[(E)-(2-fluorophenyl)methylidene]-14-hydroxy-5-thia-3,13-diazaheptacyclo[13.7.1.19,13.02,9.02,14.03,7.019,23]tetracosa-1(22),15(23),16,18,20-pentaen-10-one (4f) Light brown solid, 88% (0.160 g), m.p. 150–152 °C, IR (KBr) υmax 3419, 1724, 1680, 1598 cm−1; 1H-NMR (300 MHz, CDCl3): δH 3.02 (d, J = 12.3 Hz, 1H, H-6a), 3.16–3.31 (m, 3H, H-4a, H-6b and H-12a), 3.43 (d, J = 11.1 Hz, 1H, H-24a), 3.67 (dd, J = 17.7, 1.8 Hz, 1H, H-12b), 3.82 (d, J = 11.4 Hz, 1H, H-24b), 4.46–4.77 (m, 3H, H-4b and H-8 and H-7), 6.12–6.22 (m, 3H, H-25 and ArH), 6.76–6.86 (m, 2H, ArH), 7.08–7.31 (m, 4H, ArH), 7.51–7.62 (m, 4H, ArH), 7.79 (d, J = 8.4 Hz, 1H, ArH), 7.88 (d, J = 6.9 Hz, 1H, ArH); 13C-NMR (75 MHz, CDCl3): δc 37.7, 46.3, 53.3, 56.3, 57.3, 71.6, 73.8, 96.0, 103.7, 115.5, 116.8, 121.5, 122.1, 123.6, 123.8, 124.7, 125.2, 126.2, 127.1, 127.8, 128.2, 128.5, 129.7, 130.5, 130.9, 131.2, 131.9, 134.1, 135.1, 137.0, 138.4, 159.5, 162.8, 196.2. Anal. calcd for C34H26F2N2O2S: C, 72.32; H, 4.64; N, 4.96. Found: C, 72.20; H, 4.75; N, 4.80%.
14-Hydroxy-8-(3-nitrophenyl)-11-[(E)-(3-nitrophenyl)methylidene]-5-thia-3,13-diazaheptacyclo[13.7.1.19,13.02,9.02,14.03,7.019,23]tetracosa-1(22),15(23),16,18,20-pentaen-10-one (4g) Dark brown solid, 91% (0.154 g), m.p. 176–178 °C, IR (KBr) υmax 3424, 1716, 1686, 1616 cm−1; 1H-NMR (400 MHz, CDCl3): δH 3.02–3.09 (m, 2H, H-4a and H-6a), 3.23 (d, J = 17.6Hz, 1H, H-12a), 3.28 (dd, J = 12.4, 6.4 Hz, 1H, H-6b), 3.48 (d, J = 11.6 Hz, 1H, H-24a), 3.65 (dd, J = 17.6, 2.8 Hz, 1H, H-12b), 3.85 (d, J = 11.6 Hz, 1H, H-24b), 4.37–4.40 (m, 2H, H-4b and H-8), 4.75–4.80 (m, 1H, H-7), 6.16 (s, 1H, H-25), 6.69 (d, J = 8.0 Hz, 1H, ArH), 7.12 (s, 1H, ArH), 7.24–7.30 (m, 2H, ArH), 7.50–7.51 (m, 3H, ArH), 7.65–7.69 (m, 1H, ArH), 7.84–7.88 (m, 2H, ArH), 7.95 (d, J = 7.6 Hz, 1H, ArH), 7.99 (dd, J = 8.0, 1.6 Hz, 1H, ArH), 8.18 (dd, J = 7.2, 1.2 Hz, 1H, ArH), 8.46–8.48 (m, 1H, ArH); 13C-NMR (100 MHz, CDCl3): δc 37.9, 50.9, 53.2, 56.4, 56.9, 72.2, 73.5, 96.3, 104.1, 121.8, 123.2, 123.3, 124.2, 124.5, 125.2, 126.2, 127.2, 128.1, 128.7, 129.2, 130.2, 131.3, 133.1, 134.1, 134.9, 135.1, 135.9, 136.0, 136.9, 138.2, 139.0, 148.0, 148.9, 196.4. Anal. calcd for C34H26N4O6S: C, 66.01; H, 4.24; N, 9.06. Found: C, 66.19; H, 4.45; N, 9.19%.
8-(2,4-Dichlorophenyl)-11-[(E)-(2,4-dichlorophenyl)methylidene]-14-hydroxy-5-thia-3,13-diazaheptacyclo[13.7.1.19,13.02,9.02,14.03,7.019,23]tetracosa-1(22),15(23),16,18,20-pentaen-10-one (4h) Light brown solid, 90% (0.145 g), m.p. 144–146 °C, IR (KBr) υmax 3409, 1686, 1605 cm−1; 1H-NMR (300 MHz, CDCl3): δH 3.07–3.16 (m, 2H, H-4a and H-6a), 3.27–3.49 (m, 3H, H-6b, H-12a and H-24a), 3.67 (dd, J = 18.0, 3.0 Hz, 1H, H-12b), 3.82 (d, J = 11.4 Hz, 1H, H-24b), 4.36–4.99 (m, 3H, H-4b, H-7 and H-8), 5.92 (d, J = 8.4 Hz, 1H, ArH), 6.40 (s, 1H, H-25), 6.93 (dd, J = 8.4, 2.1 Hz, 1H, ArH), 7.15–7.48 (m, 3H, ArH), 7.43–7.48 (m, 1H, ArH), 7.54–7.64 (m, 3H, ArH), 7.71 (d, J = 8.1 Hz, 1H, ArH), 7.81 (d, J = 8.1 Hz, 1H, ArH), 7.91 (m, J = 6.6 Hz, 1H, ArH); 13C-NMR (75 MHz, CDCl3): δc 36.8, 46.1, 52.6, 56.3, 57.2, 74.0, 74.2, 96.2, 103.6, 121.6, 125.3, 126.5, 126.7, 127.4,127.6, 128.2,128.7, 129.1, 129.6, 130.3, 131.0, 131.1, 131.3, 131.7, 133.5, 133.8, 134.3, 134.6, 135.1, 135.2, 136.9, 137.3, 138.3, 195.3. Anal. calcd for C34H24Cl4N2O2S: C, 61.28; H, 3.63; N, 4.20. Found: C, 61.46; H, 3.49; N, 4.33%.
