Pharmacological Properties of Riparin IV in Models of Pain and Inflammation
Abstract
:1. Introduction
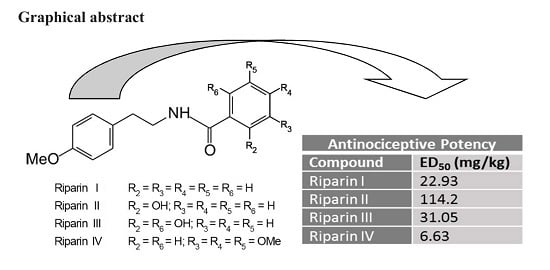
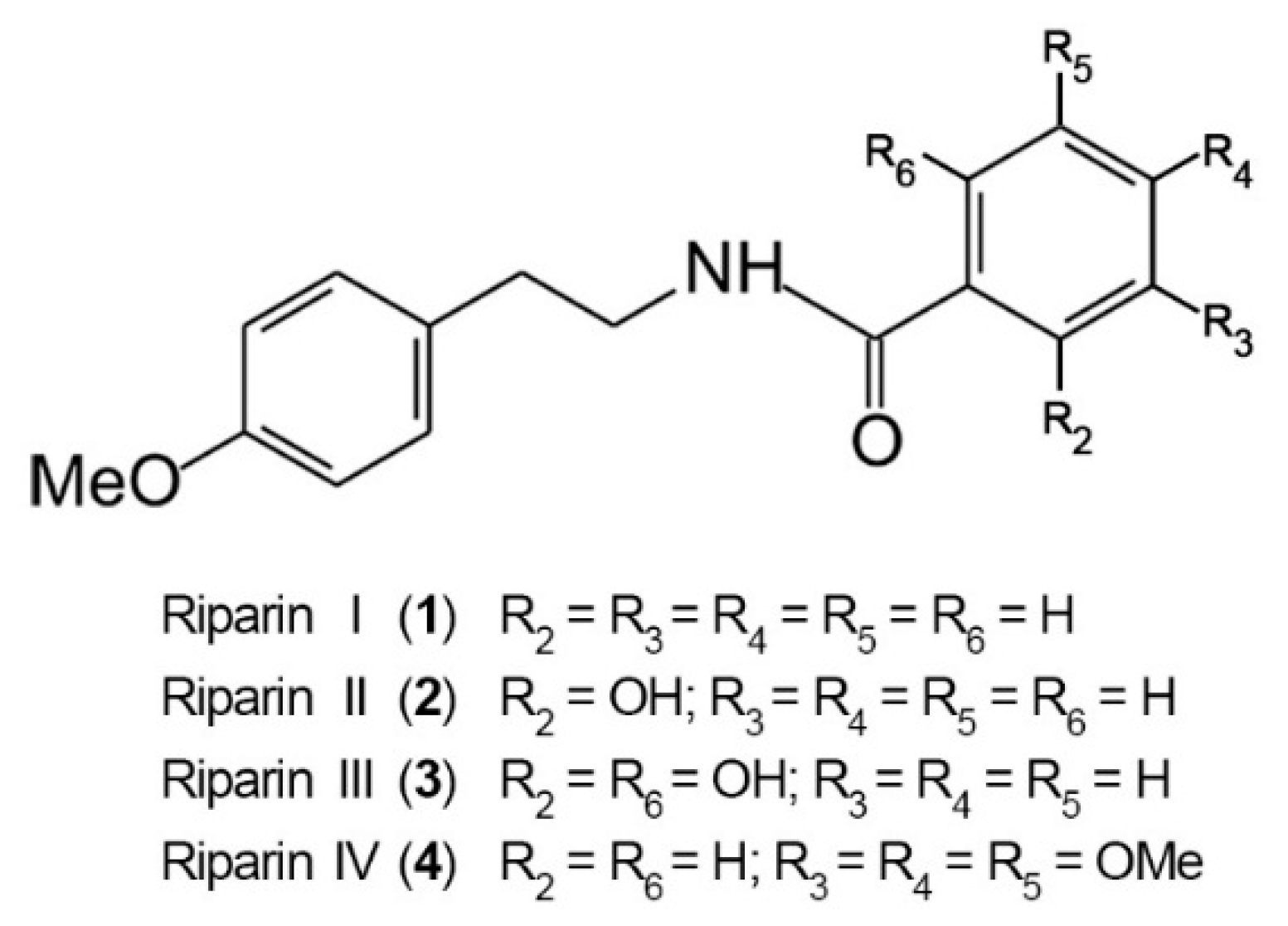
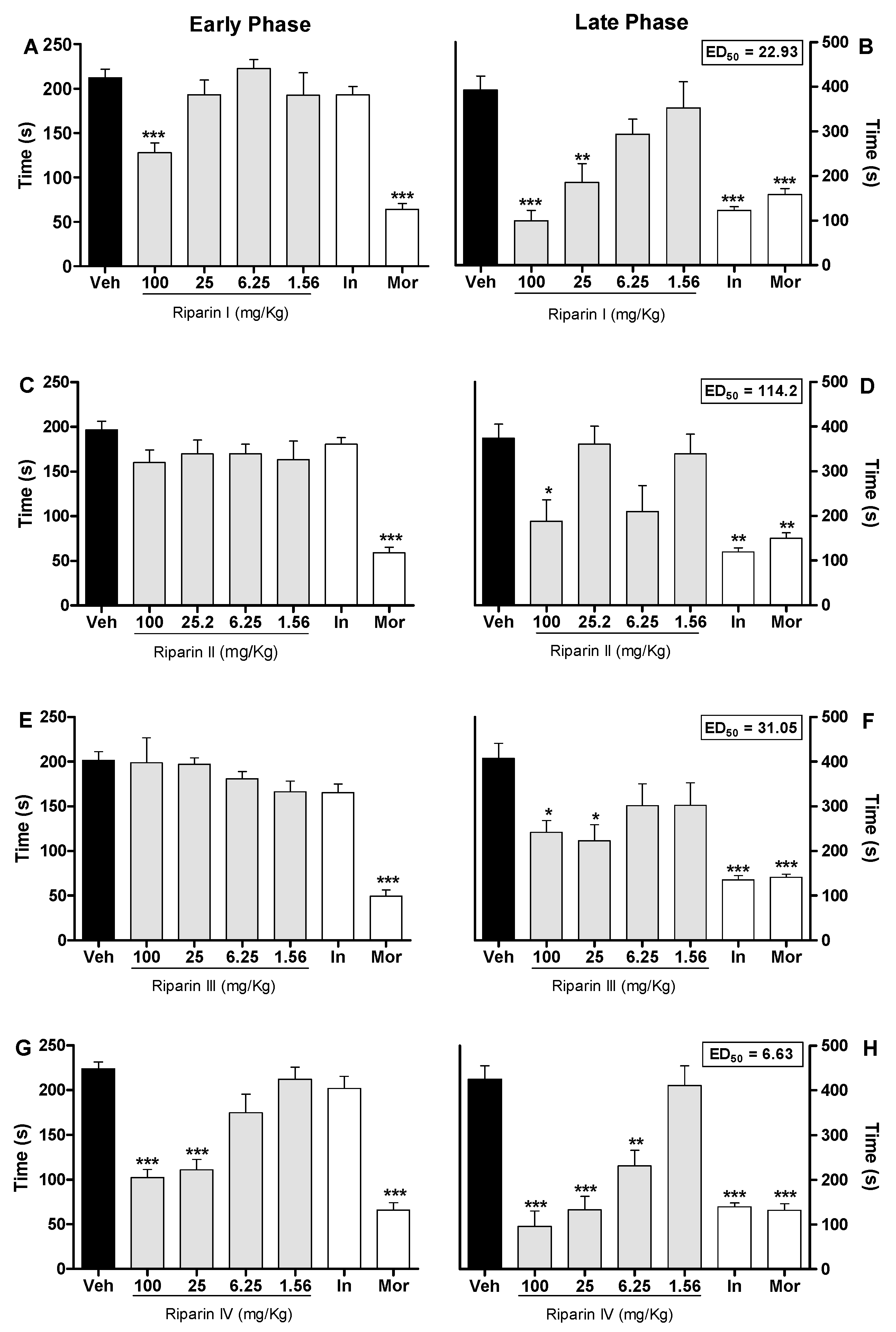
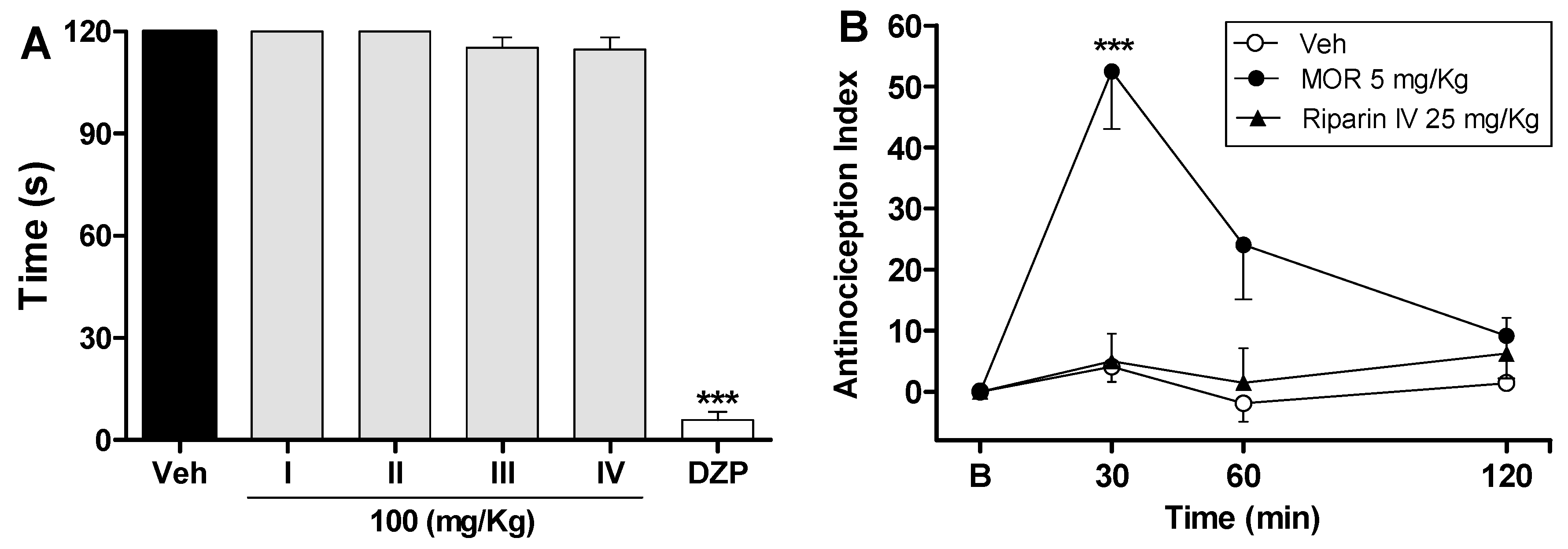
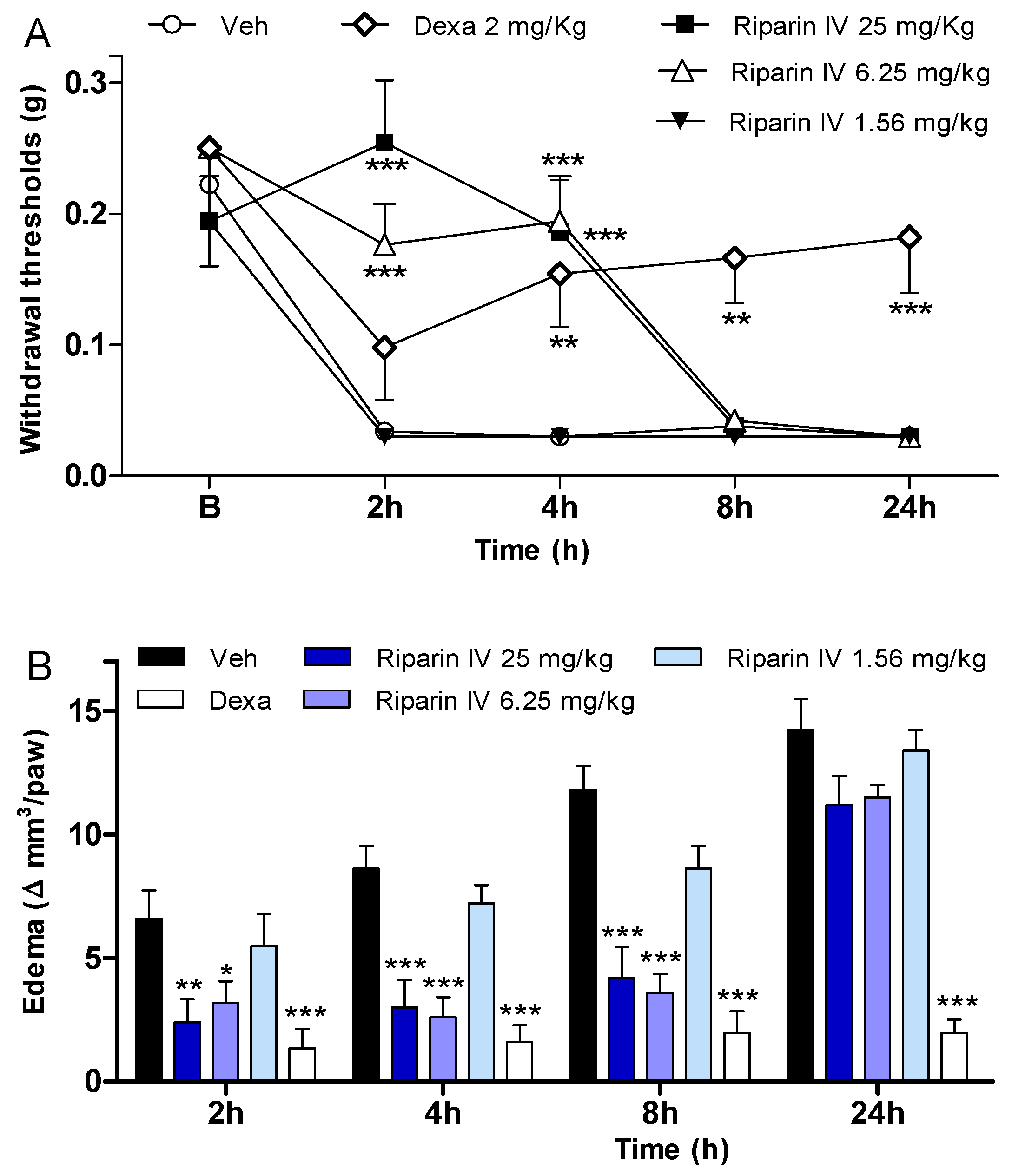
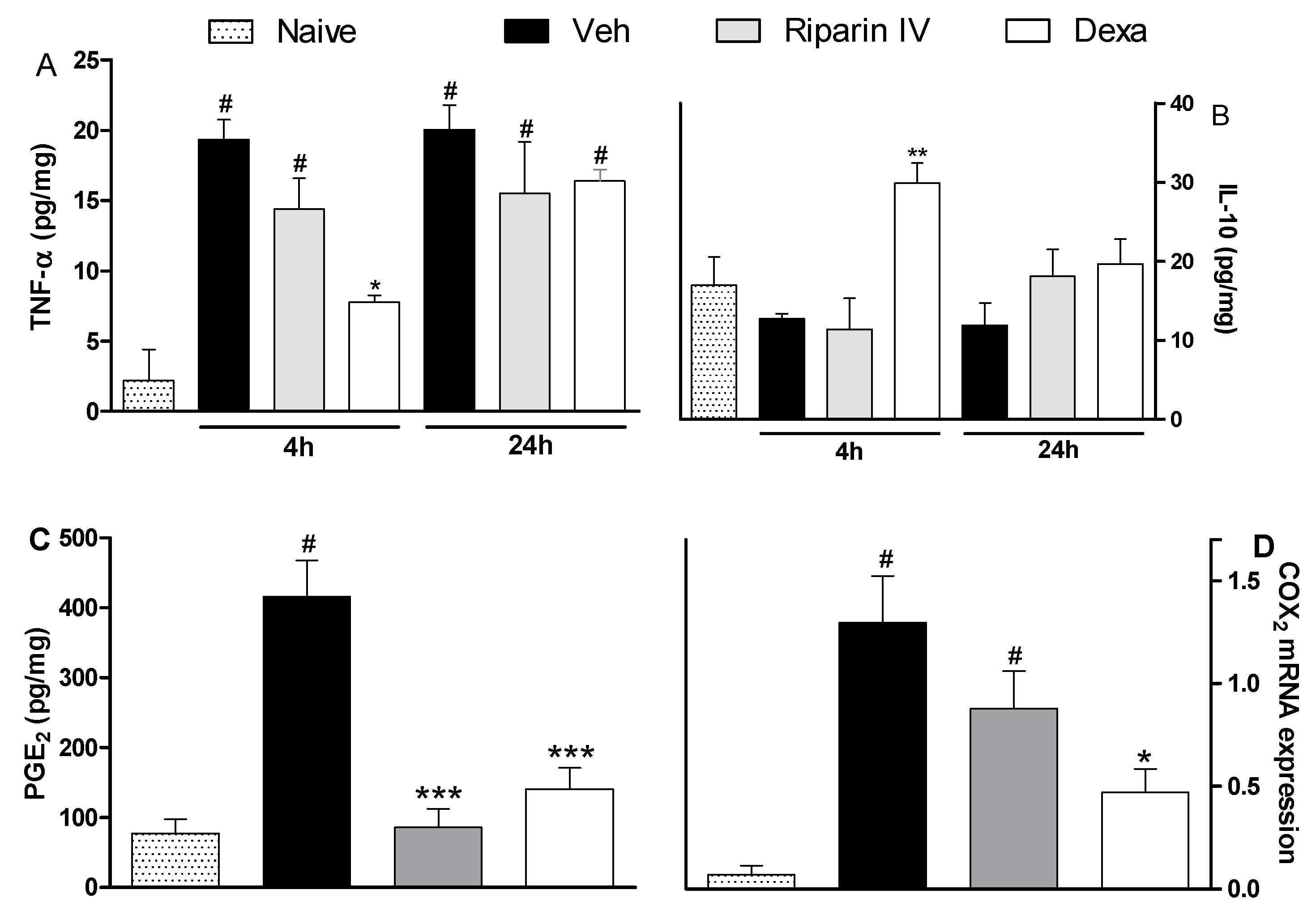
2. Results and Discussion
3. Materials and Methods
3.1. Animals
3.2. Compounds, Dilutions and Administration
3.3. Formalin Test
3.4. Tail Flick Test
3.5. Motor Function Assay
3.6. Inflammatory Model
3.7. Inflammatory Hyperalgesia Evaluation
3.8. Plesthismometer Test
3.9. Cytokine Measurement by ELISA
3.10. Measurement of PGE2 in Paw Skin
3.11. Real-Time PCR
3.12. Cytotoxicity to Mammalian Cells
3.13. Assessment of Cytokine and Nitric Oxide Production by Macrophages
3.14. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Khatami, M. Chronic Inflammation: Synergistic Interactions of Recruiting Macrophages (TAMs) and Eosinophils (Eos) with Host Mast Cells (MCs) and Tumorigenesis in CALTs. M-CSF, Suitable Biomarker for Cancer Diagnosis! Cancers (Basel) 2014, 6, 297–322. [Google Scholar] [CrossRef] [PubMed]
- Rainsford, K.D. Anti-inflammatory drugs in the 21st century. Subcell. Biochem. 2007, 42, 3–27. [Google Scholar] [PubMed]
- Schäcke, H.; Döcke, W.D.; Asadullah, K. Mechanisms involved in the side effects of glucocorticoids. Pharmacol. Ther. 2002, 96, 23–43. [Google Scholar]
