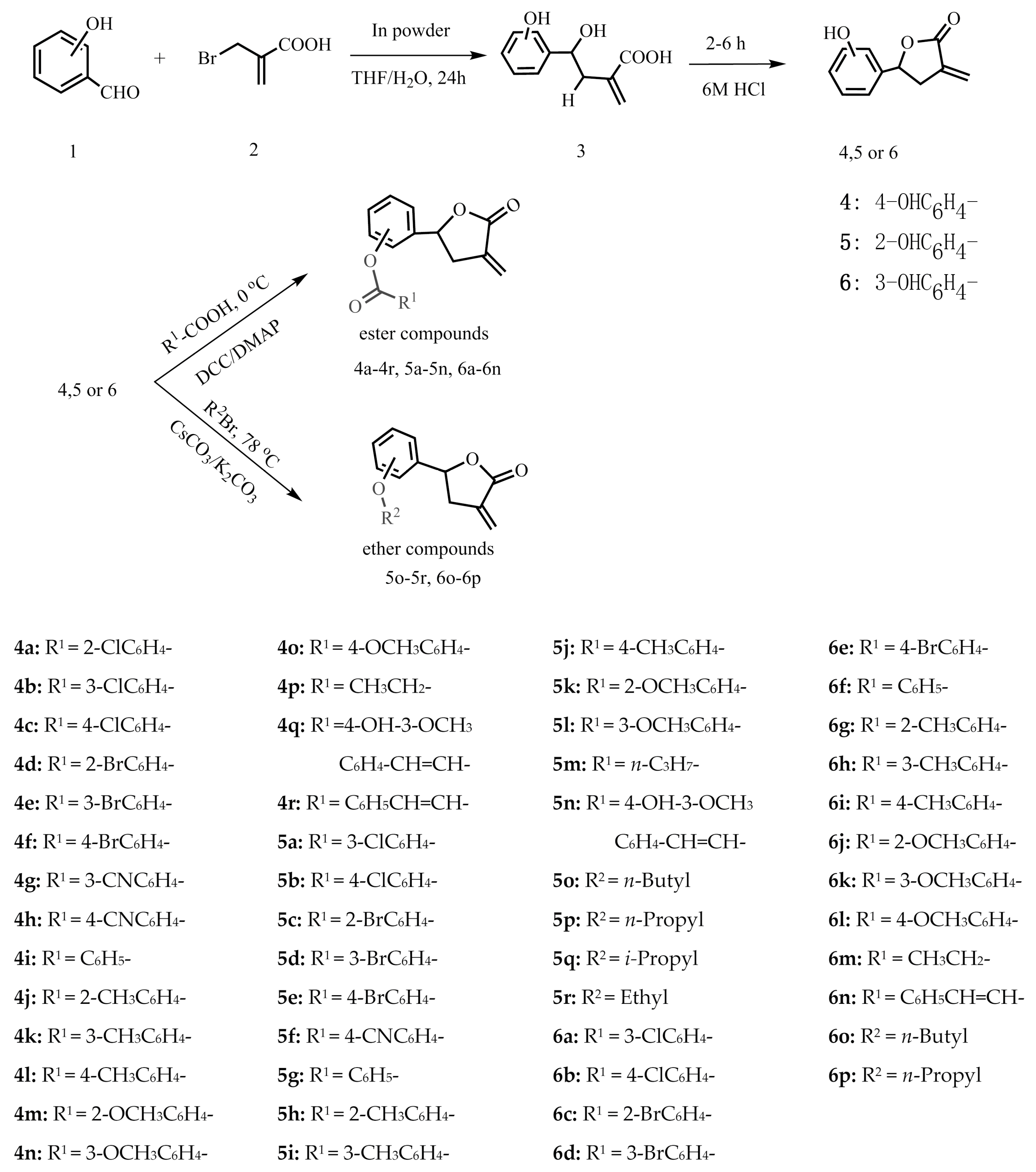
3.2.2. General Synthetic Procedure for Ester Compounds
4-Dimethylaminopyridine (DMAP, 30.0 mg, 0.2 mmol) and the appropriate intermediate compounds 4, 5 or 6 (196.0 mg, 1.1 mmol) were added to anhydrous CH2Cl2 (15.0 mL) containing the respective carboxylic acid (1.1 mmol). Then the mixture was cooled to 0 °C. N,N-dicyclohexyl-carbodiimide (DCC, 226.0 mg, 1.1 mmol) dissolved in anhydrous CH2Cl2 (10.0 mL) was added dropwise into the mixture over a period of 10 min at 0 °C and the mixture was then stirred at room temperature until the reaction was complete according to the TLC analysis. Then, the mixture was filtered. Finally, the residual organic layers were extracted by ethyl acetate (3 × 30 mL) and dried over anhydrous Na2SO4. After filtering, the solution was evaporated under vacuum. The target compounds were purified by column chromatography on silica gel eluting with 0%–40% ethyl acetate in petroleum ether. The structures of all ester derivatives were characterized by 1H-NMR, 13C-NMR, and HR-ESI-MS, and the data are listed below.
4-[4-(2-Chlorobenzoyloxy)phenyl]-2-methylenebutyrolactone (4a) White solid; mp: 187.2–187.9 °C; 40% yield; 1H-NMR (400 MHz, CDCl3): δ 8.05 (dd, J =7.8, 1.1 Hz, 1H, ArH), 7.56–7.47 (m, 3H, ArH), 7.44–7.37 (m, 4H, ArH), 6.33 (t, J = 2.8 Hz, 1H, C=CHH), 5.61–5.52 (m, 1H, OCH), 5.56 (d, J = 7.7 Hz, 1H, C=CHH), 3.50–3.37 (m, 1H, CHHC=CH2), 3.04–2.82 (m, 1H, CHHC=CH2); 13C-NMR (125 MHz, CDCl3): δ 170.06, 163.97, 150.70, 137.72, 134.50, 133.94, 133.41, 131.97, 131.44, 128.99, 126.77, 122.83, 122.16, 77.62, 77.05, 76.73, 36.36, 1.05; HR-MS (ESI): m/z calcd for C18H13ClNaO4 ([M + Na]+) 351.0394, found 351.0395.
4-[4-(3-Chlorobenzoyloxy)phenyl]-2-methylenebutyrolactone (4b) White solid; mp: 168.8–169.2 °C; 54% yield; 1H-NMR (500 MHz, CDCl3): δ 8.24 (m, 1H, ArH), 7.69–7.56 (m, 1H, ArH), 7.54–7.34 (m, 3H, ArH), 7.31–7.19 (m, 3H, ArH), 6.37 (dd, J = 14.9, 12.1 Hz, 1H, C=CHH), 5.76 (dd, J = 14.6, 12.2 Hz, 1H, OCH), 5.60 (dd, J = 20.0, 12.6 Hz, 1H, C=CHH), 3.54–3.37 (m, 1H, CHHC=CH2), 3.02–2.88 (m, 1H, CHHC=CH2); 13C-NMR (125 MHz, CDCl3): δ 150.76, 137.71, 134.84, 133.86, 131.05, 130.22, 129.95, 128.31, 126.75, 122.74, 122.02, 77.31, 77.01, 76.75, 36.30, 29.70; HR-MS (ESI): m/z calcd for C18H13ClNaO4 ([M + Na]+) 351.0394, found 351.0395.
4-[4-(4-Chlorobenzoyloxy)phenyl]-2-methylenebutyrolactone (4c) White solid; mp: 171.3–171.6 °C; 65% yield; 1H-NMR (400 MHz, CDCl3): δ 8.30–8.02 (m, 2H, ArH), 7.68–7.14 (m, 6H, ArH), 6.33 (t, J = 2.8 Hz, 1H, C=CHH), 5.72 (t, J = 2.5 Hz, 1H, OCH), 5.60–5.47 (m, 1H, C=CHH), 3.43 (ddt, J = 17.1, 8.0, 2.4 Hz, 1H, CHHC=CH2), 2.94 (ddt, J = 17.1, 6.1, 2.9 Hz, 1H, CHHC=CH2); 13C-NMR (125 MHz, CDCl3): δ 170.01, 150.82, 140.37, 137.63, 133.95, 131.59, 129.04, 126.73, 122.80, 122.16, 77.39, 77.04, 76.72, 36.31. HR-MS (ESI): m/z calcd for C18H13ClNaO4 ([M + Na]+) 351.0394, found 351.0395.