14-Hydroxy-8-(4-methylphenyl)-11-[(E)-(4-methylphenyl)methylidene]-5-thia-3,13-diazaheptacyclo[13.7.1.19,13.02,9.02,14.03,7.019,23]tetracosa-1(22),15(23),16,18,20-pentaen-10-one (4i) Light brown solid, 93% (0.170 g), m.p. 141–143 °C, IR (KBr) υmax 3416, 1723, 1682, 1594 cm−1; 1H-NMR (400 MHz, CDCl3): δH 2.23 (s, 3H, CH3), 2.33 (s, 3H, CH3), 3.03–3.09 (m, 2H, H-4a and H-6a), 3.21 (dd, J = 12.0, 6.4 Hz, 1H, H-6b), 3.34 (d, J = 17.6 Hz, 1H, H-12a), 3.43 (d, J = 11.2 Hz, 1H, H-24a), 3.68 (dd, J = 17.6, 2.0 Hz, 1H, H-12b), 3.81 (d, J = 11.2 Hz, 1H, H-24b), 4.24–4.31 (m, 2H, H-4b and H-8), 4.65–4.69 (m, 1H, H-7), 6.29 (s, 1H, H-25), 6.35 (d, J = 8.0 Hz, 2H, ArH), 6.88 (d, J = 8.0 Hz, 2H, ArH), 7.17 (d, J = 8.0 Hz, 2H, ArH), 7.30–7.34 (m, 1H, ArH), 7.43 (d, J = 8.0 Hz, 2H, ArH), 7.52–7.61 (m, 3H, ArH), 7.72 (d, J = 8.0 Hz, 1H, ArH), 7.82 (d, J = 6.8 Hz, 1H, ArH); 13C-NMR (100 MHz, CDCl3): δc 21.5, 21.7, 38.1, 51.0, 53.3, 56.4, 56.8, 72.7, 73.0, 96.2, 104.1, 121.5, 125.2, 126.3, 126.7, 127.9, 128.4, 129.0, 129.4, 129.8, 130.2, 131.2, 131.5, 132.1, 133.9, 134.5, 136.5, 137.0, 137.6, 138.5, 139.2, 196.4. Anal. calcd for C36H32N2O2S: C, 77.67; H, 5.79; N, 5.03. Found: C, 77.79; H, 5.68; N, 5.12%.
14-Hydroxy-8-(4-methoxyphenyl)-11-[(E)-(4-methoxyphenyl)methylidene]-5-thia-3,13-diazaheptacyclo[13.7.1.19,13.02,9.02,14.03,7.019,23]tetracosa-1(22),15(23),16,18,20-pentaen-10-one (4j) Pale yellow solid, 87% (0.152 g), m.p. 137–139 °C, IR (KBr) υmax 3422, 1720, 1684, 1600 cm−1; 1H-NMR (300 MHz, CDCl3): δH 3.03–3.11 (m, 2H, H-4a and H-6a), 3.22 (dd, J = 12.0, 6.6 Hz, 1H, H-6b), 3.34 (d, J = 17.1 Hz, 1H, H-12a), 3.42–3.49 (m, 1H, H-24a), 3.68–3.70 (m, 2H, H-12b and H-24b), 3.73 (s, 3H, OCH3), 3.79 (s, 3H, OCH3), 4.23–4.30 (m, 2H, H-4b and H-8), 4.63–4.68 (m, 1H, H-7), 6.27 (s, 1H, H-25), 6.47 (d, J = 8.7 Hz, 2H, ArH), 6.62 (d, J = 8.7 Hz, 2H, ArH), 6.89 (d, J = 8.7 Hz, 2H, ArH), 7.31–7.36 (m, 1H, ArH), 7.45–7.63 (m, 5H, ArH), 7.71 (d, J = 8.1 Hz, 1H, ArH), 7.82 (d, J = 6.9 Hz, 1H, ArH); 13C-NMR (75 MHz, CDCl3): δc 38.0, 50.6, 53.4, 55.6, 55.7, 56.4, 56.8, 72.7, 72.9, 96.1, 104.1, 113.8, 114.4, 121.5, 125.1, 126.2, 126.6, 127.1, 127.9, 128.3, 129.0, 130.5, 131.0, 131.2, 132.1, 134.7, 136.1, 137.0, 138.6, 159.3, 160.2, 196.7. Anal. calcd for C36H32N2O4S: C, 73.45; H, 5.48; N, 4.76. Found: C, 73.31; H, 5.34; N, 4.87%.