- Hapgood, J.P.; Avenant, C.; Moliki, J.M. Glucocorticoid-independent modulation of GR activity: Implications for immunotherapy. Pharmacol. Ther. 2016, 165, 93–113. [Google Scholar] [CrossRef] [PubMed]
- Martínez-González, J.; Badimon, L. Mechanisms underlying the cardiovascular effects of COX-inhibition: benefits and risks. Curr. Pharm. Des. 2007, 13, 2215–2227. [Google Scholar] [CrossRef] [PubMed]
- Moore, N.; Pollack, C.; Butkerait, P. Adverse drug reactions and drug-drug interactions with over-the-counter NSAIDs. Ther. Clin. Risk Manag. 2015, 11, 1061–1075. [Google Scholar] [PubMed]
- Kang, S.Y.; Yoon, S.Y.; Roh, D.H.; Jeon, M.J.; Seo, H.S.; Uh, D.K.; Kwon, Y.B.; Kim, H.W.; Han, H.J.; Lee, H.J.; et al. The anti-arthritic effect of ursolic acid on zymosan-induced acute inflammation and adjuvant-induced chronic arthritis models. J. Pharm. Pharmacol. 2008, 60, 1347–1354. [Google Scholar] [CrossRef] [PubMed]
- Brustolim, D.; Vasconcelos, J.; Freitas, L.A.; Teixeira, M.M.; Farias, M.T.; Ribeiro, Y.M.; Tomassini, T.C.; Oliveira, G.G.; Pontes de Carvalho, L.C.; Ribeiro dos Santos, R.; et al. Activity of physalin F in a collagen-induced arthritis model. J. Nat. Prod. 2010, 73, 1323–1326. [Google Scholar] [CrossRef] [PubMed]
- Kingston, D.G. Modern natural products drug discovery and its relevance to biodiversity conservation. J. Nat. Prod. 2011, 74, 496–511. [Google Scholar] [CrossRef] [PubMed]