4-[4-(2-Bromobenzoyloxy)phenyl]-2-methylenebutyrolactone (4d) White crystal; mp: 174.5–174.9 °C; 62% yield; 1H-NMR (500 MHz, CDCl3): δ 8.04 (dd, J = 7.5, 1.7 Hz, 1H, ArH), 7.91–7.68 (m, 1H, ArH), 7.56–7.08 (m, 6H, ArH), 6.36 (t, J = 2.8 Hz, 1H, C=CHH), 5.74 (t, J = 2.4 Hz, 1H, OCH), 5.61–5.50 (m, 1H, C=CHH), 3.56–3.08 (m, 1H, CHHC=CH2), 3.05–2.67 (m, 1H, CHHC=CH2). 13C-NMR (125 MHz, CDCl3): δ 134.69, 133.30, 131.85, 127.36, 126.70, 122.73, 122.03, 77.32, 77.01, 76.75, 36.35, 29.70, 14.99.HR-MS (ESI): m/z calcd for C18H13BrNaO4 ([M + Na]+) 394.9890, found 394.9889.
4-[4-(3-Bromobenzoyloxy)phenyl]-2-methylenebutyrolactone (4e) White crystal; mp: 176.5–176.8 °C; 45% yield; 1H-NMR (500 MHz, CDCl3): δ 8.37 (s, 1H, ArH), 8.16 (d, J = 7.7 Hz, 1H, ArH), 7.80 (d, J = 7.9 Hz, 1H, ArH), 7.56–7.19 (m, 4H, ArH), 6.36 (t, J = 2.8 Hz, 1H, C=CHH), 5.75 (t, J = 2.4 Hz, 1H, OCH), 5.66–5.52 (m, 1H, C=CHH), 5.42 (dd, J = 10.6, 5.3 Hz, 1H, ArH), 3.57–3.37 (m, 1H, CHHC=CH2), 2.95 (s, 1H, CHHC=CH2); 13C-NMR (125 MHz, CDCl3): δ 136.73, 133.16, 132.05, 131.69, 130.22, 128.79, 126.91, 126.53, 122.77, 122.20, 78.63, 77.34, 77.03, 76.78, 40.03, 36.36, 29.72, 15.00. HR-MS (ESI): m/z calcd for C18H13BrNaO4 ([M + Na]+) 394.9890, found 394.9889.
4-[4-(4-Bromobenzoyloxy)phenyl]-2-methylenebutyrolactone (4f) White crystal; mp: 175.1–175.6 °C; 52% yield; 1H-NMR (500 MHz, CDCl3): δ 8.10 (d, J = 8.5 Hz, 2H, ArH), 7.72 (t, J = 12.5 Hz, 4H, ArH), 6.38 (t, J = 2.8 Hz, 1H, C=CHH), 5.76 (t, J = 2.4 Hz, 1H, OCH), 5.65–5.54 (m, 1H, C=CHH), 5.43 (dd, J = 10.7, 5.2 Hz, 2H, ArH), 3.55–3.36 (m, 1H, CHHC=CH2), 3.04–2.93 (m, 1H, CHHC=CH2); 13C-NMR (125 MHz, CDCl3): δ 150.78, 136.95, 132.05, 131.69, 129.05, 128.28, 126.76, 122.75, 122.08, 78.62, 77.28, 77.03, 76.78, 40.03, 36.36, 15.00. HR-MS (ESI): m/z calcd for C18H13BrNaO4 ([M + Na]+) 394.9890, found 394.9889.
4-[4-(3-Benzonitrile)phenyl]-2-methylenebutyrolactone (4g) Yellow oil; 54% yield; 1H-NMR (500 MHz, CDCl3): δ 8.52 (s, 1H, ArH), 8.45 (d, J = 8.0 Hz, 1H, ArH), 7.95 (d, J = 7.8 Hz, 1H, ArH), 7.70 (t, J = 7.9 Hz, 1H, ArH), 7.29 (t, J = 4.3 Hz, 4H, ArH), 6.36 (t, J = 2.8 Hz, 1H, 1H, C=CHH), 5.75 (t, J = 2.4 Hz, 1H, OCH), 5.65–5.56 (m, 1H, C=CHH), 3.46 (ddd, J = 10.5, 5.7, 2.4 Hz, 1H, CHHC=CH2), 2.96 (ddt, J = 17.0, 6.1, 2.9 Hz, 1H, CHHC=CH2), 13C-NMR (125 MHz, CDCl3): δ 163.13, 150.51, 138.01, 136.69, 134.16, 133.82, 130.69, 129.73, 126.83, 122.82, 121.90, 113.36, 77.26, 77.01, 76.76, 49.17, 36.29, 33.95, 25.62. HR-MS (ESI): m/z calcd for C19H13NNaO4 ([M + Na]+) 342.0736, found 342.0740.
4-[4-(4-Benzonitrile)phenyl]-2-methylenebutyrolactone (4h) Yellow oil; 57% yield; 1H-NMR (500 MHz, CDCl3): δ 8.51–8.39 (m, 1H, ArH), 8.34 (t, J = 7.3 Hz, 2H, ArH), 7.98 (d, J = 7.8 Hz, 1H, ArH), 7.85 (s, 2H, ArH), 7.73 (t, J = 7.9 Hz, 1H, ArH), 6.37 (t, J = 2.8 Hz, 1H, C=CHH), 6.25 (t, J = 2.9 Hz, 1H, ArH), 5.75 (t, J = 2.4 Hz, 1H, OCH), 5.71–5.64 (m, 2H, ArH), 5.61–5.55 (m, 1H, C=CHH), 3.49–3.41 (m, 1H, CHHC=CH2), 3.38–3.28 (m, 1H, CHHC=CH2). 13C-NMR (125 MHz, CDCl3): δ 137.00, 133.71, 132.43, 130.67, 129.88, 127.05, 126.80, 123.06, 122.84, 121.89, 77.26, 77.01, 76.75, 73.73, 49.17, 36.28, 33.95, 29.69, 25.64. HR-MS (ESI): m/z calcd for C19H13NNaO4 ([M + Na]+) 342.0736, found 342.0740.
4-(4-Benzoyloxyphenyl)-2-methylenebutyrolactone 4-(4-Benzoyloxyphenyl)-2-methylenebutyrolactone (4i) White solid; mp: 223.3–223.7 °C; 55% yield; 1H-NMR (500 MHz, CDCl3): δ 8.25 (d, J = 8.3 Hz, 5H, ArH), 7.70 (t, J = 7.4 Hz, 4H, ArH), 6.38 (s, 1H, C=CHH), 5.76 (d, J = 2.4 Hz, 1H, OCH), 5.63–5.55 (m, 1H, C=CHH), 4.11 (dd, J = 15.3, 7.9 Hz, 1H, CHHC=CH2), 3.47 (dd, J = 17.1, 8.1 Hz, 1H, CHHC=CH2); 13C-NMR (125 MHz, CDCl3): δ 133.76, 130.21, 128.64, 126.72, 122.12, 78.70, 77.28, 77.03, 76.77, 40.04, 36.41, 29.72, 15.00. HR-MS (ESI): m/z calcd for C18H14NaO4 ([M + Na]+) 317.0784, found 317.0788.