8-(4-Bromophenyl)-11-[(E)-(4-bromophenyl)methylidene]-14-hydroxy-5-thia-3,13-diazaheptacyclo[13.7.1.19,13.02,9.02,14.03,7.019,23]tetracosa-1(22),15(23),16,18,20-pentaen-10-one (4k) Light brown solid, 92% (0.146 g), m.p. 171–173 °C, IR (KBr) υmax 3418, 1724, 1680, 1597 cm−1; 1H-NMR (300 MHz, CDCl3): δH 3.00–3.06 (m, 2H, H-4a and H-6a), 3.20–3.30 (m, 1H, H-6b and H-12a), 3.44 (d, J = 11.4 Hz, 1H, H-24a), 3.63 (dd, J = 17.7, 2.1 Hz, 1H, H-12b), 3.81 (d, J = 11.4 Hz, 1H, H-24b), 4.22–4.29 (m, 2H, H-4b and H-8), 4.62–4.68 (m, 1H, H-7), 6.16 (s, 1H, H-25), 6.26 (d, J = 8.4 Hz, 2H, ArH), 7.21–7.60 (m, 10H, ArH), 7.75 (d, J = 8.1 Hz, 1H, ArH), 7.80 (d, J = 6.9 Hz, 1H, ArH); 13C-NMR (75 MHz, CDCl3): δc 38.0, 50.7, 53.2, 56.4, 56.8, 72.4, 73.1, 96.2, 104.1, 121.7, 122.1, 123.3, 125.1, 126.3, 126.9, 128.1, 128.5, 130.4, 131.2, 131.4, 131.5, 132.2, 133.0, 133.7, 134.9, 135.9, 135.4, 136.9, 138.3, 196.5. Anal. calcd for C34H26Br2N2O2S: C, 59.49; H, 3.82; N, 4.08. Found: C, 59.62; H, 3.90; N, 4.21%.
8-(4-Chlorophenyl)-11-[(E)-(4-chlorophenyl)methylidene]-14-hydroxy-5-thia-3,13-diazaheptacyclo[13.7.1.19,13.02,9.02,14.03,7.019,23]tetracosa-1(22),15(23),16,18,20-pentaen-10-one (4l) Brown solid, 95% (0.165 g), m.p. 154–156 °C, IR (KBr) υmax 3402, 1719, 1686, 1601 cm−1; 1H-NMR (400 MHz, CDCl3): δH 2.99–3.04 (m, 2H, H-4a and H-6a), 3.19–3.29 (m, 2H, H-6b and H-12a), 3.43 (d, J = 11.6 Hz, 1H, H-24a), 3.62 (dd, J = 17.6, 2.4 Hz, 1H, H-12b), 3.80 (d, J = 11.2 Hz, 1H, H-24b), 4.23–4.27 (m, 2H, H-4b and H-8), 4.61–4.66 (m, 1H, H-7), 6.17 (s, 1H, H-25), 6.32 (d, J = 8.4 Hz, 2H, ArH), 7.05 (d, J = 8.4 Hz, 2H, ArH), 7.27–7.35 (m, 3H, ArH), 7.48 (d, J = 8.4 Hz, 2H, ArH), 7.55–7.69 (m, 3H, ArH), 7.73 (d, J = 8.4 Hz, 1H, ArH), 7.79 (d, J = 6.8 Hz, 1H, ArH); 13C-NMR (100 MHz, CDCl3): δc 38.0, 50.7, 53.2, 56.4, 56.8, 72.4, 73.1, 96.2, 104.1, 121.7, 125.1, 126.3, 126.9, 128.1, 128.4, 128.5, 128.8, 129.3, 130.9, 131.2, 132.5, 133.6, 133.9, 134.3, 134.8, 134.9, 135.4, 136.9, 138.3, 196.5. Anal. calcd for C34H26Cl2N2O2S: C, 68.34; H, 4.39; N, 4.69. Found: C, 68.45; H, 4.51; N, 4.78%.
8-(4-Fluorophenyl)-11-[(E)-(4-fluorophenyl)methylidene]-14-hydroxy-5-thia-3,13-diazaheptacyclo[13.7.1.19,13.02,9.02,14.03,7.019,23]tetracosa-1(22),15(23),16,18,20-pentaen-10-one (4m) White solid, 93% (0.169 g), m.p. 132–134 °C, IR (KBr) υmax 3416, 1682, 1601 cm−1; 1H-NMR (400 MHz, CDCl3): δH 3.02–3.09 (m, 2H, H-4a and H-6a), 3.21–3.26 (m, 1H, H-6b), 3.30 (d, J = 17.6 Hz, 1H, H-12a), 3.45 (d, J = 11.2 Hz, 1H, H-24a), 3.67 (dd, J = 17.2, 2.0 Hz, 1H, H-12b), 3.82 (d, J = 11.6 Hz, 1H, H-24b), 4.25–4.31 (m, 2H, H-4b and H-8), 4.64–4.68 (m, 1H, H-7), 6.20 (s, 1H, H-25), 6.39–6.42 (m, 2H, ArH), 6.76–6.81 (m, 2H, ArH), 7.04–7.08 (m, 2H, ArH), 7.31–7.34 (m, 1H, ArH), 7.51–7.56 (m, 3H, ArH), 7.59 (d, J = 6.8 Hz, 2H, ArH), 7.74 (d, J = 8.4 Hz, 1H, ArH), 7.81 (d, J = 7.2 Hz, 1H, ArH); 13C-NMR (100 MHz, CDCl3): δC 38.0, 50.6, 53.3, 56.4, 56.9, 72.6, 73.1, 96.2, 104.1, 115.4, 116.0, 121.6, 125.1, 126.2, 126.8, 128.0, 128.4, 130.3, 131.0, 131.2, 131.9, 132.5, 133.0, 134.5, 135.0, 137.0,138.4, 161.5,163.9, 196.7. Anal. calcd for C34H26F2N2O2S: C, 72.32; H, 4.64; N, 4.96. Found: C, 73.20; H, 4.79; N, 4.78%.
