Decomposition of Intermolecular Interactions in the Crystal Structure of Some Diacetyl Platinum(II) Complexes: Combined Hirshfeld, AIM, and NBO Analyses
Abstract
:1. Introduction
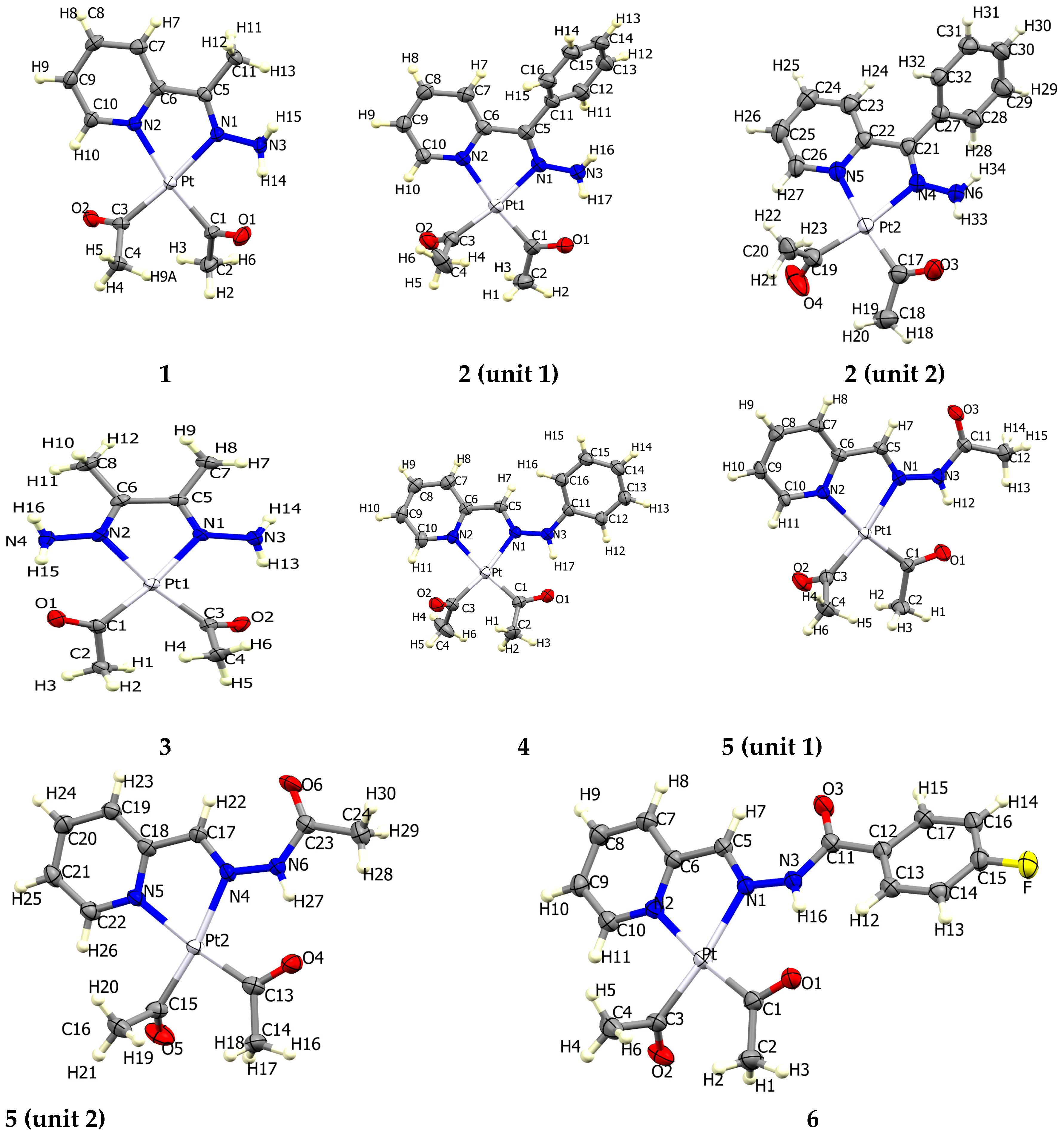
2. Results and Discussion
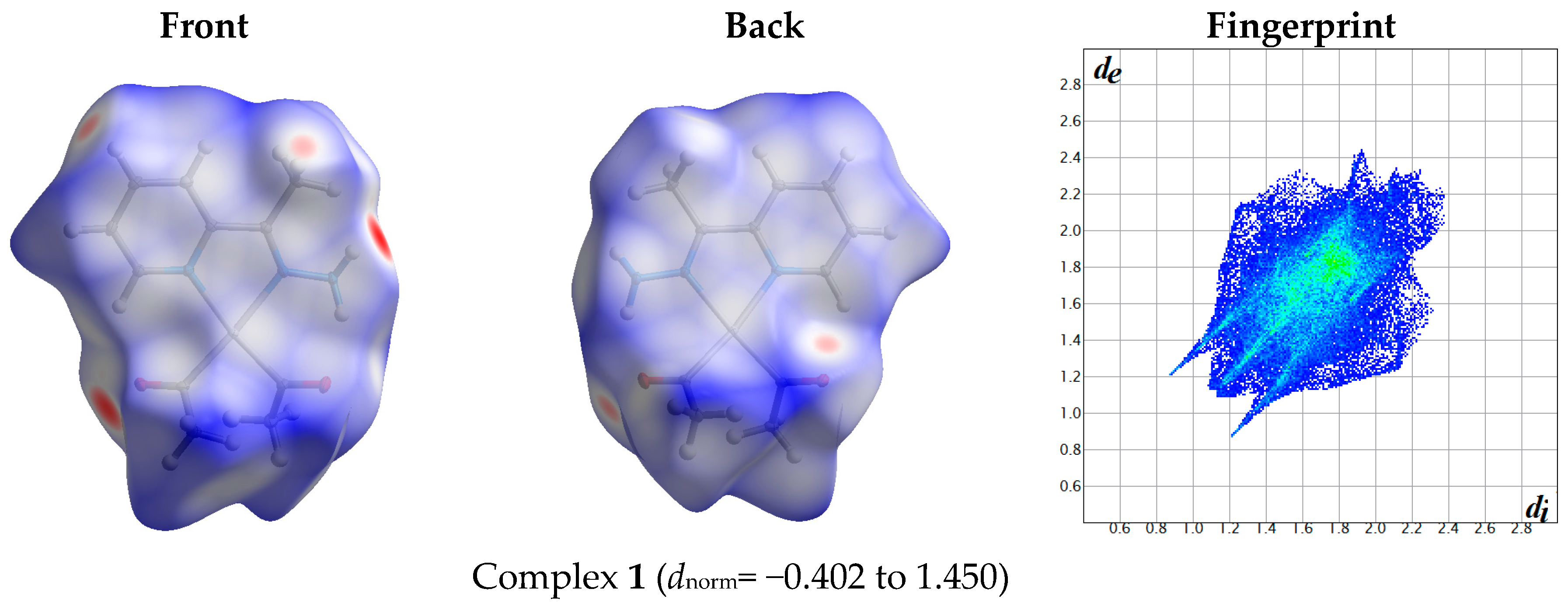
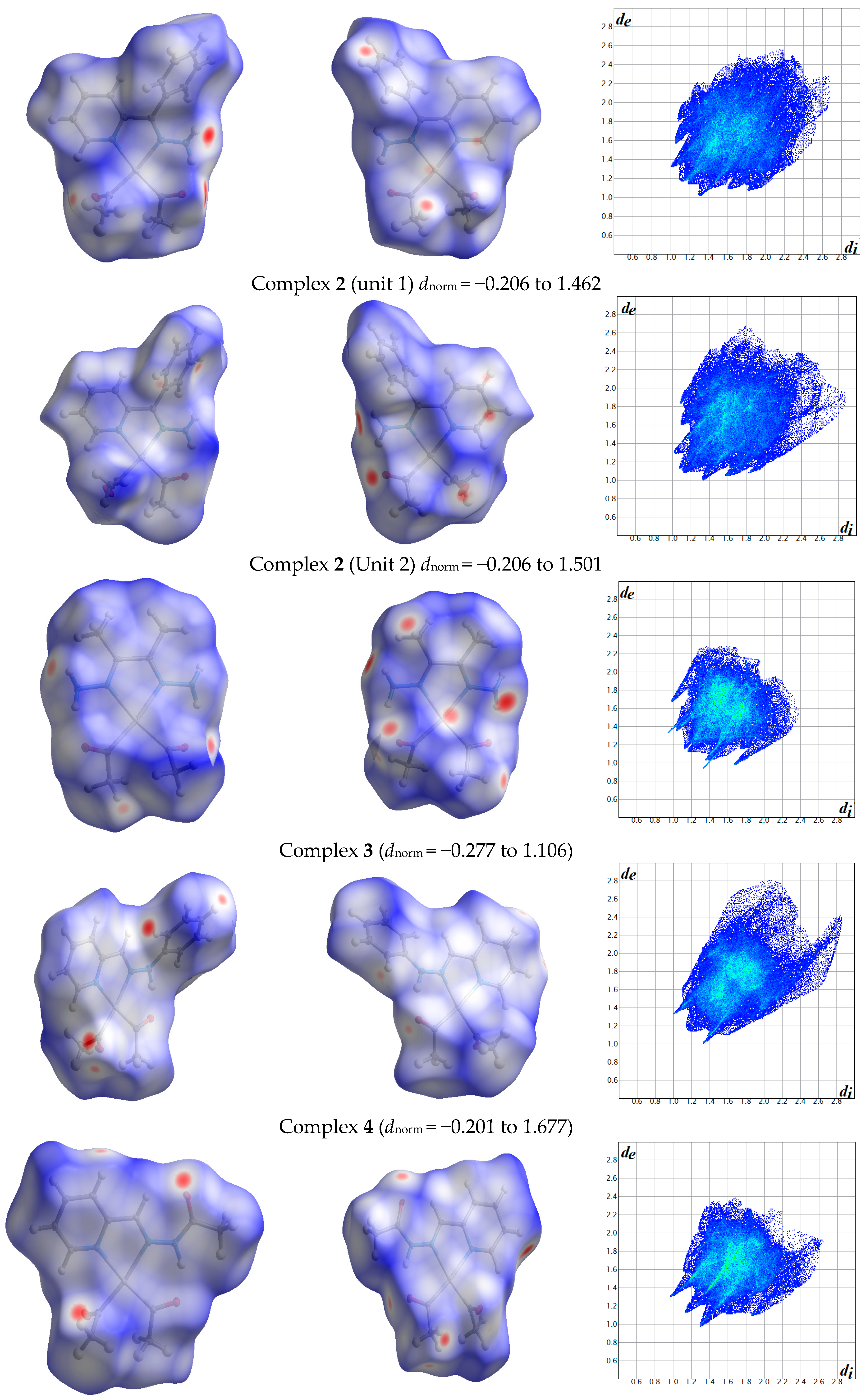
2.1. Hirshfeld Analysis
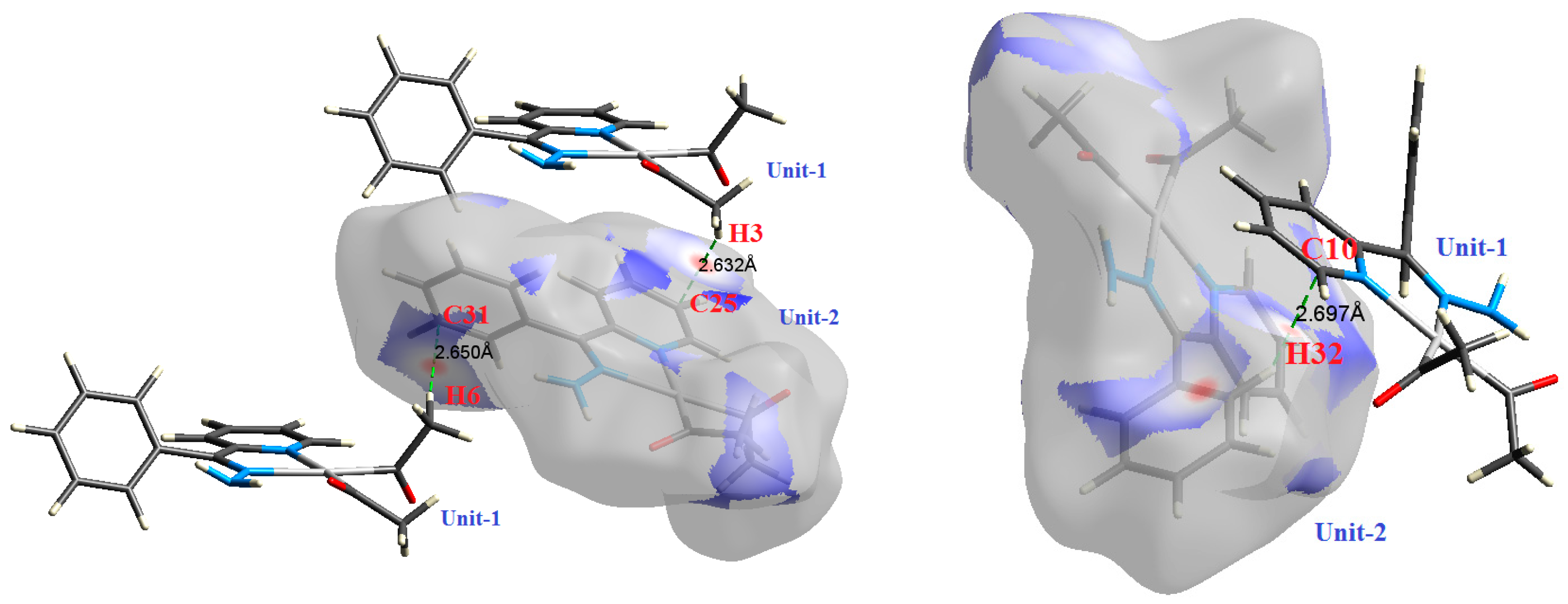
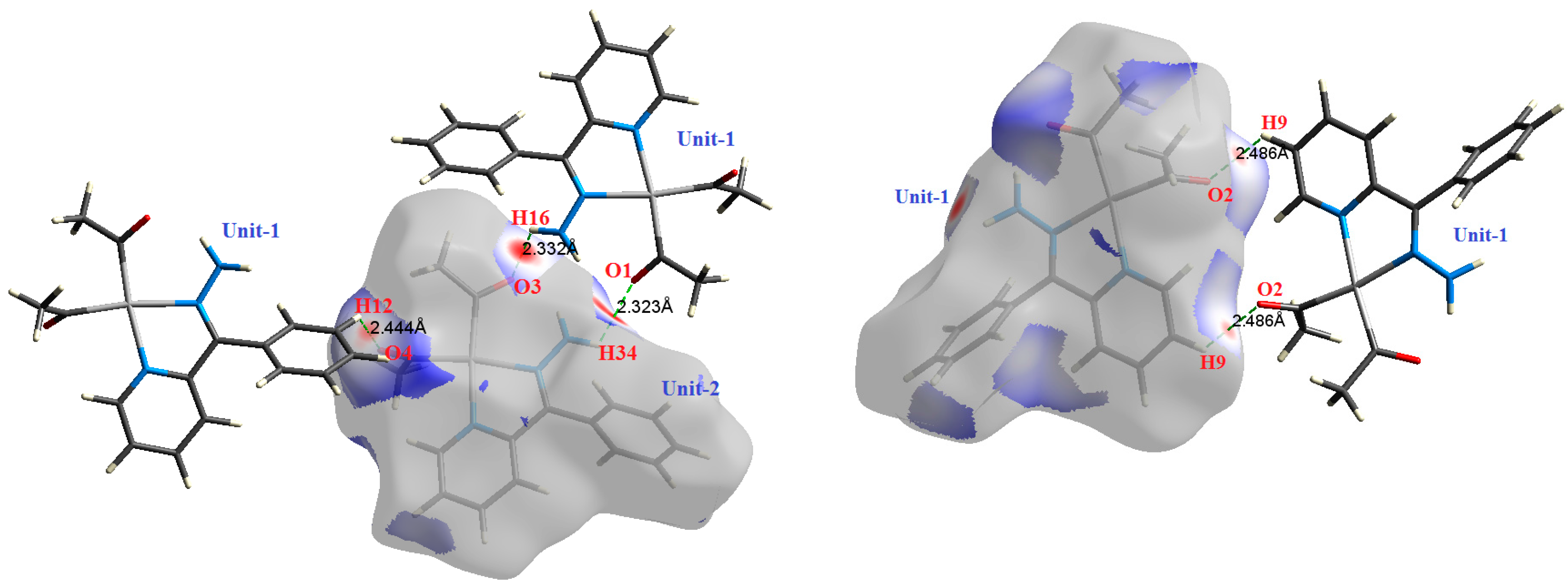
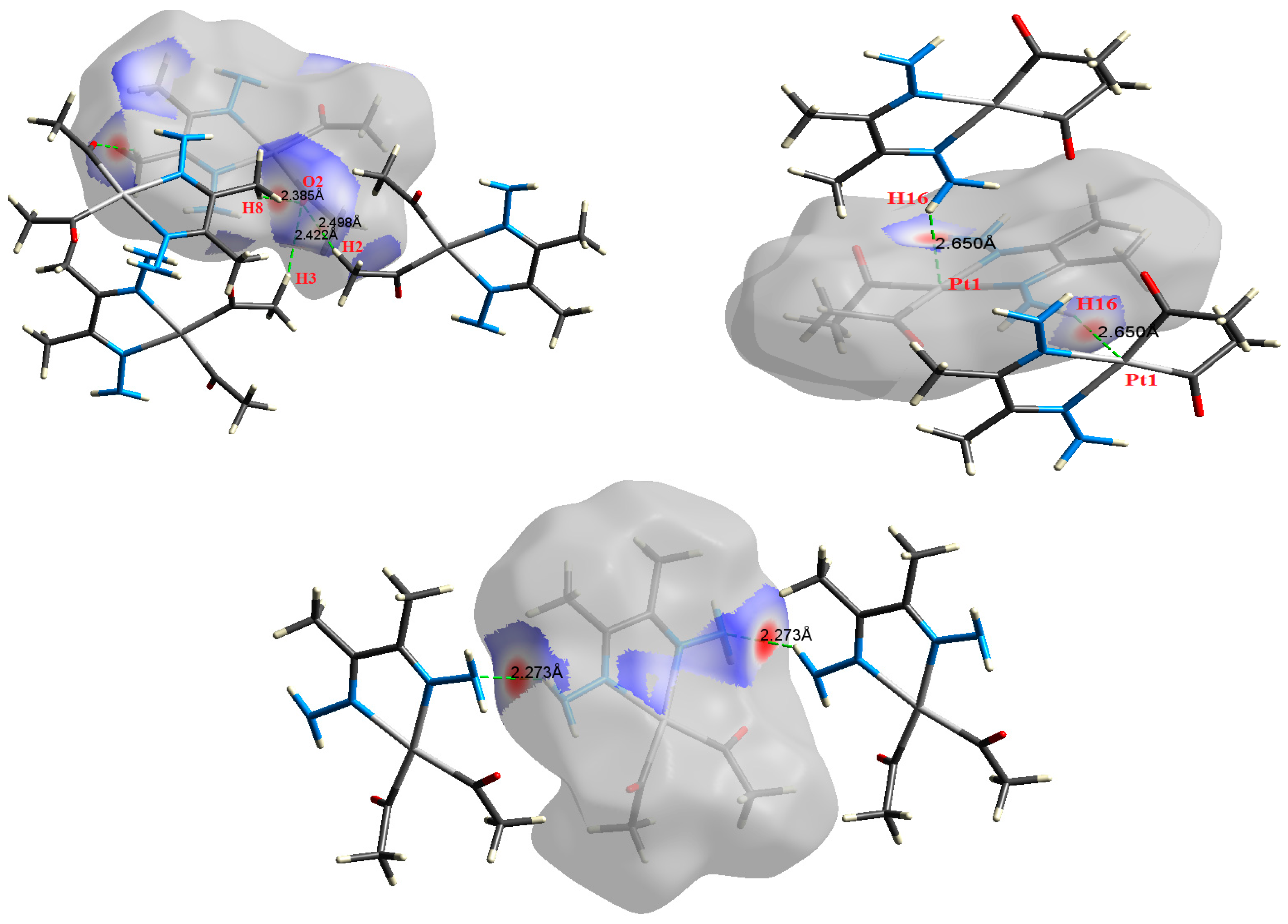
2.2. AIM Study
2.3. NBO Charges
3. Computational Details
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Desiraju, G.R.; Steiner, T. The Weak Hydrogen Bond in Structural Chemistry and Biology; Oxford University Press: Oxford, UK, 1999. [Google Scholar]
- Bader, R.F.W. Atoms in Molecules: A Quantum Theory, 2nd ed.; Oxford University Press: Oxford, UK, 1994. [Google Scholar]
- Bader, R.F.W. A bond path: A universal indicator of bonded interactions. J. Phys. Chem. A 1998, 102, 7314–7323. [Google Scholar] [CrossRef]
- Parthasarathi, R.; Subramanian, V.; Sathyamurthy, N. Hydrogen bonding without borders: An atoms-in-molecules perspective. J. Phys. Chem. A 2006, 110, 3349–3351. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, B.; Vancamp, L.; Trosko, J.E.; Mansour, V.H. Platinum compounds: A new class of potent antitumour agents. Nature 1969, 222, 385–386. [Google Scholar] [CrossRef] [PubMed]
- O’Dwyer, P.J.; Stevenson, J.P.; Johnson, S.W. Cisplatin. Chemistry and Biochemistry of a Leading Anticancer Drug; Lippert, B., Ed.; Wiley-VCH: Weinheim, Germany, 1999; pp. 31–69. [Google Scholar]
- Watson, M.; Barret, A.; Spence, R.; Twelves, C. Oncology, 2nd ed.; Oxford University Press: Oxford, UK, 2006. [Google Scholar]
- Wheate, N.J.; Walker, S.; Craig, G.E.; Oun, R. The status of platinum anticancer drugs in the clinic and in clinical trials. Dalton Trans. 2010, 39, 8113–8127. [Google Scholar] [CrossRef] [PubMed]