4-[4-(2-Methylbenzoyloxy)phenyl]-2-methylenebutyrolactone (4j) White crystals; mp: 189.6–190.1 °C; 53% yield; 1H-NMR (500 MHz, CDCl3): δ 8.18 (d, J = 7.8 Hz, 1H, ArH), 7.58–7.46 (m, 1H, ArH), 7.40 (t, J = 14.7 Hz, 2H, ArH), 7.35 (t, J = 7.7 Hz, 2H, ArH), 7.28 (t, J = 6.3 Hz, 2H, ArH), 6.35 (t, J = 2.8 Hz, 1H, C=CHH), 5.73 (t, J = 2.4 Hz, 1H, C=CHH), 5.64–5.40 (m, 1H, OCH), 3.45 (ddt, J = 17.1, 8.0, 2.4 Hz, 1H, CHHC=CH2), 3.05–2.85 (m, 1H, CHHC=CH2), 2.70 (s, 3H, ArCH3); 13C-NMR (125 MHz, CDCl3): δ 170.00, 165.67, 151.00, 141.44, 137.39, 134.02, 132.91, 126.65, 125.97, 125.11, 122.96, 122.66, 122.35, 77.47, 77.30, 77.05, 76.79, 36.27, 21.95; HR-MS (ESI): m/z calcd for C19H16NaO4 ([M + Na]+) 331.0940, found 331.0937.
4-[4-(3-Methylbenzoyloxy)phenyl]-2-methylenebutyrolactone (4k) White crystals; mp: 186.2–186.6 °C; 52% yield; 1H-NMR (500 MHz, CDCl3): δ 8.00 (d, J = 8.9 Hz, 2H, ArH), 7.57–7.14 (m, 6H, ArH), 6.33 (t, J = 2.8 Hz, 1H, C=CHH), 5.72 (t, J = 2.5 Hz, 1H, C=CHH), 5.58–5.50 (m, 1H, OCH), 3.43 (ddt, J = 17.1, 8.0, 2.4 Hz, 1H, CHHC=CH2), 3.05–2.82 (m, 1H, CHHC=CH2), 2.45 (s, 3H, ArCH3); 13C-NMR (125 MHz, CDCl3): δ 170.03, 165.28, 151.07, 138.51, 137.37, 134.55, 134.01, 130.71, 129.19, 128.53, 127.37, 126.67, 122.26, 77.48, 77.28, 77.02, 76.77, 36.32, 21.30. HR-MS (ESI): m/z calcd for C19H16NaO4 ([M + Na]+) 331.0940, found 331.0937.
4-[4-(4-Methylbenzoyloxy)phenyl]-2-methylenebutyrolactone (4l) White crystals; mp: 184.5–185.1 °C; 60% yield; 1H-NMR (500 MHz, CDCl3): δ 8.11 (d, J = 8.2 Hz, 2H, ArH), 7.56–7.19 (m, 6H, ArH), 6.35 (t, J = 2.8 Hz, 1H, C=CHH), 5.74 (t, J = 2.5 Hz, 1H, C=CHH), 5.64–5.40 (m, 1H, OCH), 3.45 (ddt, J = 17.1, 8.0, 2.4 Hz, 1H, CHHC=CH2), 3.08–2.82 (m, 1H, CHHC=CH2), 2.48 (s, 3H, ArCH3); 13C-NMR (125 MHz, CDCl3): δ 169.99, 165.13, 151.11, 144.63, 138.51, 137.30, 134.03, 130.24, 129.34, 127.37, 126.58, 122.65, 122.27, 77.48, 77.27, 77.02, 76.76, 36.32, 21.77. HR-MS (ESI): m/z calcd for C19H16NaO4 ([M + Na]+) 331.0940, found 331.0937.
4-[4-(2-Methoxylbenzoyloxy)phenyl]-2-methylenebutyrolactone (4m) White crystals; mp: 179.8–180.4 °C; 60% yield; 1H-NMR (500 MHz, CDCl3): δ 8.02 (dd, J = 8.0, 1.7 Hz, 1H, ArH), 7.56 (td, J = 8.2, 1.8 Hz, 1H, ArH), 7.40–6.97 (m, 6H, ArH), 6.32 (t, J = 2.8 Hz, 1H, C=CHH), 5.71 (t, J = 2.5 Hz, 1H, C=CHH), 5.59–5.48 (m, 1H, OCH), 3.94 (s, 3H, ArOCH3), 3.42 (ddt, J = 17.1, 8.0, 2.4 Hz, 1H, CHHC=CH2), 2.93 (ddt, J = 9.3, 6.0, 2.9 Hz, 1H, CHHC=CH2); 13C-NMR (125 MHz, CDCl3): δ 170.08, 164.25, 159.97, 151.08, 137.20, 134.54, 134.06, 132.25, 126.56, 122.67, 122.37, 120.25, 112.25, 77.55, 77.30, 77.04, 76.79, 56.07, 36.34; HR-MS (ESI): m/z calcd for C19H16NaO5 ([M + Na]+) 347.0889, found 347.0891.
4-[4-(3-Methoxylbenzoyloxy)phenyl]-2-methylenebutyrolactone (4n) White crystals; mp: 185.7–186.2 °C; 44% yield; 1H-NMR (500 MHz, CDCl3): δ 7.85 (d, J = 7.7 Hz, 1H, ArH), 7.74 (s, 4H, ArH), 6.38 (s, 1H, C=CHH), 5.76 (s, 1H, C=CHH), 5.66–5.56 (m, 1H, OCH), 5.44 (dd, J = 10.7, 5.2 Hz, 3H, ArH), 4.35 (s, 3H, ArOCH3), 4.11 (dd, J = 17.0, 9.5 Hz, 1H, CHHC=CH2), 3.47 (dd, J = 17.1, 8.1 Hz, 1H, CHHC=CH2); 13C-NMR (125 MHz, CDCl3): δ 170.03, 164.28, 159.77, 129.67, 126.76, 122.64, 122.10, 120.32, 114.60, 78.69, 77.28, 77.03, 76.78, 55.56, 40.04, 36.41, 29.72, 15.00. HR-MS (ESI): m/z calcd for C19H16NaO5 ([M + Na]+) 347.0889, found 347.0891.
4-[4-(4-Methoxylbenzoyloxy)phenyl]-2-methylenebutyrolactone (4o) White crystals; mp: 184.5–185.2 °C; 51% yield; 1H-NMR (500 MHz, CDCl3): δ 8.28–8.00 (m, 2H, ArH), 7.53–7.30 (m, 2H, ArH), 7.28–7.14 (m, 2H, ArH), 7.02–6.95 (m, 2H, ArH), 6.32 (t, J = 2.8 Hz, 1H, C=CHH), 5.71 (t, J = 2.5 Hz, 1H, C=CHH), 5.58–5.41 (m, 1H, OCH), 3.90 (s, 3H, ArOCH3), 3.49–3.33 (m, 1H, CHHC=CH2), 2.94 (ddd, J = 14.2, 6.3, 3.1 Hz, 1H, CHHC=CH2);13C-NMR (125 MHz, CDCl3): δ 170.08, 164.83, 164.05, 159.97, 151.16, 137.23, 134.05, 132.36, 126.64, 122.70, 122.33, 121.55, 113.92, 77.46, 77.06, 76.74, 55.56, 36.32. HR-MS (ESI): m/z calcd for C19H16NaO5 ([M + Na]+) 347.0889, found 347.0891.
4-[4-(Propionyloxy)phenyl]-2-methylenebutyrolactone (4p) Colourless oil; 68% yield; 1H-NMR (500 MHz, CDCl3): δ 6.35 (t, J = 2.8 Hz, 1H, C=CHH), 5.74 (t, J = 2.4 Hz, 1H, C=CHH), 5.63–5.46 (m, 1H, OCH), 5.39 (dd, J = 10.7, 5.2 Hz, 3H,CH3CH2), 3.44 (dd, J = 17.1, 8.1 Hz, 1H, CHHC=CH2), 3.03–2.90 (m, 1H, CHHC=CH2), 1.67 (s, 2H,CH3CH2); 13C-NMR (125 MHz, CDCl3): δ 178.99, 172.87, 150.84, 136.53, 126.63, 122.64, 78.68, 77.39, 77.06, 76.81, 39.98, 36.33, 27.75, 14.97. HR-MS (ESI): m/z calcd for C14H14NaO4 ([M + Na]+) 269.0784, found 269.0786.