14-Hydroxy-8-(naphthyl)-11-[(E)-naphthylmethylidene]-5-thia-3,13-diazaheptacyclo-[13.7.1.19,13.02,9.02,14.03,7.019,23]tetracosa-1(22),15(23),16,18,20-pentaen-10-one (4n) White solid, 89% (0.149 g), m.p. 158–160 °C, IR (KBr) υmax 3423, 1721, 1684, 1590 cm−1; 1H-NMR (300 MHz, CDCl3): δH 2.96 (d, J = 12.3 Hz, 1H, H-6a), 3.13 (dd, J = 12.3, 6.3 Hz, 1H, H-6b), 3.30 (d, J = 18.0 Hz, 1H, H-12a), 3.41–3.52 (m, 2H, H-4a and H-24a), 3.73 (dd, J = 18.0, 2.1 Hz, 1H, H-12b), 3.88 (d, J = 11.4 Hz, 1H, H-24b), 4.66–4.77 (m, 2H, H-4b and H-7), 5.32 (d, J = 10.2 Hz, 1H, H-8), 6.22 (d, J = 6.9 Hz, 1H, ArH), 6.69 (d, J = 8.4 Hz, 1H, ArH), 6.93 (s, 1H, H-25), 7.11–7.91 (m, 16H, ArH), 8.15 (d, J = 6.9 Hz, 1H, ArH), 8.92 (d, J = 8.7 Hz, 1H, ArH); 13C-NMR (75 MHz, CDCl3): δc 37.6, 44.8, 53.2, 56.6, 57.8, 73.8, 75.5, 97.0, 104.0, 121.6, 124.8, 125.0, 125.2, 125.4, 126.1, 126.3, 126.4, 127.0, 127.1, 128.1, 128.4, 128.5, 128.8, 129.2, 129.4, 131.2, 131.3, 131.4, 133.3, 133.6, 134.0, 134.1, 134.4, 134.7, 134.8, 137.2, 138.5, 196.4. Anal. calcd for C42H32N2O2S: C, 80.23; H, 5.13; N, 4.46. Found: C, 80.38; H, 5.29; N, 4.35%.
14-Hydroxy-8-(phenyl)-11-[(E)-phenylmethylidene]-6-thia-3,13-diazaheptacyclo-[13.7.1.19,13.02,9.02,14.03,7.019,23]tetracosa-1(22),15(23),16,18,20-pentaen-10-one (6a) White solid, 93% (0.178 g), m.p. 135–137 °C, IR (KBr) υmax 3418, 1721, 1692, 1599 cm−1; 1H-NMR (300 MHz, CDCl3): δH 2.79–2.99 (m, 5H, H-4a, H-4b, H-5a, H-24a and H-24b), 3.40 (d, J = 17.4 Hz, 1H, H-12a), 3.67 (dd, J = 17.4, 2.7 Hz, 1H, H-12b), 4.25 (dd, J = 12.6, 2.1 Hz, 1H, H-5b), 4.91 (d, J = 6.9 Hz, 1H, H-8), 5.70 (d, J = 7.2 Hz, 1H, H-7), 5.94 (brs, 1H, OH), 6.28 (s, 1H, H-25), 6.40–6.43 (m, 2H, ArH), 7.05–7.15 (m, 3H, ArH), 7.25–7.39 (m, 4H, ArH), 7.51–7.62 (m, 6H, ArH), 7.74 (dd, J = 6.9, 2.1 Hz, 1H, ArH); 13C-NMR (75 MHz, CDCl3): δc 33.9, 53.1, 53.5, 53.9, 57.6, 75.2, 78.0, 95.1, 103.8, 121.7, 124.2, 126.3, 126.8, 127.8, 128.1, 128.2, 128.5, 128.9, 129.0, 129.1, 130.0, 131.2, 133.3, 134.1, 136.0, 136.5, 137.2, 137.3, 138.2, 196.4. Anal. calcd for C34H28N2O2S: C, 77.24; H, 5.34; N, 5.30. Found: C, 77.43; H, 5.20; N, 5.17%.
14-Hydroxy-8-(2-methylphenyl)-11-[(E)-(2-methylphenyl)methylidene]-6-thia-3,13-diazaheptacyclo[13.7.1.19,13.02,9.02,14.03,7.019,23]tetracosa-1(22),15(23),16,18,20-pentaen-10-one (6b) Pale yellow solid, 90% (0.165 g), m.p. 179–181 °C, IR (KBr) υmax 3398, 1719, 1680, 1598 cm−1; 1H-NMR (300 MHz, CDCl3): δH 1.56 (s, 3H, CH3), 2.79–2.96 (m, 7H, CH3, H-4a, H-4b, H-24a and H-24b), 3.23 (d, J = 12.6 Hz, 1H, H-5a), 3.38 (d, J = 18.0 Hz, 1H, H-12a), 3.77 (dd, J = 17.7, 2.7 Hz, 1H, H-12b), 4.63 (d, J = 12.6 Hz, 1H, H-5b), 5.07 (d, J = 7.8 Hz, 1H, H-8), 5.46 (d, J = 8.1 Hz, 1H, H-7), 6.06 (d, J = 7.5 Hz, 1H, ArH), 6.55 (s, 1H, H-25), 6.84–6.91 (m, 2H, ArH), 7.00 (d, J = 7.2 Hz, 1H, ArH), 7.15–7.26 (m, 3H, ArH), 7.39 (d, J = 6.9 Hz, 1H, ArH), 7.44 (d, J = 7.8 Hz, 1H, ArH), 7.56–7.62 (m, 3H, ArH), 7.67 (d, J = 8.1 Hz, 1H, ArH), 7.78 (dd, J = 6.3, 2.7 Hz, 1H, ArH); 13C-NMR (75 MHz, CDCl3): δc 19.6, 21.0, 33.4, 50.4, 52.8, 53.1, 57.7, 75.7, 80.3, 95.5, 103.6, 121.9, 124.1, 125.4, 126.2, 126.6, 126.8, 127.4, 127.7, 128.3, 128.5, 128.6, 129.0, 130.1, 131.3, 131.7, 132.7, 133.3, 135.7, 135.9, 136.0, 137.3, 137.6, 138.5, 139.1, 195.8. Anal. calcd for C36H32N2O2S: C, 77.67; H, 5.79; N, 5.03. Found: C, 77.51; H, 5.91; N, 5.15%.