- Dhar, S.; Lippard, S.J. Bioinorganic Medicinal Chemistry; Alessio, E., Ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2011; pp. 79–95. [Google Scholar]
- Wang, X.; Guo, Z. Bioinorganic Medicinal Chemistry; Alonso, E., Ed.; Wiley-VCH: Weinheim, Germany, 2011; pp. 97–149. [Google Scholar]
- Štarha, P.; Trávníček, Z.; Popa, A.; Popa, I.; Muchová, T.; Brabec, V. How to modify 7-azaindole to form cytotoxic Pt(II) complexes: Highly in vitro anticancer effective cisplatin derivatives involving halogen-substituted 7-azaindole. J. Inorg. Biochem. 2012, 115, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Ferri, N.; Cazzaniga, S.; Mazzarella, L.; Curigliano, G.; Lucchini, G.; Zerla, D.; Gandolfi, R.; Facchetti, G.; Pellizzoni, M.; Rimoldi, I. Cytotoxic effect of (1-methyl-1H-imidazol-2-yl)-methanamine and its derivatives in PtII complexes on human carcinoma cell lines: A comparative study with cisplatin. Bioorg. Med. Chem. 2013, 21, 2379–2386. [Google Scholar] [CrossRef] [PubMed]
- Icsel, C.; Yilmaz, V.T.; Ari, F.; Ulukaya, E.; Harrison, W.T.A. Trans-Dichloridopalladium(II) and platinum(II) complexes with 2-(hydroxymethyl)pyridine and 2-(2-hydroxyethyl)pyridine: Synthesis, structural characterization, DNA binding and in vitro cytotoxicity studies. Eur. J. Med. Chem. 2013, 60, 386–394. [Google Scholar] [CrossRef] [PubMed]
- Spackman, M.A.; McKinnon, J.J. Fingerprinting intermolecular interactions in molecular crystals. CrystEngComm 2002, 4, 378–392. [Google Scholar] [CrossRef]
- McKinnon, J.J.; Jayatilaka, D.; Spackman, M.A. Towards quantitative analysis of intermolecular interactions with Hirshfeld surfaces. Chem. Commun. 2007, 3814–3816. [Google Scholar] [CrossRef]