[4-(4-Hydroxy-3-methoxycinnamoyloxy)phenyl]-2-methylenebutyrolactone (4q) Yellow oil; 45% yield; 1H-NMR (400 MHz, CDCl3): δ 7.80 (d, J = 15.9 Hz, 1H, ArOH), 7.36 (d, J = 8.6 Hz, 2H, ArH), 7.21 (dd, J = 21.2, 12.6 Hz, 2H, ArH), 7.16–7.05 (m, 2H, CH=CH), 6.95 (d, J = 8.2 Hz, 1H, ArH), 6.47 (d, J = 15.9 Hz, 1H, ArH), 6.32 (t, J = 2.8 Hz, 1H, C=CHH), 6.10 (s, 1H, ArH), 5.71 (t, J = 2.5 Hz, 1H, C=CHH), 5.58–5.44 (m, 1H, OCH),4.02–3.82 (m, 3H, ArOCH3), 3.49–3.29 (m, 1H, CHHC=CH2), 2.93 (ddt, J = 12.2, 6.0, 2.9 Hz, 1H, CHHC=CH2); 13C-NMR (125 MHz, CDCl3): δ 170.02, 165.56, 151.01, 148.60, 147.01, 137.172, 134.06, 126.63, 123.51, 122.18, 114.92, 114.22, 109.66, 76.72, 56.01, 49.16, 36.28, 33.94, 30.90, 29.69, 25.62. HR-MS (ESI): m/z calcd for C21H18NaO6 ([M + Na]+) 389.0997, found 389.0995.
4-[4-(Cinnamoyloxy)phenyl]-2-methylenebutyrolactone (4r) Yellow oil; 43% yield; 1H-NMR (400 MHz, CDCl3): δ 7.88 (d, J = 16.0 Hz, 1H, ArH), 7.64–7.53 (m, 2H, ArH), 7.47–7.40 (m, 2H, ArH), 7.39–7.32 (m, 2H, ArH), 7.28–7.16 (m, 2H, CH=CH), 6.63 (d, J = 16.0 Hz, 1H, ArH), 6.32 (t, J = 2.8 Hz, 1H, C=CHH), 5.71 (t, J = 2.5 Hz, 1H, C=CHH), 5.63–5.46 (m, 1H, OCH), 3.41 (ddt, J = 17.1, 8.0, 2.4 Hz, 1H, CHHC=CH2), 2.93 (ddt, J = 9.4, 6.0, 2.9 Hz, 1H, CHHC=CH2); 13C-NMR (100 MHz, CDCl3): δ 170.06, 165.31, 150.88, 146.99, 137.31, 134.05, 130.86, 129.05, 128.37, 126.65, 122.72, 122.16, 116.99, 77.44, 77.06, 76.74, 36.30, 33.94, 30.91, 29.63. HR-MS (ESI): m/z calcd for C20H16NaO4 ([M + Na]+) 343.0940, found 343.0941.
4-[2-(3-Chlorobenzoyloxy)phenyl]-2-methylenebutyrolactone (5a) White solid; mp: 201.3–201.8 °C; 54% yield; 1H-NMR (500 MHz, CDCl3): δ 8.16 (t, J = 1.8 Hz, 1H, ArH), 8.08 (d, J = 7.8 Hz, 1H, ArH), 7.71–7.59 (m, 1H, ArH), 7.53–7.45 (m, 2H, ArH), 7.43 (dt, J = 7.8, 3.9 Hz, 1H, ArH), 7.34 (dd, J = 9.4, 4.9 Hz, 1H, ArH), 7.21 (dd, J = 8.0, 0.7 Hz, 1H, ArH), 6.24 (t, J = 2.9 Hz, 1H, C=CHH), 5.68 (dd, J = 8.4, 6.2 Hz, 1H, C=CHH), 5.63 (t, J = 2.5 Hz, 1H, OCH), 3.32 (ddt, J = 17.4, 8.4, 2.6 Hz, 1H, CHHC=CH2), 2.90 (ddt, J = 17.4, 5.9, 2.9 Hz, 1H, CHHC=CH2); 13C-NMR (125 MHz, CDCl3): δ 169.96, 163.75, 147.46, 135.04, 134.19, 133.51, 132.32, 130.46, 129.67, 128.34, 126.85, 126.40, 123.09, 122.85, 77.31, 77.06, 76.80, 35.20; HR-MS (ESI): m/z calcd for C18H14ClO4 ([M + Na]+) 325.0574, found 325.0575.
4-[2-(4-Chlorobenzoyloxy)phenyl]-2-methylenebutyrolactone (5b) White solid; mp: 208.7–209.3 °C; 48% yield; 1H-NMR (400 MHz, CDCl3): δ 8.17–8.07 (m, 2H, ArH), 7.59–7.45 (m, 4H, ArH), 7.26 (s, 2H, ArH), 6.22 (s, 1H, C=CHH), 5.67 (dd, J = 8.4, 6.1 Hz, 1H, OCH), 5.61 (s, 1H, C=CHH), 3.30 (ddt, J = 17.4, 8.4, 2.6 Hz, 1H, CHHC=CH2), 2.90 (ddt, J = 17.4, 5.9, 2.9 Hz, 1H, CHHC=CH2); 13C-NMR (125 MHz, CDCl3): δ 168.96, 165.75, 146.45, 137.04, 134.29, 131.58, 129.66, 129.25, 128.81, 128.26, 126.76, 126.40, 123.09, 122.97, 77.35, 77.03, 76.72, 30.87. HR-MS (ESI): m/z calcd for C18H14ClO4 ([M + Na]+) 325.0572, found 325.0575.
4-[2-(2-Bromobenzoyloxy)phenyl]-2-methylenebutyrolactone (5c) White crystal; mp: 204.6–205.1 °C; 43% yield; 1H-NMR (500 MHz, CDCl3): δ 8.05 (dd, J = 7.5, 1.9 Hz, 1H, ArH), 7.79 (dd, J = 7.7, 1.2 Hz, 1H, ArH), 7.56–7.16 (m, 6H, ArH), 6.25 (t, J = 2.8 Hz, 1H, C=CHH), 5.76 (dd, J = 8.3, 6.2 Hz, 1H, OCH), 5.66 (t, J = 2.5 Hz, 1H, C=CHH), 3.60–3.22 (m, 1H, CHHC=CH2), 2.93 (ddt, J = 17.4, 5.9, 2.8 Hz, 1H, CHHC=CH2); 13C-NMR (125 MHz, CDCl3): δ 164.19, 147.43, 134.88, 132.39, 131.97, 130.59, 129.59, 127.57, 126.81, 126.29, 122.88, 122.69, 122.42, 77.28, 77.03, 76.78, 73.60, 35.33.HR-MS (ESI): m/z calcd for C18H14BrO4 ([M + H]+) 373.0070, found 373.0072.
4-[2-(3-Bromobenzoyloxy)phenyl]-2-methylenebutyrolactone (5d) White crystals; mp: 189.7–190.4 °C; 45% yield; 1H-NMR (500 MHz, CDCl3): δ 8.34 (t, J = 1.6 Hz, 1H, ArH), 8.14 (d, J = 7.8 Hz, 1H, ArH), 7.91–7.76 (m, 1H, ArH), 7.54–7.40 (m, 3H, ArH), 7.36 (td, J = 7.6, 0.7 Hz, 1H, ArH), 7.23 (dd, J = 8.0, 0.6 Hz, 1H, ArH), 6.25 (t, J = 2.9 Hz, 1H, C=CHH), 5.69 (dd, J = 8.3, 6.2 Hz, 1H, OCH), 5.65 (t, J = 2.5 Hz, 1H, C=CHH), 3.33 (ddt, J = 17.4, 8.4, 2.5 Hz, 1H, CHHC=CH2), 2.92 (ddt, J = 17.4, 5.9, 2.8 Hz, 1H, CHHC=CH2); 13C-NMR (125 MHz, CDCl3): δ 169.89, 163.60, 147.50, 137.07, 133.54, 133.12, 130.69, 130.42, 129.65, 128.77, 126.83, 126.42, 123.15, 77.32, 77.06, 76.81, 73.61, 35.20. HR-MS (ESI): m/z calcd for C18H14BrO4 ([M + H]+) 373.0070, found 373.0069.