- Nonato, F.R.; Santana, D.G.; Melo, F.M.; Santos, G.G.L.; Brustolim, D.; Camargo, E.A.; Sousa, D.P.; Soares, M.B.P.; Villarreal, C.F. Anti-inflammatory properties of rose oxide. Int. Immunopharmacol. 2012, 14, 779–784. [Google Scholar] [CrossRef] [PubMed]
- Lima, M.S.; Quintans-Júnior, L.J.; Santana, W.A.; Martins Kaneto, C.; Pereira Soares, M.B.; Villarreal, C.F. Anti-inflammatory effects of carvacrol: Evidence for a key role of interleukin-10. Eur. J. Pharmacol. 2013, 699, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef] [PubMed]
- Atanasov, A.G.; Waltenberger, B.; Pferschy-Wenzig, E.M.; Linder, T.; Wawrosch, C.; Uhrin, P.; Temml, V.; Wang, L.; Schwaiger, S.; Heiss, E.H.; et al. Discovery and resupply of pharmacologically active plant-derived natural products: A review. Biotechnol. Adv. 2015, 33, 1582–1614. [Google Scholar] [CrossRef] [PubMed]
- Barbosa-Filho, J.M.; Yoshida, M.; Gottlieb, O.R.; Barbosa, R.C.S.B.C.; Giesbrecht, A.M.; Young, C.M. Benzoyl esters and amides, styrylpyrones and neolignans from the fruits of Aniba riparia. Phytochemistry 1987, 26, 2615–2617. [Google Scholar] [CrossRef]
- Sousa, F.C.F.; Melo, C.T.V.; Monteiro, A.P.; Lima, V.T.M.; Gutierrez, S.J.C.; Pereira, B.A.; Barbosa-Filho, J.M.; Vasconcelos, S.M M.; Fonteles, M.F.; Viana, G.S.B. Antianxiety and antidepressant effects of riparin III from Aniba riparia (Nees) Mez (Lauraceae) in mice. Pharmacol. Biochem. Behav. 