14-Hydroxy-8-(2-methoxylphenyl)-11-[(E)-(2-methoxylphenyl)methylidene]-6-thia-3,13-diazaheptacyclo[13.7.1.19,13.02,9.02,14.03,7.019,23]tetracosa-1(22),15(23),16,18,20-pentaen-10-one (6c) White solid, 84% (0.147 g), m.p. 175–177 °C, IR (KBr) υmax 3416, 1721, 1681, 1601 cm−1; 1H-NMR (300 MHz, CDCl3): δH 2.72–2.97 (m, 4H, H-4a, H-4b, H-24a and H-24b), 3.09 (d, J = 12.6 Hz, 1H, H-5a), 3.26 (d, J = 17.4 Hz, 1H, H-12a), 3.56 (s, 3H, OCH3), 3.62 (d, J = 17.4 Hz, 1H, H-12b), 3.92 (s, 3H, OCH3), 4.62 (d, J = 12.6 Hz, 1H, H-5b), 5.05 (d, J = 6.9 Hz, 1H, H-8), 5.96 (d, J = 6.9 Hz, 1H, H-7), 6.01 (d, J = 7.5 Hz, 1H, ArH), 6.51 (s, 1H, H-25), 6.55–6.62 (m, 2H, ArH), 6.90–7.59 (m, 9H, ArH), 7.66 (d, J = 6.6 Hz, 1H, ArH), 7.73 (d, J = 8.1 Hz, 1H, ArH); 13C-NMR (75 MHz, CDCl3): δc 33.7, 51.8, 52.9, 53.1, 55.2, 55.9, 57.5, 75.7, 76.9, 94.6, 103.5, 110.3, 111.9, 119.8, 121.2, 121.5, 123.3, 124.4, 125.2, 126.1, 126.5, 128.0, 128.4, 129.1, 129.9, 130.5, 131.2, 132.2, 133.0, 136.3, 137.3, 138.5, 157.7, 158.5, 195.9. Anal. calcd for C36H32N2O4S: C, 73.45; H, 5.48; N, 4.76. Found: C, 73.60; H, 5.32; N, 4.68%.
8-(2-Bromophenyl)-11-[(E)-(2-bromophenyl)methylidene]-14-hydroxy-6-thia-3,13-diazaheptacyclo[13.7.1.19,13.02,9.02,14.03,7.019,23]tetracosa-1(22),15(23),16,18,20-pentaen-10-one (6d) White solid, 92% (0.146 g), m.p. 172–174 °C, IR (KBr) υmax 3396, 1718, 1681, 1602 cm−1; 1H-NMR (300 MHz, CDCl3): δH 2.83–2.99 (m, 4H, H-4a, H-4b, H-24a and H-24b), 3.29 (d, J = 12.3 Hz, 1H, H-5a), 3.40 (d, J = 17.7 Hz, 1H, H-12a), 3.68 (dd, J = 17.7, 2.7 Hz, 1H, H-12b), 4.65 (d, J = 12.3 Hz, 1H, H-5b), 5.37 (d, J = 8.4 Hz, 1H, H-8), 5.41 (d, J = 8.7 Hz, 1H, H-7), 5.91–5.94 (m, 1H, ArH), 6.11 (brs, 1H, OH), 6.54 (s, 1H, H-25), 6.96–6.99 (m, 2H, ArH), 7.11–7.78 (m, 10H, ArH), 7.82 (d, J = 7.5 Hz, 1H, ArH); 13C-NMR (75 MHz, CDCl3): δc 33.4, 52.4, 53.1, 53.4, 57.4, 75.6, 79.2, 95.0, 103.4, 121.7, 124.1, 124.3, 126.6, 126.9, 127.2, 127.4, 127.8, 128.4, 128.6, 128.7, 129.3, 129.6, 130.1, 131.5, 133.0, 133.8, 134.5, 134.6, 135.2, 135.7, 136.7, 137.1, 138.3, 194.8. Anal. calcd for C34H26Br2N2O2S: C, 59.49; H, 3.82; N, 4.08. Found: C, 59.32; H, 3.73; N, 4.17%.
8-(2-Chlorophenyl)-11-[(E)-(2-chlorophenyl)methylidene]-14-hydroxy-6-thia-3,13-diazaheptacyclo[13.7.1.19,13.02,9.02,14.03,7.019,23]tetracosa-1(22),15(23),16,18,20-pentaen-10-one (6e) White solid, 87% (0.150 g), m.p. 134–136 °C, IR (KBr) υmax 3398, 1725, 1685, 1603 cm−1; 1H-NMR (300 MHz, CDCl3): δH 2.83–3.02 (m, 4H, H-4a, H-4b, H-24a and H-24b), 3.25 (d, J = 12.3 Hz, 1H, H-5a), 3.37 (d, J = 17.7 Hz, 1H, H-12a), 3.69 (dd, J = 17.7, 2.7 Hz, 1H, H-12b), 4.64 (dd, J = 12.3, 2.1 Hz, 1H, H-5b), 5.39 (d, J = 8.4 Hz, 1H, H-8), 5.49 (d, J = 8.1 Hz, 1H, H-7), 6.03–6.09 (m, 1H, ArH), 6.55 (s, 1H, H-25), 6.91–7.14 (m, 3H, ArH), 7.20–7.63 (m, 8H, ArH), 7.70 (d, J = 8.1 Hz, 1H, ArH), 7.81 (d, J = 7.8 Hz, 1H, ArH); 13C-NMR (75 MHz, CDCl3): δc 33.6, 52.1, 53.5, 53.7, 57.6, 75.5, 79.8, 95.2, 103.6, 121.6, 124.0, 124.2, 126.5, 126.9, 127.1, 127.5, 127.8, 128.2, 128.6, 128.8, 129.5, 129.8, 130.2, 131.7, 133.1, 133.6, 134.3, 134.5, 135.1, 135.8, 136.4, 137.3, 138.6, 194.3. Anal. calcd for C34H26Cl2N2O2S: C, 68.34; H, 4.39; N, 4.69. Found: C, 68.47; H, 4.30; N, 4.81%.