4-[2-(4-Bromobenzoyloxy)phenyl]-2-methylenebutyrolactone (5e) White crystals; mp: 184.7–185.2 °C; 53% yield; 1H-NMR (500 MHz, CDCl3): δ 8.05 (dd, J = 23.8, 8.5 Hz, 2H, ArH), 7.70 (t, J = 11.9 Hz, 2H, ArH), 7.56–7.20 (m, 4H, ArH), 6.25 (t, J = 2.8 Hz, 1H, C=CHH), 5.69 (dd, J = 8.3, 6.2 Hz, 1H, OCH), 5.63 (t, J = 2.4 Hz, 1H, C=CHH), 3.32 (ddt, J = 17.4, 8.4, 2.5 Hz, 1H, CHHC=CH2), 2.92 (ddt, J = 17.4, 5.9, 2.8 Hz, 1H, CHHC=CH2); 13C-NMR (125 MHz, CDCl3): δ 169.90, 164.22, 147.58, 133.55, 132.27, 131.65, 130.89, 130.62, 129.58, 127.66, 126.75, 126.44, 122.93, 77.27, 77.02, 76.76, 73.69, 35.16. HR-MS (ESI): m/z calcd for C18H14BrO4 ([M + H]+) 373.0070, found 373.0069.
4-[2-(4-Benzonitrile)phenyl]-2-methylenebutyrolactone (5f) Yellow oil; 65% yield; 1H-NMR (500 MHz, CDCl3): δ 8.31 (d, J = 8.5 Hz, 2H, ArH), 7.86 (d, J = 8.5 Hz, 2H, ArH), 7.55–7.43 (m, 2H, ArH), 7.41–7.35 (m, 1H, ArH), 7.30–7.24 (m, 1H, ArH), 6.23 (t, J = 2.9 Hz, 1H, C=CHH), 5.68 (t, J = 7.3 Hz, 1H, OCH), 5.64 (t, J = 2.5 Hz, 1H, C=CHH), 3.33 (ddt, J = 17.4, 8.5, 2.5 Hz, 1H, CHHC=CH2), 3.02–2.93 (m, 1H, CHHC=CH2); 13C-NMR (125 MHz, CDCl3): δ 163.38, 147.52, 133.47, 132.59, 130.68, 129.48, 127.05, 126.81, 23.12, 122.85, 120.68, 117.59, 115.82, 77.30, 77.04, 76.79, 75.28, 73.86, 35.02. HR-MS (ESI): m/z calcd for C19H13NNaO4 ([M + Na]+) 342.0736, found 342.0741.
4-(2-Benzoyloxyphenyl)-2-methylenebutyrolactone (5g) White solid; mp: 166.3–167.0 °C; 57% yield; 1H-NMR (500 MHz, CDCl3): δ 8.22 (d, J = 7.3 Hz, 2H, ArH), 7.71 (t, J = 7.5 Hz, 1H, ArH), 7.57 (t, J = 7.8 Hz, 2H, ArH), 7.49 (t, J = 7.4 Hz, 1H, ArH), 7.47–7.42 (m, 1H, ArH), 7.35 (dd, J = 11.1, 4.0 Hz, 1H, ArH), 7.25 (d, J = 8.1 Hz, 1H, ArH), 6.24 (t, J = 2.8 Hz, 1H, C=CHH), 5.73 (dd, J = 8.2, 6.3 Hz, 1H, OCH), 5.63 (t, J = 2.4 Hz, 1H, C=CHH), 3.34 (ddt, J = 17.4, 8.4, 2.5 Hz, 1H, CHHC=CH2), 2.92 (ddt, J = 17.4, 5.9, 2.8 Hz, 1H, CHHC=CH2); 13C-NMR (125 MHz, CDCl3): δ 133.76, 130.21, 128.64, 126.72, 122.12, 78.70, 77.28, 77.03, 76.77, 40.04, 36.41, 29.72, 15.00. HR-MS (ESI): m/z calcd for C18H15O4 ([M + H]+) 295.0964, found 295.0964.
4-[2-(2-Methylbenzoyloxy)phenyl]-2-methylenebutyrolactone (5h) White crystals; mp: 172.8–173.4 °C; 47% yield; 1H-NMR (400 MHz, CDCl3): δ 8.21–8.12 (m, 1H, ArH), 7.55–7.49 (m, 1H, ArH), 7.49–7.39 (m, 2H, ArH), 7.38–7.28 (m, 3H, ArH), 7.26–7.19 (m, 1H, ArH), 6.22 (t, J = 2.9 Hz, 1H, C=CHH), 5.70 (dd, J = 8.3, 6.1 Hz, 1H, OCH), 5.61 (t, J = 2.5 Hz, 1H, C=CHH), 3.32 (ddt, J = 17.4, 8.4, 2.6 Hz, 1H, CHHC=CH2), 2.90 (ddt, J = 17.4, 5.9, 2.9 Hz, 1H, CHHC=CH2), 2.68 (s, 3H, ArCH3); 13C-NMR (125 MHz, CDCl3): δ 170.05, 165.29, 147.67, 141.92, 133.66, 133.33, 132.55, 131.16, 129.52, 127.60, 126.52, 126.17, 122.96, 77.40, 77.08, 76.77, 73.66, 35.30, 22.08.HR-MS (ESI): m/z calcd for C19H16NaO4 ([M + Na]+) 331.0940, found 337.0940.
4-[2-(3-Methylbenzoyloxy)phenyl]-2-methylenebutyrolactone (5i) White crystals; mp: 179.3–179.9 °C; 45% yield; 1H-NMR (500 MHz, CDCl3): δ 7.99 (d, J = 8.7 Hz, 2H, ArH), 7.48 (t, J = 8.0 Hz, 2H, ArH), 7.45–7.39 (m, 2H, ArH), 7.32 (t, J = 7.6 Hz, 1H, ArH), 7.21 (d, J = 8.0 Hz, 1H, ArH), 6.22 (t, J = 2.9 Hz, 1H, C=CHH), 5.70 (dd, J = 8.3, 6.2 Hz, 1H, OCH), 5.60 (t, J = 2.5 Hz, 1H, C=CHH), 3.31 (ddt, J = 17.4, 8.4, 2.5 Hz, 1H, CHHC=CH2), 2.89 (ddt, J = 17.4, 5.9, 2.8 Hz, 1H, CHHC=CH2), 2.46 (s, 3H, ArCH3); 13C-NMR (125 MHz, CDCl3): δ 170.06, 165.06, 147.69, 138.77, 134.93, 133.65, 132.48, 130.76, 129.52, 128.69, 127.36, 126.56, 126.16, 122.93, 77.30, 77.05, 76.79, 73.66, 35.28, 21.32. HR-MS (ESI): m/z calcd for C19H16NaO4 ([M + Na]+) 337.0940, found 337.0941.
4-[2-(4-Methylbenzoyloxy)phenyl]-2-methylenebutyrolactone (5j) White crystals; mp: 181.2–181.6 °C; 46% yield; 1H-NMR (400 MHz, CDCl3): δ 8.08 (d, J = 8.2 Hz, 2H, ArH), 7.49–7.38 (m, 2H, ArH), 7.36–7.28 (m, 3H, ArH), 7.24–7.19 (m, 1H, ArH), 6.22 (t, J = 2.9 Hz, 1H, C=CHH), 5.70 (dd, J = 8.3, 6.1 Hz, 1H, OCH), 5.60 (t, J = 2.5 Hz, 1H, C=CHH), 3.31 (ddt, J = 17.4, 8.4, 2.6 Hz, 1H, CHHC=CH2), 2.89 (ddt, J = 17.4, 5.9, 2.9 Hz, 1H, CHHC=CH2), 2.47 (s, 3H, ArCH3); 13C-NMR (125 MHz, CDCl3): δ 170.26, 163.06, 149.69,145.15, 138.77, 133.65, 132.49, 130.29, 129.53, 128.49, 126.51, 126.05, 122.95, 77.35, 77.03, 76.72, 73.71, 35.28, 21.83. HR-MS (ESI): m/z calcd for C19H16NaO4 ([M + Na]+) 331.0940, found 331.0937.