2004, 78, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Araújo, F.L.O.; Melo, C.T.V.; Rocha, N.F.M.; Moura, B.A.; Leite, C.P.; Amaral, J.F.; Barbosa-Filho, J.M.; Gutierrez, S.J.C.; Vasconcelos, S.M.M.; Viana, G.S.B.; et al. Antinociceptive effects of (O-methyl)-N-benzoyl tyramine (riparin I) from Aniba riparia (Nees) Mez (Lauraceae) in mice. Naunyn-Schmied Arch. Pharmacol. 2009, 380, 337–344. [Google Scholar]
- Carvalho, A.M.R.; Rocha, N.F.M.; Vasconcelos, L.F.; Rios, E.R.V.; Dias, M.L.; Silva, M.I.G.; Fonteles, M.M.F.; Barbosa-Filho, J.M.; Gutierrez, S.J.C.; Sousa, F.C.F. Evaluation of the anti-inflammatory activity of riparin II (O-methil-N-2-hidroxi-benzoyl tyramine) in animal models. Chem. Biol. Interact. 2013, 205, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.O.; Damasceno, S.R.; Silva, I.S.; Silva, V.G.; Brito, C.F.; Teixeira, A.E.; Nunes, G.B.; Camara, C.A.; Filho, J.M.; Gutierrez, S.J.; et al. Riparin A, a compound from Aniba riparia, attenuate the inflammatory response by modulation of neutrophil migration. Chem. Biol. Interact. 2015, 229, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Barbosa-Filho, J.M.; Silva, E.C.; Bhattacharyya, J. Synthesis of several new phenylethylamides of substituted benzoic acids. Quim. Nova 1990, 13, 332–334. [Google Scholar]
- Hunskaar, S.; Hole, K. The formalin test in mice: Dissociation between inflammatory and non-inflammatory pain. Pain 1987, 30, 103–114. [Google Scholar] [CrossRef]