- Spackman, M.A.; McKinnon, J.J.; Jayatilaka, D. Electrostatic potentials mapped on Hirshfeld surfaces provide direct insight into intermolecular interactions in crystals. CrystEngComm 2008, 10, 377–388. [Google Scholar] [CrossRef]
- Spackman, M.A.; Jayatilaka, D. Hirshfeld surface analysis. CrystEngComm 2009, 11, 19–32. [Google Scholar] [CrossRef]
- Hirshfeld, F.L. Bonded-atom fragments for describing molecular charge densities. Theor. Chim. Acta 1977, 44, 129–133. [Google Scholar] [CrossRef]
- Martin, A.D.; Hartlieb, K.J.; Sobolev, A.N.; Raston, C.L. Hirshfeld surface analysis of substituted phenols. Cryst. Growth Des. 2010, 10, 5302–5306. [Google Scholar] [CrossRef]
- Chattopadhyay, B.; Mukherjee, A.K.; Narendra, N.; Hemantha, H.P.; Sureshbabu, V.V.; Helliwell, M.; Mukherjee, M. Supramolecular architectures in 5,5′-substituted hydantoins: Crystal structures and Hirshfeld surface analyses. Cryst. Growth Des. 2010, 10, 4476–4484. [Google Scholar] [CrossRef]
- Seth, S.K.; Sarkar, D.; Kar, T. Use of π–π forces to steer the assembly of chromone derivatives into hydrogen-bonded supramolecular layers: Crystal structures and Hirshfeld surface analyses. CrystEngComm 2011, 13, 4528–4535. [Google Scholar] [CrossRef]
- Seth, S.K.; Saha, I.; Estarellas, C.; Frontera, A.; Kar, T.; Mukhopadhyay, S. Supramolecular self-assembly of M-IDA complexes involving lone-pair···π interactions: Crystal structures, Hirshfeld surface analysis, and DFT calculations [H2IDA = iminodiacetic acid, M = Cu(II), Ni(II)]. Cryst. Growth Des. 2011, 11, 3250–3265. [Google Scholar] [CrossRef]
- McKi, J.J.; Spackman, M.A.; Mitchell, A.S. Novel tools for visualizing and exploring intermolecular interactions in molecular crystals. Acta Crystallogr. B 2004, 60, 627–668. [Google Scholar]