8-(2-Fluorophenyl)-11-[(E)-(2-fluorophenyl)methylidene]-14-hydroxy-6-thia-3,13-diazaheptacyclo[13.7.1.19,13.02,9.02,14.03,7.019,23]tetracosa-1(22),15(23),16,18,20-pentaen-10-one (6f) Pale yellow solid, 90% (0.163 g), m.p. 162–164 °C, IR (KBr) υmax 3418, 1724, 1690, 1599 cm−1; 1H-NMR (300 MHz, CDCl3): δH 2.77–3.01 (m, 4H, H-4a, H-4b, H-24a and H-24b), 3.13 (d, J = 12.6 Hz, 1H, H-5a), 3.24 (d, J = 17.7 Hz, 1H, H-12a), 3.65 (dd, J = 17.7, 2.1 Hz, 1H, H-12b), 4.53 (d, J = 12.6 Hz, 1H, H-5b), 4.98 (d, J = 7.2 Hz, 1H, H-8), 5.78 (d, J = 7.2 Hz, 1H, H-7), 5.96 (brs, 1H, OH), 6.17–6.24 (m, 2H, ArH and H-25), 6.77–6.86 (m, 2H, ArH), 7.08–7.32 (m, 5H, ArH), 7.54–7.64 (m, 5H, ArH), 7.80 (d, J = 7.8 Hz, 1H, ArH); 13C-NMR (75 MHz, CDCl3): δC 33.7, 50.4, 53.1, 53.4, 57.5, 57.6, 75.8, 94.7, 103.7, 115.5, 116.8, 121.6, 122.0, 123.6, 123.9, 124.2, 124.7, 126.3, 127.0, 128.0, 128.5, 129.7, 130.4, 130.5, 130.9, 131.2, 132.5, 135.2, 135.7, 137.3, 138.2, 160.2, 161.7, 195.7. Anal. calcd for C34H26F2N2O2S: C, 72.32; H, 4.64; N, 4.96. Found: C, 72.47; H, 4.52; N, 4.87%.
14-Hydroxy-8-(3-nitrophenyl)-11-[(E)-(3-nitrophenyl)methylidene]-6-thia-3,13-diazaheptacyclo[13.7.1.19,13.02,9.02,14.03,7.019,23]tetracosa-1(22),15(23),16,18,20-pentaen-10-one (6g) Dark brown solid, 89% (0.150 g), m.p. 180–182 °C, IR (KBr) υmax 3416, 1719, 1694, 1601 cm−1; 1H-NMR (400 MHz, CDCl3): δH 2.76–3.02 (m, 5H, H-4a, H-4b, H-5a, H-24a and H-24b), 3.31 (d, J = 17.6 Hz, 1H, H-12a), 3.67 (d, J = 17.6 Hz, 1H, H-12b), 4.36 (d, J = 12.0 Hz, 1H, H-5b), 4.97 (d, J = 7.2 Hz, 1H, H-8), 5.74 (d, J = 7.2 Hz , 1H, H-7), 6.15 (s, 1H, H-25), 6.71 (d, J = 7.2 Hz, 1H, ArH), 7.12 (s, 1H, ArH), 7.23–7.34 (m, 2H, ArH), 7.51–7.73 (m, 4H, ArH), 7.88 (d, J = 8.4 Hz, 1H, ArH), 7.93 (d, J = 8.0 Hz, 1H, ArH), 8.00 (d, J = 8.0 Hz, 1H, ArH), 8.17–8.24 (m, 2H, ArH), 8.46 (s, 1H, ArH); 13C-NMR (100 MHz, CDCl3): δC 34.0, 48.2, 52.9, 53.3, 53.6, 57.6, 75.5, 95.0, 103.9, 121.8, 123.0, 123.3, 123.8, 124.1, 124.5, 124.9, 126.2, 127.0, 128.1, 128.8, 129.3, 130.1, 131.2, 133.2, 133.8, 134.9, 135.6, 136.2, 136.5, 138.0, 139.4, 147.9, 148.7, 196.1. Anal. calcd for C34H26N4O6S: C, 66.01; H, 4.24; N, 9.06. Found: C, 66.15; H, 4.10; N, 9.21%.
8-(2,4-Dichlorophenyl)-11-[(E)-(2,4-dichlorophenyl)methylidene]-14-hydroxy-6-thia-3,13-diazaheptacyclo[13.7.1.19,13.02,9.02,14.03,7.019,23]tetracosa-1(22),15(23),16,18,20-pentaen-10-one (6h) Light brown solid, 91% (0.147 g), m.p. 146–147 °C, IR (KBr) υmax 3380, 1723, 1690, 1601 cm−1; 1H-NMR (400 MHz, CDCl3): δH 2.86–2.99 (m, 4H, H-4a, H-4b, H-24a, and H-24b), 3.21 (d, J = 12.0 Hz, 1H, H-5a), 3.33 (d, J = 18.0 Hz, 1H, H-12a), 3.66 (dd, J = 17.6, 2.8 Hz, 1H, H-12b), 4.59 (d, J = 12.4 Hz, 1H, H-5b), 5.30 (d, J = 8.4 Hz, 1H, H-8), 5.41 (d, J = 8.0 Hz 1H, H-7), 5.99 (d, J = 8.4 Hz 1H, ArH), 6.43 (s, 1H, H-25), 6.94 (dd, J = 8.4, 2.0 Hz, 1H, ArH), 7.15–7.28 (m, 2H, ArH), 7.37 (d, J = 8.8 Hz, 1H, ArH), 7.45 (d, J = 7.2 Hz, 1H, ArH), 7.51–7.62 (m, 4H, ArH), 7.71 (d, J = 8.4 Hz, 1H, ArH), 7.80 (dd, J = 6.4, 4.0 Hz, 1H, ArH); 13C-NMR (100 MHz, CDCl3): δC 33.5, 50.4, 52.7, 53.1, 57.4, 75.6, 78.7, 95.0, 103.5, 121.7, 124.1, 126.6, 126.7, 127.4, 127.5, 128.4, 128.7, 129.6, 129.8, 130.4, 130.9, 131.0, 131.3, 132.0, 133.6, 134.3, 134.6, 135.1, 135.2, 135.3, 136.9, 137.0, 138.1, 194.8. Anal. calcd for C34H24Cl4N2O2S: C, 61.28; H, 3.63; N, 4.20. Found: C, 61.15; H, 3.72; N, 4.35%.