- Shibata, M.; Ohkubo, T.; Takahaski, H.; Inoki, R. Modified formalin test: characteristic biphasic pain response. Pain 1989, 38, 347–352. [Google Scholar] [CrossRef]
- Hughes, J.P.; Rees, S.; Kalindjian, S.B.; Philpott, K.L. Principles of early drug discovery. Br. J. Pharmacol. 2011, 162, 1239–1249. [Google Scholar] [CrossRef] [PubMed]
- Jensen, T.S.; Yaksh, T.L. Comparison of antinociceptive action of morphine in the periaqueductal gray, medial and paramedical medulla in rat. Brain Res. 1986, 363, 99–113. [Google Scholar] [CrossRef]
- Le Bars, D.; Gozariu, M.; Cadden, S.W. Animal models of nociception. Pharmacol. Rev. 2001, 53, 597–652. [Google Scholar] [PubMed]
- Woolf, C.J.; Allchorne, A.; Safieh-Garabedian, B.; Poole, S. Cytokines, nerve growth factor and inflammatory hyperalgesia: The contribution of tumour necrosis factor alpha. Br. J. Pharmacol. 1997, 121, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Dinarello, C.A. Proinflammatory cytokines. Chest 2000, 118, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Cunha, F.Q.; Ferreira, S.H. Peripheral hyperalgesic cytokines. Adv. Exp. Med. Biol. 2003, 521, 22–39. [Google Scholar] [PubMed]
- Conti, B.; Tabarean, I.; Andrei, C.; Bartfai, T. Cytokines and fever. Front. Biosci. 2004, 9, 1433–1449. [Google Scholar] [CrossRef] [PubMed]