- Rohl, A.L.; Moret, M.; Kaminsky, W.; Claborn, K.; Mckinnon, J.J.; Kahr, B. Hirshfeld surfaces identify inadequacies in computations of intermolecular interactions in crystals: Pentamorphic 1,8-dihydroxyanthraquinone. Cryst. Growth Des. 2008, 8, 4517–4525. [Google Scholar] [CrossRef]
- Parkin, A.; Barr, G.; Dong, W.; Gilmore, C.J.; Jayatilaka, D.; Mckinnon, J.J.; Spackman, M.A.; Wilson, C.C. Comparing entire crystal structures: Structural genetic fingerprinting. CrystEngComm 2007, 9, 648–652. [Google Scholar] [CrossRef]
- Fabbiani, F.P.A.; Byrne, L.T.; Mckinnon, J.J.; Spackman, M.A. Solvent inclusion in the structural voids of form II carbamazepine: Single-crystal X-ray diffraction, NMR spectroscopy, and Hirshfeld surface analysis. CrystEngComm 2007, 9, 728–731. [Google Scholar] [CrossRef]
- Spackman, M.A.; Byrom, P.G. A novel definition of a molecule in a crystal. Chem. Phys. Lett. 1997, 267, 215–220. [Google Scholar] [CrossRef]
- McKinnon, J.J.; Mitchell, A.S.; Spackman, M.A. Hirshfeld surfaces: A new tool for visualising and exploring molecular crystals. Chem. Eur. J. 1998, 4, 2136–2141. [Google Scholar] [CrossRef]
- Bernstein, J.; Davis, R.E.; Shimoni, L.; Chang, N.L. Patterns in hydrogen bonding: Functionality and graph set analysis in crystals. Angew. Chem. Int. Ed. 1995, 34, 1555–1573. [Google Scholar] [CrossRef]
- Grabowsky, S.; Dean, P.M.; Skelton, B.W.; Sobolev, A.N.; Spackman, M.A.; White, A.H. Crystal packing in the 2-R,4-oxo-[1,3-a/b]-naphthodioxanes—Hirshfeld surface analysis and melting point correlation. CrystEngComm 2012, 14, 1083–1093. [Google Scholar] [CrossRef]
- Carrol, M.T.; Chang, C.; Bader, R.F.W. Prediction of the structures of hydrogen-bonded complexes using the Laplacian of the charge density. Mol. Phys. 1988, 63, 387–405. [Google Scholar] [CrossRef]