4-[2-(2-Methoxylbenzoyloxy)phenyl]-2-methylenebutyrolactone (5k) White crystals; mp: 177.8–178.6 °C; 51% yield; 1H-NMR (500 MHz, CDCl3): δ 8.00 (dd, J = 7.7, 1.6 Hz, 1H, ArH), 7.59 (td, J = 8.5, 1.7 Hz, 1H, ArH), 7.41 (ddd, J = 15.7, 9.2, 4.6 Hz, 2H, ArH), 7.33–7.19 (m, 2H, ArH), 7.07 (dd, J = 12.2, 5.2 Hz, 2H, ArH), 6.24 (t, J = 2.9 Hz, 1H, C=CHH), 5.79 (dd, J = 8.2, 6.2 Hz, 1H, OCH), 5.61 (t, J = 2.5 Hz, 1H, C=CHH), 3.95 (s, 3H, ArOCH3), 3.36 (ddt, J = 17.4, 8.3, 2.5 Hz, 1H, CHHC=CH2), 2.87 (ddt, J = 17.4, 5.9, 2.9 Hz, 1H, CHHC=CH2); 13C-NMR (125 MHz, CDCl3): δ 170.21, 164.40, 159.83, 147.61, 134.84, 133.89, 132.54, 129.32, 126.40, 125.81, 122.97, 122.72, 120.46, 118.38, 112.25, 77.31, 77.06, 76.80, 73.71, 56.01, 35.45. HR-MS (ESI): m/z calcd for C19H16NaO5 ([M + Na]+) 347.0889, found 347.0889.
4-[2-(3-Methoxylbenzoyloxy)phenyl]-2-methylenebutyrolactone (5l) White crystals; mp: 180.3–180.8 °C; 50% yield; 1H-NMR (500 MHz, CDCl3): δ 7.81 (d, J = 7.7 Hz, 1H, ArH), 7.76–7.65 (m, 1H, ArH), 7.55–7.40 (m, 3H, ArH), 7.35 (t, J = 7.2 Hz, 1H, ArH), 7.30–7.22 (m, 2H, ArH), 6.25 (t, J = 2.9 Hz, 1H, C=CHH), 5.72 (dd, J = 8.3, 6.2 Hz, 1H, OCH), 5.63 (t, J = 2.5 Hz, 1H, C=CHH), 3.92 (s, 3H, ArOCH3), 3.42–3.22 (m, 1H, CHHC=CH2), 3.02–2.76 (m, 1H, CHHC=CH2); 13C-NMR (125 MHz, CDCl3): δ 169.99, 164.76, 159.88, 147.70, 133.63, 132.44, 129.86, 129.54, 126.60, 126.24, 122.92, 122.56, 120.63, 114.65, 77.28, 77.02, 76.77, 73.69, 55.57, 35.24. HR-MS (ESI): m/z calcd for C19H16NaO5 ([M + Na]+) 347.0889, found 347.0889.
4-[2-(Butyryloxy)phenyl]-2-methylenebutyrolactone (5m) Colourless oil; 78% yield; 1H-NMR (500 MHz, CDCl3): δ 7.41–7.33 (m, 2H, ArH), 7.30–7.21 (m, 1H, ArH), 7.16–7.05 (m, 1H, ArH), 6.31 (t, J = 2.8 Hz, 1H, C=CHH), 5.68 (t, J = 2.4 Hz, 1H, C=CHH), 5.61 (dd, J = 8.3, 6.3 Hz, 1H, OCH), 3.34 (ddt, J = 17.4, 8.4, 2.5 Hz, 1H, CHHC=CH2), 2.86 (ddt, J = 17.4, 5.9, 2.8 Hz, 1H, CHHC=CH2), 2.61–2.50 (m, 2H, CH3CH2CH2), 1.78 (dt, J = 14.8, 7.4 Hz, 2H, CH3CH2CH2), 1.05 (dd, J = 9.0, 5.9 Hz, 3H, CH3CH2CH2); 13C-NMR (125 MHz, CDCl3): δ 171.75, 147.64, 133.87, 131.95, 129.47, 126.31, 77.34, 77.08, 76.83, 73.94, 36.12, 35.23, 18.41, 13.68. HR-MS (ESI): m/z calcd for C15H16NaO4 ([M + Na]+) 283.0840, found 283.0841.
4-[2-(Cinnamoyloxy)phenyl]-2-methylenebutyrolactone (5n) Yellow oil; 48% yield; 1H-NMR (500 MHz, CDCl3): δ 7.84 (d, J = 15.9 Hz, 1H, ArH), 7.49–7.39 (m, 2H, ArH), 7.32 (t, J = 7.5 Hz, 1H, ArH), 7.29 (s, 1H, ArH), 7.24–7.17 (m, 2H, ArH), 7.01–6.97 (m, 1H, ArH), 6.48 (d, J = 15.9 Hz, 1H, ArH), 6.30 (t, J = 2.7 Hz, 1H, C=CHH), 5.71 (dd, J = 8.2, 6.4 Hz, 1H, OCH), 5.66 (d, J = 7.3 Hz, 1H, C=CHH), 4.00 (s, 2H, CH=CH), 3.39 (dd, J = 17.5, 8.5 Hz, 1H, CHHC=CH2), 2.99–2.84 (m, 1H, CHHC=CH2); 13C-NMR (125 MHz, CDCl3): δ 148.74, 147.72, 129.49, 127.04, 126.21, 123.67, 122.95, 122.72, 114.93, 113.54, 109.70, 77.27, 77.01, 76.76, 74.01, 56.04, 35.16, 26.33. HR-MS (ESI): m/z calcd for C21H19O6 ([M + H]+) 367.1173, found 317.1176.
4-[3-(3-Chlorobenzoyloxy)phenyl]-2-methylenebutyrolactone (6a) White solid; mp: 205.7–206.4 °C; 52% yield; 1H-NMR (500 MHz, CDCl3): δ 8.20 (s, 1H, ArH), 8.10 (d, J = 7.7 Hz, 1H, ArH), 7.65 (dd, J = 8.0, 1.0 Hz, 1H, ArH), 7.55–7.45 (m, 2H, ArH), 7.30–7.19 (m, 3H, ArH), 6.35 (t, J = 2.8 Hz, 1H, C=CHH), 5.74 (t, J = 2.4 Hz, 1H, C=CHH), 5.62–5.54 (m, 1H, OCH), 3.52–3.41 (m, 1H, CHHC=CH2), 2.97 (ddt, J = 12.2, 6.0, 2.9 Hz, 1H, CHHC=CH2); 13C-NMR (125 MHz, CDCl3): δ 163.87, 151.10, 141.83, 134.86, 133.76, 131.04, 130.35, 129.90, 128.31, 122.93, 121.78, 118.66, 77.41, 76.88, 76.76, 36.21, 29.70. HR-MS (ESI): m/z calcd for C18H13ClNaO4 ([M + Na]+) 351.0394, found 351.0395.