- Fehrenbacher, J.C.; Vasko, M.R.; Duarte, D.B. Models of inflammation: Carrageenan- or complete Freund’s Adjuvant (CFA)-induced edema and hypersensitivity in the rat. Curr. Protoc. Pharmacol. 2012. Chapter 5, Unit 5.4. [Google Scholar] [CrossRef] [Green Version]
- Verri-Jr, W.A.; Cunha, T.M.; Parada, C.A.; Poole, S.; Cunha, F.Q.; Ferreira, S.H. Hypernociceptive role of cytokines and chemokines: Targets for analgesic drug development? Pharmacol. Ther. 2006, 112, 116–138. [Google Scholar] [CrossRef] [PubMed]
- Vane, J.R.; Bakhle, Y.S.; Botting, R.M. Cyclooxygenases 1 and 2. Annu. Rev. Pharmacol. Toxicol. 1998, 38, 97–120. [Google Scholar] [CrossRef] [PubMed]
- McCleskey, E.W.; Gold, M.S. Ion channels of nociception. Annu. Rev. Physiol. 1999, 61, 835–856. [Google Scholar] [CrossRef] [PubMed]
- Basbaum, A.I. Distinct neurochemical features of acute and persistent pain. Proc. Natl. Acad. Sci. USA 1999, 96, 7739–7743. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, N.; Kobayashi, K. Macrophages in inflammation. Curr. Drug Targets Inflamm. Allergy 2005, 4, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Coleman, J.W. Nitric oxide in immunity and inflammation. Int. Immunopharmacol. 2001, 1, 1397–1406. [Google Scholar] [CrossRef]
- Korhonen, R.; Lahti, A.; Kankaanranta, H.; Moilanen, E. Nitric oxide production and signaling in inflammation. Curr. Drug Targets Inflamm. Allergy 2005, 4, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Giroux, M.; Descoteaux, A. Cyclooxygenase-2 expression in macrophages: Modulation by protein kinase C-alpha. J. Immunol. 2000, 165, 3985–3991. [Google Scholar] [CrossRef] [PubMed]