- Carroll, M.T.; Chang, C.; Bader, R.F.W. An analysis of the hydrogen bond in BASE-HF complexes using the theory of atoms in molecules. Mol. Phys. 1998, 65, 695–722. [Google Scholar] [CrossRef]
- Koch, U.; Popelier, P. Characterization of C-H···O hydrogen bonds on the basis of the charge density. J. Chem. Phys. 1995, 99, 9747–9754. [Google Scholar] [CrossRef]
- Espinosa, E.; Molins, D.E.; Lecomte, C. Hydrogen bond strengths revealed by topological analyses of experimentally observed electron densities. Chem. Phys. Lett. 1998, 285, 170–173. [Google Scholar] [CrossRef]
- Bader, R.F.W.; Essen, H. The characterization of atomic interactions. J. Chem. Phys. 1984, 80, 1943–1960. [Google Scholar] [CrossRef]
- Popelier, P.L.A. Atoms in Molecules: An Introduction; Pearson Education Limited: Harlow, UK, 2000. [Google Scholar]
- Parthasarathi, R.; Subramanian, V.; Sathyamurthy, N. Hydrogen bonding in phenol, water, and phenol−water clusters. J. Phys. Chem. A 2005, 109, 843–850. [Google Scholar] [CrossRef] [PubMed]
- Rozas, I.; Alkorta, I.; Elguero, J. Behavior of ylides containing N, O, and C atoms as hydrogen bond acceptors. J. Am. Chem. Soc. 2000, 122, 11154–11161. [Google Scholar] [CrossRef]
- Espinosa, E.; Alkorta, I.; Elguero, J.; Molins, E. From weak to strong interactions: A comprehensive analysis of the topological and energetic properties of the electron density distribution involving X–H···F–Y system. J. Chem. Phys. 2002, 117, 5529–5542. [Google Scholar] [CrossRef]
- Wolff, S.K.; Grimwood, D.J.; McKinnon, J.J.; Turner, M.J.; Jayatilaka, D.; Spackman, M.A. Crystal Explorer, version 3.1; University of Western Australia: Crawley, Australia, 2012. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Montgomery, J.A.; Vreven, J.T.; Kudin, K.N.; Burant, J.C.; et al. Gaussian 03; revision C01; Gaussian Inc.: Wallingford, CT, USA, 2004. [Google Scholar]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, J.E.; Weinhold, F. Analysis of the geometry of the hydroxymethyl radical by the different hybrids for different spins natural bond orbital procedure. J. Mol. Struct. (Theochem) 1988, 169, 41–62. [Google Scholar] [CrossRef]