4-[3-(4-Chlorobenzoyloxy)phenyl]-2-methylenebutyrolactone (6b) White solid; mp: 209.8–210.5 °C; 64% yield; 1H-NMR (400 MHz, CDCl3): δ 8.22–8.03 (m, 2H, ArH), 7.61–7.36 (m, 3H, ArH), 7.33–7.14 (m, 3H, ArH), 6.32 (t, J = 2.8 Hz, 1H, C=CHH), 5.71 (t, J = 2.5 Hz, 1H, C=CHH), 5.56 (dd, J = 7.8, 6.7 Hz, 1H, OCH), 3.44 (ddt, J = 17.1, 8.1, 2.5 Hz, 1H, CHHC=CH2), 2.94 (ddt, J = 17.1, 6.2, 2.9 Hz, 1H, CHHC=CH2); 13C-NMR (125 MHz, CDCl3): δ 164.24, 151.14, 141.80, 140.37, 133.73, 131.58, 130.12, 129.05, 127.72, 122.93, 121.85, 118.72, 77.52 ,76.91, 76.74, 36.21. HR-MS (ESI): m/z calcd for C18H13ClNaO4 ([M + Na]+) 351.0394, found 351.0394.
4-[3-(2-Bromobenzoyloxy)phenyl]-2-methylenebutyrolactone (6c) White crystals; mp: 175.3–175.8 °C; 56% yield; 1H-NMR (500 MHz, CDCl3): δ 8.06 (dd, J = 7.6, 1.7 Hz, 1H, ArH), 7.83–7.72 (m, 1H, ArH), 7.61–7.42 (m, 2H, ArH), 7.30 (d, J = 10.2 Hz, 4H, ArH), 6.38 (t, J = 2.8 Hz, 1H, C=CHH), 5.76 (dd, J = 6.3, 3.9 Hz, 1H, C=CHH), 5.67–5.40 (m, 1H, OCH), 3.62–3.30 (m, 1H, CHHC=CH2), 3.05–2.88 (m, 1H, CHHC=CH2); 13C-NMR (125 MHz, CDCl3): δ 134.74, 133.35, 130.12, 127.40, 126.70, 122.91, 118.69, 77.28, 77.02, 76.77, 36.35, 29.71, 19.20. HR-MS (ESI): m/z calcd for C18H13BrNaO4 ([M + Na]+) 394.9889, found 394.9892.
4-[3-(3-Bromobenzoyloxy)phenyl]-2-methylenebutyrolactone (6d) White crystals; mp: 178.6–178.9 °C; 63% yield; 1H-NMR (500 MHz, CDCl3): δ 8.31 (d, J = 52.5 Hz, 1H, ArH), 8.10 (dd, J = 52.3, 7.5 Hz, 1H, ArH), 7.84–7.72 (m, 1H, ArH), 7.54–7.34 (m, 2H, ArH), 7.33–7.17 (m, 3H, ArH), 6.36 (t, J = 2.8 Hz, 1H, C=CHH), 5.74 (t, J = 2.5 Hz, 1H, C=CHH), 5.66–5.46 (m, 1H, OCH), 3.60–3.36 (m, 1H, CHHC=CH2), 2.97 (ddt, J = 17.1, 6.1, 2.9 Hz, 1H, CHHC=CH2); 13C-NMR (125 MHz, CDCl3): δ 136.72, 133.14, 132.05, 131.69, 130.17, 128.76, 126.91, 126.53, 122.94, 121.78, 118.66, 77.37, 76.87, 76.76, 36.21, 29.72, 15.00. HR-MS (ESI): m/z calcd for C18H13BrNaO4 ([M + Na]+) 394.9894, found 394.9890.
4-[3-(4-Bromobenzoyloxy)phenyl]-2-methylenebutyrolactone (6e) White crystals; mp: 168.6–169.4 °C; 41% yield; 1H-NMR (500 MHz, CDCl3): δ 8.10 (d, J = 8.5 Hz, 2H, ArH), 8.00 (d, J = 8.4 Hz, 2H, ArH), 7.69 (dd, J = 24.8, 8.4 Hz, 4H, ArH), 6.37 (t, J = 2.8 Hz, 1H, C=CHH), 5.76 (t, J = 2.4 Hz, 1H, C=CHH), 5.61 (s, 1H, OCH), 4.35 (t, J = 6.8 Hz, 1H, CHHC=CH2), 3.48 (dd, J = 17.0, 8.1 Hz, 1H, CHHC=CH2). 13C-NMR (125 MHz, CDCl3): δ 150.78, 137.95, 133.05, 131.69, 129.05, 127.28, 126.76, 122.75, 122.08, 78.62, 77.28, 77.03, 76.78, 40.03, 36.36, 15.00. HR-MS (ESI): m/z calcd for C18H13BrNaO4 ([M + Na]+) 394.9890, found 394.9889.
4-(3-Benzoyloxyphenyl)-2-methylenebutyrolactone (6f) White solid; mp: 204.5–204.8 °C; 52% yield; 1H-NMR (500 MHz, CDCl3): δ 8.22 (d, J = 7.3 Hz, 2H, ArH), 7.68 (t, J = 7.5 Hz, 1H, ArH), 7.49 (t, J = 7.8 Hz, 1H, ArH), 7.32–7.20 (m, 5H, ArH), 6.35 (t, J = 2.8 Hz, 1H, C=CHH), 5.73 (t, J = 2.4 Hz, 1H, C=CHH), 5.62–5.52 (m, 1H, OCH), 3.46 (ddt, J = 17.1, 8.1, 2.4 Hz, 1H, CHHC=CH2), 2.98 (ddt, J = 9.2, 6.0, 2.8 Hz, 1H, CHHC=CH2); 13C-NMR (125 MHz, CDCl3): δ 135.76, 131.21, 128.69, 126.77, 122.62, 78.70, 77.28, 77.03, 76.77, 42.04, 36.51, 29.79, 15.20. HR-MS (ESI): m/z calcd for C18H14NaO4 ([M + Na]+) 317.0785, found 317.0784.
4-[3-(2-Methylbenzoyloxy)phenyl]-2-methylenebutyrolactone (6g) White crystals; mp: 178.3–178.9 °C; 57% yield; 1H-NMR (500 MHz, CDCl3): δ 8.18 (d, J = 7.8 Hz, 1H, ArH), 7.54–7.46 (m, 2H, ArH), 7.36 (t, J = 7.7 Hz, 2H, ArH), 7.24 (dd, J = 13.8, 7.7 Hz, 3H, ArH), 6.35 (t, J = 2.8 Hz, 1H, C=CHH), 5.73 (t, J = 2.4 Hz, 1H, C=CHH), 5.64–5.51 (m, 1H, OCH), 3.46 (ddt, J = 17.1, 8.1, 2.4 Hz, 1H, CHHC=CH2), 2.98 (ddt, J = 17.1, 6.1, 2.9 Hz, 1H, CHHC=CH2), 2.70 (s, 3H, ArCH3); 13C-NMR (125 MHz, CDCl3): δ 165.62, 151.30, 141.68, 141.44, 133.81, 132.91, 132.02, 131.21, 130.02, 128.21, 125.98, 122.71, 122.06, 118.92, 77.26, 77.01, 76.76, 36.23, 21.96. HR-MS (ESI): m/z calcd for C19H16NaO4 ([M + Na]+) 331.0940, found 331.0940.
4-[3-(3-Methylbenzoyloxy)phenyl]-2-methylenebutyrolactone (6h) White crystals; mp: 177.6–178.2 °C; 68% yield; 1H-NMR (500 MHz, CDCl3): δ 8.03 (s, 1H, ArH), 7.46 (ddd, J = 15.1, 9.9, 5.9 Hz, 3H, ArH), 7.28 (d, J = 9.6 Hz, 4H, ArH), 6.35 (t, J = 2.8 Hz, 1H, C=CHH), 5.73 (t, J = 2.4 Hz, 1H, C=CHH), 5.66–5.52 (m, 1H, OCH), 3.50–3.39 (m, 1H, CHHC=CH2), 3.03–2.93 (m, 1H, CHHC=CH2), 2.48 (s, 3H, ArCH3); 13C-NMR (125 MHz, CDCl3): δ 170.05, 165.28, 152.07, 136.51, 137.36, 134.45, 134.61, 130.71, 129.69, 128.53, 127.37, 126.67, 122.26, 77.48, 77.28, 77.02, 76.77, 36.32, 21.30. HR-MS (ESI): m/z calcd for C19H16NaO4 ([M + Na]+) 331.0942, found 331.0940.