14-hydroxy-8-(4-methylphenyl)-11-[(E)-(4-methylphenyl)methylidene]-6-thia-3,13-diazaheptacyclo[13.7.1.19,13.02,9.02,14.03,7.019,23]tetracosa-1(22),15(23),16,18,20-pentaen-10-one (6i) Orange solid, 92% (0.168 g), m.p. 190–192 °C, IR (KBr) υmax 3394, 1723, 1682, 1598 cm−1; 1H-NMR (300 MHz, CDCl3): δH 2.23 (s, 3H, CH3), 2.32 (s, 3H, CH3), 2.79–2.97 (m, 5H, H-4a, H-4b, H-5a, H-24a and H-24b), 3.40 (d, J = 17.4 Hz, 1H, H-12a), 3.67 (dd, J = 17.4, 2.8 Hz, 1H, H-12b), 4.23 (d, J = 12.3 Hz, 1H, H-5b), 4.87 (d, J = 7.2 Hz, 1H, H-8), 5.67 (d, J = 7.2 Hz, 1H, H-7), 6.29 (s, 1H, H-25), 6.36 (d, J = 8.1 Hz, 2H, ArH), 6.89 (d, J = 7.8 Hz, 2H, ArH), 7.15 (d, J = 8.1 Hz, 2H, ArH), 7.28–7.33 (m, 1H, ArH), 7.40 (d, J = 8.1 Hz, 2H, ArH), 7.52–7.61 (m, 4H, ArH), 7.72 (dd, J = 6.9, 1.8 Hz, 1H, ArH); 13C-NMR (75 MHz, CDCl3): δC 21.4, 21.7, 33.8, 53.1, 53.5, 53.7, 57.5, 75.0, 78.0, 95.0, 103.8, 121.6, 124.2, 126.3, 126.6, 128.1, 128.4, 128.9, 129.0, 129.7, 130.2, 131.2, 131.5, 132.3, 134.2, 136.1, 136.7, 137.2, 138.4, 139.2, 196.3. Anal. calcd for C36H32N2O2S: C, 77.67; H, 5.79; N, 5.03. Found: C, 77.84; H, 5.62; N, 5.16%.
14-Hydroxy-8-(4-methoxyphenyl)-11-[(E)-(4-methoxyphenyl)methylidene]-6-thia-3,13-diazaheptacyclo[13.7.1.19,13.02,9.02,14.03,7.019,23]tetracosa-1(22),15(23),16,18,20-pentaen-10-one (6j) White solid, 86% (0.150 g), m.p. 144–146 °C, IR (KBr) υmax 3398, 1719, 1681, 1600 cm−1; 1H-NMR (300 MHz, CDCl3): δH 2.80–2.99 (m, 5H, H-4a, H-4b, H-5a, H-24a and H-24b), 3.39 (d, J = 17.4 Hz, 1H, H-12a), 3.69 (dd, J = 17.4, 2.4 Hz, 1H, H-12b), 3.74 (s, 3H, OCH3), 3.79 (s, 3H, OCH3), 4.22 (d, J = 12.6, 2.1 Hz, 1H, H-5b), 4.85 (d, J = 7.5 Hz, 1H, H-8), 5.64 (d, J = 7.5 Hz, 1H, H-7), 5.95 (s, 1H, OH), 6.28 (s, 1H, H-25), 6.46 (d, J = 8.7 Hz, 2H, ArH), 6.62 (d, J = 8.7 Hz, 2H, ArH), 6.88 (d, J = 8.7 Hz, 2H, ArH), 7.27–7.35 (m, 1H, ArH), 7.43 (d, J = 8.7 Hz, 2H, ArH), 7.51–7.58 (m, 3H, ArH), 7.61 (d, J = 6.6 Hz, 1H, ArH), 7.71 (dd, J = 6.3, 2.7 Hz, 1H, ArH); 13C-NMR (75 MHz, CDCl3): δC 33.8, 53.1, 53.4, 53.5, 55.6, 55.7, 57.4, 74.9, 78.0, 95.0, 103.8, 113.8, 114.4, 121.6, 124.1, 126.3, 126.6, 127.0, 128.1, 128.4, 129.3, 130.0, 131.1, 131.2, 132.1, 136.1, 136.4, 137.2, 138.3, 159.2, 160.3, 196.3. Anal. calcd for C36H32N2O4S: C, 73.45; H, 5.48; N, 4.76. Found: C, 73.59; H, 5.32; N, 4.60%.