- Foster, J.P.; Weinhold, F. Natural hybrid orbitals. J. Am. Chem. Soc. 1980, 102, 7211–7218. [Google Scholar] [CrossRef]
- Reed, A.E.; Weinhold, F. Natural bond orbital analysis of near-Hartree-Fock water dimer. J. Chem. Phys. 1983, 78, 4066–4073. [Google Scholar] [CrossRef]
- Reed, A.E.; Weinhold, F. Natural localized molecular orbitals. J. Chem. Phys. 1983, 83, 1736–1740. [Google Scholar] [CrossRef]
- Reed, A.E.; Weinstock, R.B.; Weinhold, F. Natural population analysis. J. Chem. Phys. 1985, 83, 735–746. [Google Scholar] [CrossRef]
- Reed, A.E.; Curtiss, L.A.; Weinhold, F. Intermolecular interactions from a natural bond orbital, donor-acceptor viewpoint. Chem. Rev. 1988, 88, 899–926. [Google Scholar] [CrossRef]
- Weinhold, F.; Carpenter, J.E. The natural bond orbital Lewis structure concept for molecules, radicals, and radical ions. In The Structure of Small Molecules and Ions; Naaman, R., Vager, Z., Eds.; Plenum: New York, NY, USA, 1988; pp. 227–236. [Google Scholar]
- Kluge, T.; Bette, E.; Bette, M.; Schmidt, J.; Steinborn, D. Hydrazone-diacetyl platinum(II) complexes: Substituent effect on intramolecular N-H⋯O hydrogen-bond strength. J. Organomet. Chem. 2014, 762, 48–57. [Google Scholar] [CrossRef]
- McLean, A.D.; Chandler, G.S. Contracted Gaussian basis sets for molecular calculations. I. 2nd-row atoms, Z = 11–18. J. Chem. Phys. 1980, 72, 5639–5648. [Google Scholar] [CrossRef]
- Raghavachari, K.; Binkley, J.S.; Seeger, R.; Pople, J.A. Self-consistent molecular orbital methods. XX. Basis set for correlated wavefunctions. J. Chem. Phys. 1980, 72, 650–654. [Google Scholar]