4-[3-(4-Methylbenzoyloxy)phenyl]-2-methylenebutyrolactone (6i) White crystals; mp: 168.5–168.9 °C; 57% yield; 1H-NMR (500 MHz, CDCl3): δ 8.10 (d, J = 8.1 Hz, 2H, ArH), 7.48 (t, J = 7.8 Hz, 1H, ArH), 7.34 (d, J = 8.0 Hz, 1H, ArH), 7.30–7.20 (m, 4H, ArH), 6.35 (t, J = 2.8 Hz, 1H, C=CHH), 5.73 (t, J = 2.5 Hz, 1H, C=CHH), 5.70–5.40 (m, 1H, OCH), 3.56–3.36 (m, 1H, CHHC=CH2), 2.98 (ddt, J = 17.1, 6.0, 2.9 Hz, 1H, CHHC=CH2), 2.34 (d, J = 144.3 Hz, 3H, ArCH3). 13C-NMR (125 MHz, CDCl3): δ 169.99, 165.13, 151.11, 144.66, 141.64, 130.24, 130.01, 129.36, 127.37, 126.58, 122.79, 122.63, 122.00, 118.87, 77.27, 77.01, 76.76, 36.22, 21.77. HR-MS (ESI): m/z calcd for C19H16NaO4 ([M + Na]+) 331.0940, found 331.0941.
4-[3-(2-Methoxylbenzoyloxy)phenyl]-2-methylenebutyrolactone (6j) White crystals; mp: 172.3–172.8 °C; 45% yield; 1H-NMR (500 MHz, CDCl3): δ 8.07–7.99 (m, 1H, ArH), 7.61–7.56 (m, 1H, ArH), 7.32–7.20 (m, 4H, ArH), 7.08 (t, J = 7.6 Hz, 2H, ArH), 6.34 (t, J = 2.8 Hz, 1H, C=CHH), 5.73 (t, J = 2.4 Hz, 1H, C=CHH), 5.62–5.43 (m, 1H, OCH), 3.97 (s, 3H, ArOCH3), 3.47–3.38 (m, 1H, CHHC=CH2), 2.97 (ddt, J = 17.1, 6.1, 2.9 Hz, 1H, CHHC=CH2). 13C-NMR (125 MHz, CDCl3): δ 151.37, 141.52, 134.54, 133.87, 132.27, 129.92, 122.76, 122.54, 122.09, 120.27, 118.98, 112.26, 77.30, 77.02, 76.76, 56.07, 49.16, 36.23, 33.95, 25.63, 24.94. HR-MS (ESI): m/z calcd for C19H16NaO5 ([M + Na]+) 347.0889, found 347.0890.
4-[3-(3-Methoxybenzoyloxy)phenyl]-2-methylenebutyrolactone (6k) White crystals; mp: 183.7–184.2 °C; 57% yield; 1H-NMR (500 MHz, CDCl3): δ 7.82 (d, J = 7.7 Hz, 1H, ArH), 7.73 (d, J = 7.7 Hz, 1H, ArH), 7.47 (dt, J = 18.6, 7.8 Hz, 2H, ArH), 7.31–7.19 (m, 4H, ArH), 6.35 (t, J = 2.8 Hz, 1H, C=CHH), 5.73 (t, J = 2.4 Hz, 1H, C=CHH), 5.62–5.55 (m, 1H, OCH), 3.92 (s, 3H, ArOCH3), 3.46 (ddt, J = 17.0, 8.1, 2.4 Hz, 1H, CHHC=CH2), 3.01–2.91 (m, 1H, CHHC=CH2). 13C-NMR (125 MHz, CDCl3): δ 159.75, 151.34, 141.70, 133.78, 130.05, 129.67, 129.49, 122.95–122.49, 121.94, 120.39, 118.80, 114.51, 77.27, 77.01, 76.76, 55.49, 36.21, 29.70.HR-MS (ESI): m/z calcd for C19H16NaO5 ([M + Na]+) 347.0889, found 347.0889.
4-[3-(4-Methoxybenzoyloxy)phenyl]-2-methylenebutyrolactone (6l) White crystals; mp: 182.6–183.3 °C; 56% yield; 1H-NMR (500 MHz, CDCl3): δ 8.23–8.07 (m, 2H, ArH), 7.45 (t, J = 8.0 Hz, 1H, ArH), 7.29–7.14 (m, 3H, ArH), 7.02–6.92 (m, 2H, ArH), 6.32 (t, J = 2.8 Hz, 1H, C=CHH), 5.70 (t, J = 2.5 Hz, 1H, C=CHH), 5.55 (dd, J = 7.8, 6.8 Hz, 1H, OCH), 3.90 (s, 3H, ArOCH3), 3.43 (ddt, J = 17.1, 8.1, 2.5 Hz, 1H, CHHC=CH2), 3.00–2.87 (m, 1H, CHHC=CH2); 13C-NMR (125 MHz, CDCl3): δ 169.99, 164.80, 164.06, 151.44, 141.61, 133.83, 132.35, 130.00, 122.84, 122.60, 122.06, 121.52, 118.92, 113.93, 77.35, 77.06, 76.74, 55.56, 36.21.HR-MS (ESI): m/z calcd for C19H16NaO5 ([M + Na]+) 347.0889, found 347.0892.
4-(3-Propionyloxyphenyl)-2-methylenebutyrolactone (6m) Colourless oil; 56% yield; 1H-NMR (500 MHz, CDCl3): δ 7.42 (t, J = 8.3 Hz, 1H, ArH), 7.29 (s, 1H, ArH), 7.20 (d, J = 7.8 Hz, 1H, ArH), 7.09 (s, 1H, ArH), 6.34 (t, J = 2.8 Hz, 1H, C=CHH), 5.72 (t, J = 2.4 Hz, 1H, C=CHH), 5.64–5.43 (m, 1H, OCH), 3.44 (ddt, J = 17.1, 8.1, 2.4 Hz, 1H, CHHC=CH2), 2.94 (ddt, J = 17.1, 6.0, 2.8 Hz, 1H, CHHC=CH2), 2.62 (q, J = 7.5 Hz, 2H, CH3CH2), 1.29 (t, J = 7.5 Hz, 3H, CH3CH2); 13C-NMR (125 MHz, CDCl3): δ 172.81, 151.14, 141.57, 133.78, 129.92, 122.78, 122.53, 121.75, 118.63, 77.23, 77.01, 76.75, 36.20, 27.73, 9.02. HR-MS (ESI): m/z calcd for C14H14NaO4 ([M + Na]+) 269.0784, found 269.0784.
4-(3-Cinnamoyloxyphenyl)-2-methylenebutyrolactone (6n) Yellow oil; 34% yield; 1H-NMR (400 MHz, CDCl3): δ 7.87 (d, J = 16.0 Hz, 1H, ArH), 7.64–7.55 (m, 2H, CH=CH), 7.47–7.38 (m, 4H, ArH), 7.25–7.10 (m, 3H, ArH), 6.62 (d, J = 16.0 Hz, 1H, ArH), 6.31 (t, J = 2.8 Hz, 1H, C=CHH), 5.70 (t, J = 2.5 Hz, 1H, C=CHH), 5.60–5.45 (m, 1H, OCH), 3.42 (ddt, J = 17.1, 8.1, 2.5 Hz, 1H, CHHC=CH2), 2.93 (ddt, J = 17.1, 6.0, 2.9 Hz, 1H, CHHC=CH2); 13C-NMR (100 MHz, CDCl3): δ 169.99, 165.26, 151.17, 147.00, 141.64, 134.07, 133.83, 130.88, 130.01, 129.06, 128.38, 122.76, 121.88, 118.76, 116.98, 77.56,76.95, 76.78, 36.19. HR-MS (ESI): m/z calcd for C20H16NaO4 ([M + Na]+) 343.0940, found 343.0941.