- Dubuisson, D.; Dennis, S.G. The formalin test: A quantitative study of the analgesic effects of morphine, meperidine, and brain stem stimulation in rats and cats. Pain 1977, 4, 161–174. [Google Scholar] [CrossRef]
- Gama, K.B.; Quintans, J.S.; Antoniolli, A.R.; Quintans, L.J., Jr.; Santana, W.A.; Branco, A.; Soares, M.B.; Villarreal, C.F. Evidence for the involvement of descending pain-inhibitory mechanisms in the antinociceptive effect of hecogenin acetate. J. Nat. Prod. 2013, 76, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Chaplan, S.R.; Bach, F.W.; Pogrel, J.W.; Chung, J.M.; Yaksh, T.L. Quantitative assessment of tactile allodynia in the rat paw. J. Neurosci. Meth. 1994, 53, 55–63. [Google Scholar] [CrossRef]
- Cunha, T.M.; Talbot, J.; Pinto, L.G.; Vieira, S.M.; Souza, G.R.; Guerrero, A.T.; Sonego, F.; Verri, W.A., Jr.; Zamboni, D.S.; Ferreira, S.H.; et al. Caspase-1 is involved in the genesis of inflammatory hypernociception by contributing to peripheral IL-1β maturation. Mol. Pain 2010, 6, 63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Green, L.C.; Wagne, D.A.; Glogowski, J.; Skipper, P.L.; Wishnok, J.S.; Tannenbaum, S.R. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal. Biochem. 1982, 126, 131–138. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds riparins I, II, III and IV are available from the authors.


© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nascimento, O.A.; Espírito-Santo, R.F.d.; Opretzka, L.C.F.; Barbosa-Filho, J.M.; Gutierrez, S.J.C.; Villarreal, C.F.; Soares, M.B.P. Pharmacological Properties of Riparin IV in Models of Pain and Inflammation. Molecules 2016, 21, 1757. https://doi.org/10.3390/molecules21121757
Nascimento OA, Espírito-Santo RFd, Opretzka LCF, Barbosa-Filho JM, Gutierrez SJC, Villarreal CF, Soares MBP. Pharmacological Properties of Riparin IV in Models of Pain and Inflammation. Molecules. 2016; 21(12):1757. https://doi.org/10.3390/molecules21121757
Chicago/Turabian StyleNascimento, Olívia Azevêdo, Renan Fernandes do Espírito-Santo, Luíza Carolina França Opretzka, José Maria Barbosa-Filho, Stanley Juan Chavez Gutierrez, Cristiane Flora Villarreal, and Milena Botelho Pereira Soares. 2016. "Pharmacological Properties of Riparin IV in Models of Pain and Inflammation" Molecules 21, no. 12: 1757. https://doi.org/10.3390/molecules21121757
APA StyleNascimento, O. A., Espírito-Santo, R. F. d., Opretzka, L. C. F., Barbosa-Filho, J. M., Gutierrez, S. J. C., Villarreal, C. F., & Soares, M. B. P. (2016). Pharmacological Properties of Riparin IV in Models of Pain and Inflammation. Molecules, 21(12), 1757. https://doi.org/10.3390/molecules21121757