- Dunning, T.H., Jr.; Hay, P.J. Modern Theoretical Chemistry; Schaefer, H.F., Ed.; Plenum: New York, NY, USA, 1977; Volume 3, pp. 1–28. [Google Scholar]
- Hay, P.J.; Wadt, W.R. Ab initio effective core potentials for molecular calculations—Potentials for the transition-metal atoms Sc to Hg. J. Chem. Phys. 1985, 82, 270–283. [Google Scholar] [CrossRef]
- Wadt, W.R.; Hay, P.J. Ab initio effective core potentials for molecular calculations—Potentials for main group elements Na to Bi. J. Chem. Phys. 1985, 82, 284–298. [Google Scholar] [CrossRef]
- Hay, P.J.; Wadt, W.R. Ab initio effective core potentials for molecular—Potentials for K to Au including the outermost core orbitals. J. Chem. Phys. 1985, 82, 299–310. [Google Scholar] [CrossRef]
- Sample Availability: Not available.
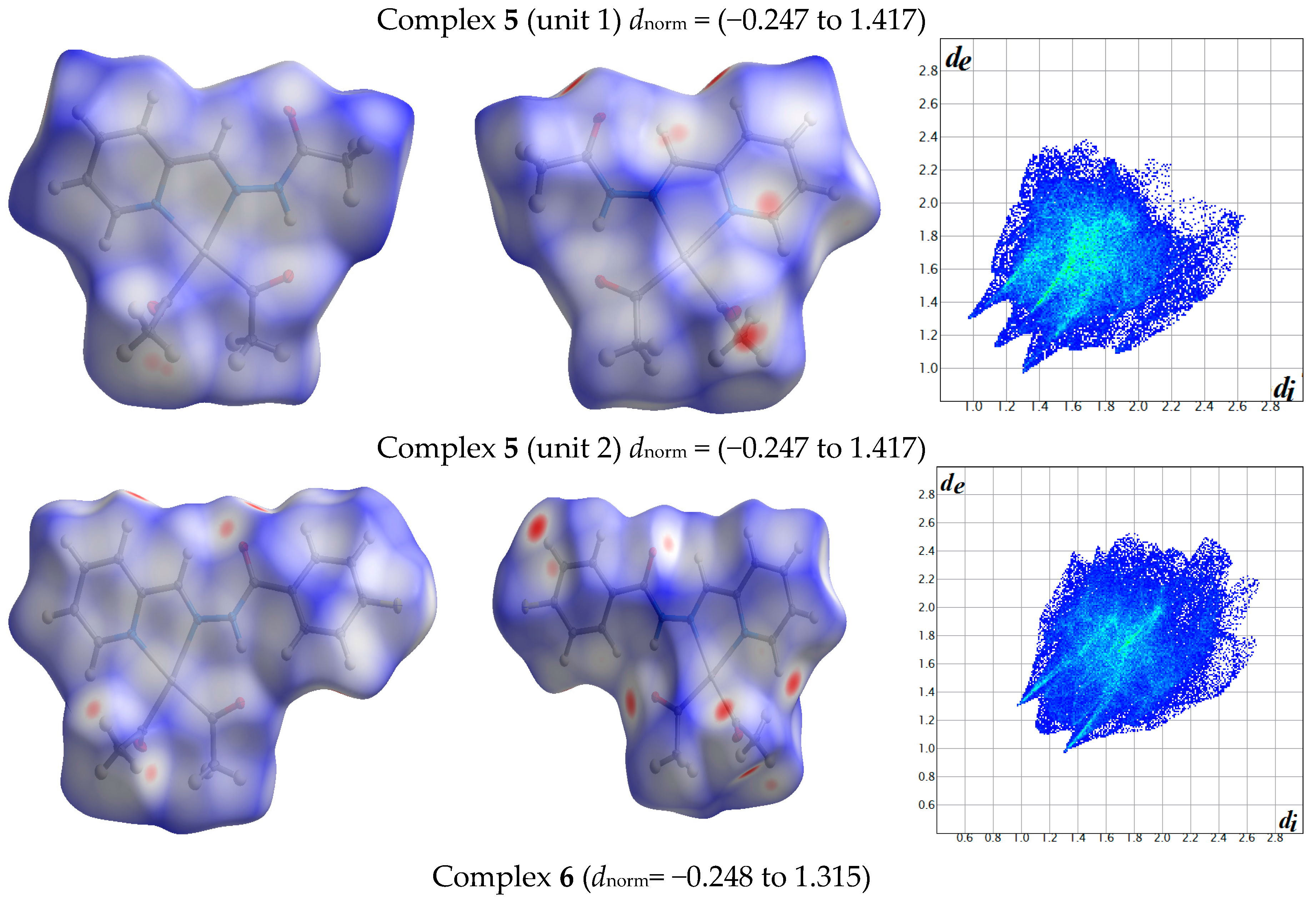

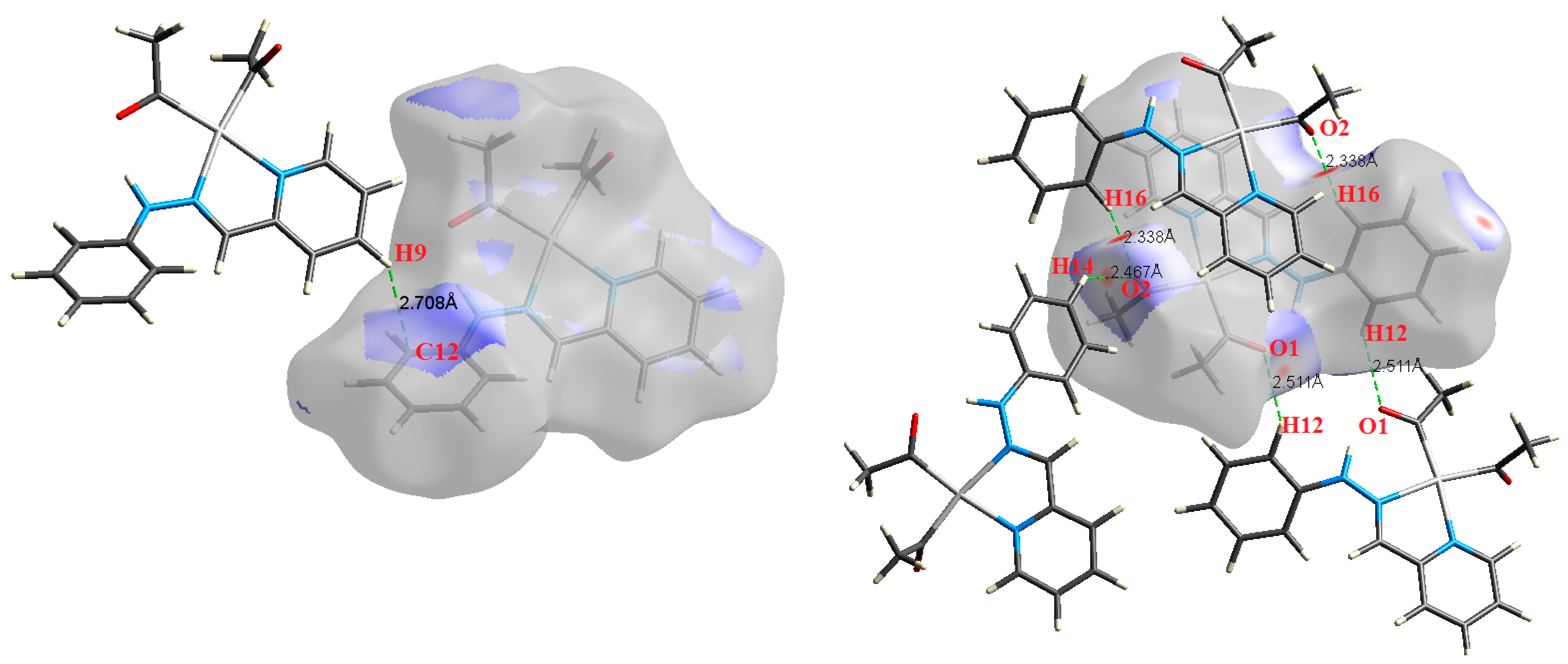



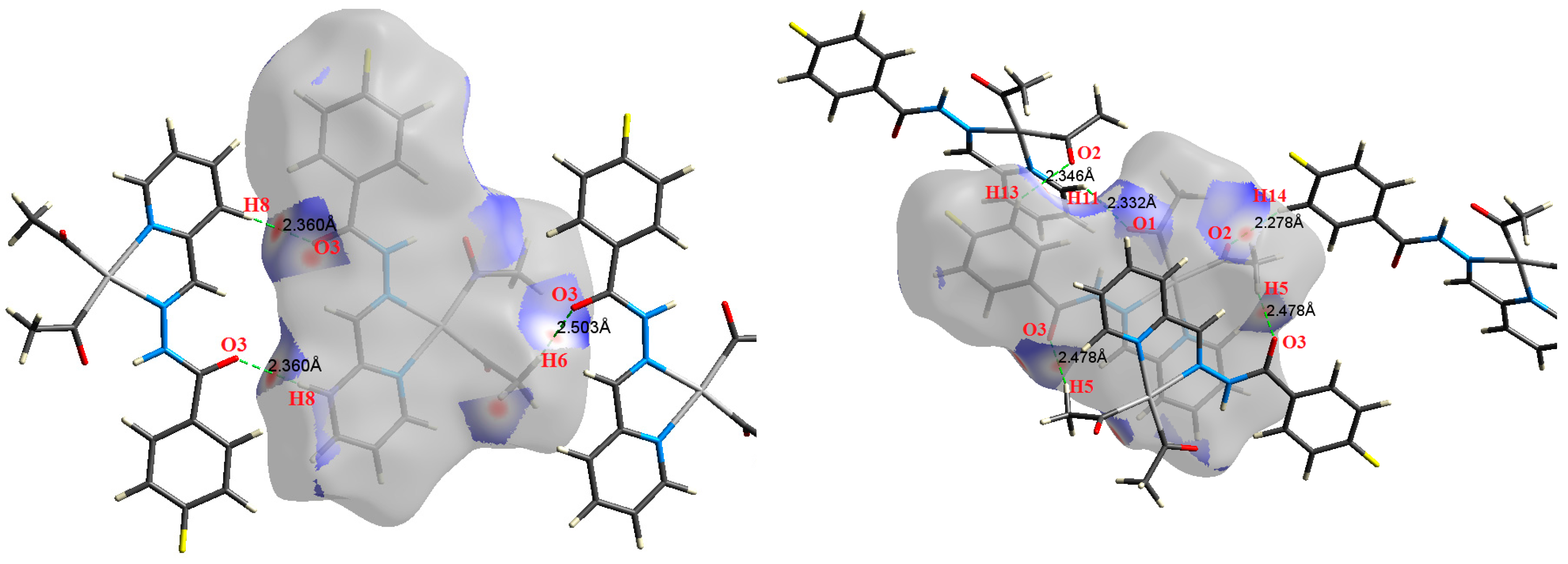
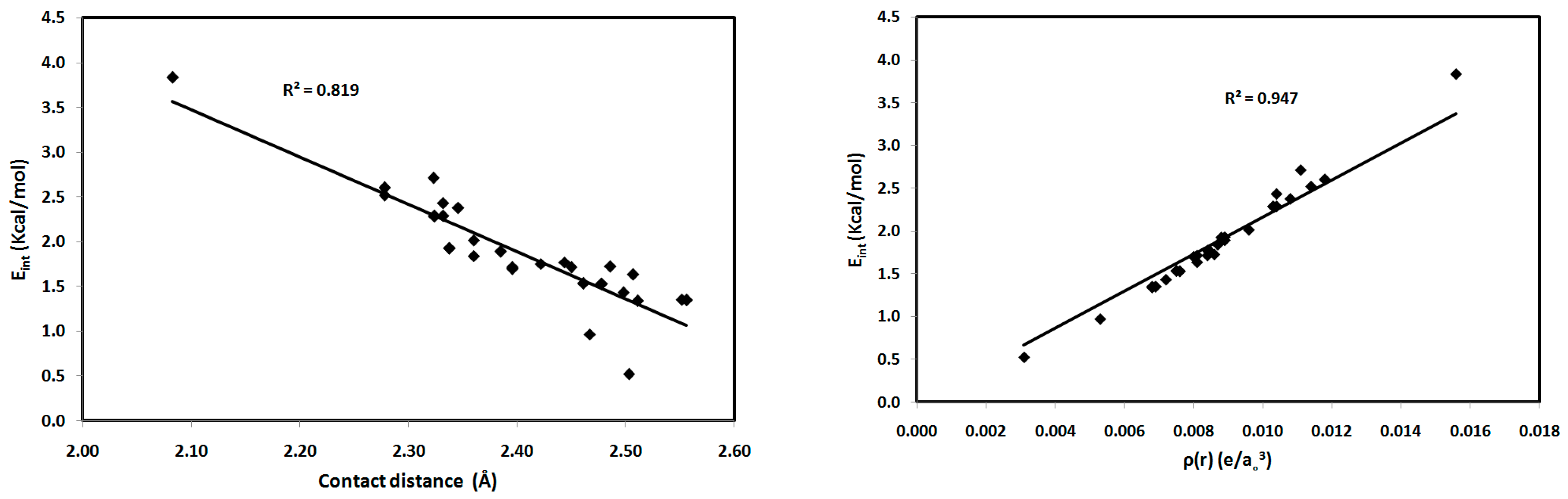


| Contact | 1 | 2 (1) | 2 (2) | 3 | 4 | 5 (1) | 5 (2) | 6 |
|---|---|---|---|---|---|---|---|---|
| C⋯H% | 5.0 (2.878) | 16.2 (2.632) | 18.2 (2.632) | 3.5 (2.876) | 18.3 (2.703) | 11.8 (2.699) | 7.5 (2.675) | 19.3 (2.669) |
| C⋯N% | 8.6 (3.392) | 1.2 (3.369) | 1.1 (3.369) | 2.3 (3.381) | 4.6 (3.383) | 3.1 (3.318) | 5.2 (3.487) | 3.9 (3.388) |
| C⋯C% | 1.6 (3.509) | 2.1 (3.523) | 1.8 (3.523) | 0.2 (3.646) | 1.1 (3.427) | 1.2 (3.315) | 2.4 (3.446) | 3.3 (3.375) |
| C⋯PT% | 2.2 (3.474) | 0.2 (3.356) | 0.2 (3.356) | 0.3 (3.629) | 2.8 (3.430) | 0.0 | 2.6 (3.682) | 0.9 (3.484) |
| H⋯H% | 57.0 (2.230) | 51.7 (2.206) | 52.4 (2.206) | 54.1 (2.288) | 53.8 (2.320) | 46.8 (2.017) | 48 (2.017) | 34.9 (2.250) |
| H⋯O% | 19.0 (2.083) | 18.0 (2.323) | 14.5 (2.323) | 21.2 (2.385) | 14.6 (2.338) | 27.3 (2.324) | 24.3 (2.324) | 19.8 (2.278) |