8-(4-Bromophenyl)-11-[(E)-(4-bromophenyl)methylidene]-14-hydroxy-6-thia-3,13-diazaheptacyclo[13.7.1.19,13.02,9.02,14.03,7.019,23]tetracosa-1(22),15(23),16,18,20-pentaen-10-one (6k) Brown solid, 90% (0.142 g ), m.p. 156–158 °C, IR (KBr) υmax 3390, 1723, 1681, 1603 cm−1; 1H-NMR (300 MHz, CDCl3): δH 2.83–2.95 (m, 5H, H-4a, H-4b, H-5a, H-24a and H-24b), 3.34 (d, J = 17.4 Hz, 1H, H-12a), 3.62 (d, J = 17.4, 2.1 Hz, 1H, H-12b), 4.20 (d, J = 12.6 Hz, 1H, H-5b), 4.83 (d, J = 7.2 Hz, 1H, H-8), 5.63 (d, J = 7.2 Hz, 1H, H-7), 6.17 (s, 1H, H-25), 6.27 (d, J = 8.4 Hz, 2H, ArH), 7.23 (d, J = 8.4 Hz, 2H, ArH), 7.32–7.41 (m, 3H, ArH), 7.49 (d, J = 8.4 Hz, 2H, ArH), 7.56–7.61 (m, 4H, ArH), 7.72–7.76 (m, 1H, ArH); 13C-NMR (75 MHz, CDCl3): δC 33.9, 53.1, 53.4, 53.5, 57.5, 75.1, 77.6, 95.0, 103.8, 121.8, 121.9, 123.3, 124.2, 126.4, 126.8, 128.3, 128.5, 130.7, 131.2, 131.4, 131.5, 132.2, 132.9, 133.5, 135.1, 135.8, 136.2, 137.1, 138.1, 196.1. Anal. calcd for C34H26Br2N2O2S: C, 59.49; H, 3.82; N, 4.08. Found: C, 59.40; H, 3.71; N, 4.24%.
8-(4-Chlorophenyl)-11-[(E)-(4-chlorophenyl)methylidene]-14-hydroxy-6-thia-3,13-diazaheptacyclo[13.7.1.19,13.02,9.02,14.03,7.019,23]tetracosa-1(22),15(23),16,18,20-pentaen-10-one (6l) Brown solid, 92% (0.160 g ), m.p. 164–166 °C, IR (KBr) υmax 3357, 1719, 1682, 1605 cm−1; 1H-NMR (400 MHz, CDCl3): δH 2.84–2.94 (m, 5H, H-4a, H-4b, H-5a, H-24a and H-24b), 3.35 (d, J = 17.6 Hz, 1H, H-12a), 3.63 (d, J = 17.6, 2.4 Hz, 1H, H-12b), 4.28 (d, J = 12.4 Hz, 1H, H-5b), 4.83 (d, J = 6.4 Hz, 1H, H-8), 5.65 (d, J = 6.4 Hz, 1H, H-7), 6.02 (s, 1H, OH), 6.15 (s, 1H, H-25), 6.71 (d, J = 7.6 Hz, 1H, ArH), 7.12 (s, 1H, ArH), 7.23–7.34 (m, 2H, ArH), 7.51–7.73 (m, 4H, ArH), 7.88 (d, J = 8.4 Hz, 2H, ArH), 7.93 (d, J = 8.0 Hz, 1H, ArH), 8.00 (d, J = 8.0 Hz, 1H, ArH), 8.17–8.24 (m, 2H, ArH ); 13C-NMR (100 MHz, CDCl3): δC 33.8, 52.7, 53.0, 53.3, 57.3, 75.2, 78.2, 94.7, 103.9, 121.4, 123.8, 126.1, 126.5, 127.9, 128.2, 128.3, 128.8, 130.3, 130.9, 131.0, 132.3, 133.2, 133.9, 134.4, 134.5, 135.9, 136.0, 136.9, 138.2, 196.1. Anal. calcd for C34H26Cl2N2O2S: C, 68.34; H, 4.39; N, 4.69. Found: C, 68.20; H, 4.57; N, 4.60%.
8-(4-Fluorophenyl)-11-[(E)-(4-fluorophenyl)methylidene]-14-hydroxy-6-thia-3,13-diazaheptacyclo[13.7.1.19,13.02,9.02,14.03,7.019,23]tetracosa-1(22),15(23),16,18,20-pentaen-10-one (6m) Light brown solid, 91% (0.165 g), m.p. 136–138 °C, IR (KBr) υmax 3424, 1723, 1682, 1598 cm−1; 1H-NMR (400 MHz, CDCl3): δH 2.85–2.99 (m, 5H, H-4a, H-4b, H-5a, H-24a and H-24b), 3.35 (d, J = 17.6 Hz, 1H, H-12a), 3.65 (dd, J = 17.6, 2.4 Hz, 1H, H-12b), 4.23 (dd, J = 12.8, 2.4 Hz, 1H, H-5b), 4.86 (d, J = 7.2 Hz, 1H, H-8), 5.64 (d, J = 7.6 Hz, 1H, H-7), 6.20 (s, 1H, H-25), 6.39–6.43 (m, 2H, ArH), 6.77–6.82 (m, 2H, ArH), 7.03–7.08 (m, 2H, ArH), 7.27–7.34 (m, 1H, ArH), 7.48–7.62 (m, 6H, ArH), 7.75 (dd, J = 6.4, 2.4, 1H, ArH); 13C-NMR (100 MHz, CDCl3): δC 33.9, 52.6, 53.1, 53.4, 57.6, 75.1, 77.9, 95.0, 103.8, 115.4, 115.9, 121.7, 124.1, 126.3, 126.8, 128.1, 128.4, 130.2, 130.6, 131.2, 131.9, 132.0, 133.1, 135.3, 135.9, 137.2, 138.1, 162.5, 162.9, 196.3. Anal. calcd for C34H26F2N2O2S: C, 72.32; H, 4.64; N, 4.96. Found: C, 73.45; H, 4.73; N, 4.82%.