| H⋯N% | 3.5 (2.773) | 6.8 (2.643) | 7.7 (2.643) | 12.1 (2.273) | 0.4 (3.448) | 4.1 (2.908) | 2.2 (3.510) | 1.5 (3.328) |
| H⋯Pt% | 2.7 (3.361) | 3.1 (2.901) | 3.4 (2.901) | 5.3 (2.650) | 0.9 (3.329) | 3.0 (2.960) | 2.2 (2.960) | 2.4 (3.057) |
| F⋯H% | 9.5 (2.544) |
| Contact Type | D (Å) | Eint (kcal/mol) | ρ(r) (e/a03) | ∇2ρ(r) (e/a05) | V(r) (a.u.) | G(r) (a.u.) | H(r) (a.u.) | |V(r)|/G(r) |
|---|---|---|---|---|---|---|---|---|
| Complex 1 | ||||||||
| C9⋯N3 | 3.404 | 0.9230 | 0.0056 | 0.0161 | −0.0029 | 0.0035 | 0.0005 | 0.844 |
| C5⋯N2 | 3.392 | 0.8683 | 0.0050 | 0.0160 | −0.0028 | 0.0034 | 0.0006 | 0.817 |
| C5⋯N2 | 3.417 | 0.8424 | 0.0049 | 0.0156 | −0.0027 | 0.0033 | 0.0006 | 0.815 |
| C9⋯N3 | 3.407 | 0.8591 | 0.0052 | 0.0148 | −0.0027 | 0.0032 | 0.0005 | 0.850 |
| O1⋯H8 | 2.360 | 2.0118 | 0.0096 | 0.0295 | −0.0064 | 0.0069 | 0.0005 | 0.930 |
| O2⋯H13 | 2.552 | 1.3478 | 0.0068 | 0.0252 | −0.0043 | 0.0053 | 0.0010 | 0.811 |
| O2⋯H15 | 2.083 | 3.8335 | 0.0156 | 0.0485 | −0.0122 | 0.0122 | −0.0001 | 1.004 |
| O2⋯H12 | 2.450 | 1.7128 | 0.0084 | 0.0272 | −0.0055 | 0.0061 | 0.0007 | 0.890 |
| Complex 2 | ||||||||
| C25⋯H3 | 2.632 | 1.0444 | 0.0071 | 0.0223 | −0.0033 | 0.0045 | 0.0011 | 0.747 |
| C31⋯H6 | 2.650 | 0.9912 | 0.0068 | 0.0209 | −0.0032 | 0.0042 | 0.0010 | 0.753 |
| C10⋯H32 | 2.697 | 0.7919 | 0.0055 | 0.0193 | −0.0025 | 0.0037 | 0.0012 | 0.686 |
| H34⋯O1 | 2.323 | 2.7102 | 0.0111 | 0.0504 | −0.0086 | 0.0106 | 0.0020 | 0.814 |
| H16⋯O3 | 2.332 | 2.4315 | 0.0104 | 0.0391 | −0.0077 | 0.0088 | 0.0010 | 0.885 |
| H12⋯O4 | 2.444 | 1.7705 | 0.0084 | 0.0328 | −0.0056 | 0.0069 | 0.0013 | 0.815 |
| H9⋯O2 | 2.486 | 1.7245 | 0.0086 | 0.0306 | −0.0055 | 0.0066 | 0.0011 | 0.836 |
| Pt2⋯H29 | 2.901 | 1.7245 | 0.0086 | 0.0306 | −0.0055 | 0.0066 | 0.0011 | 0.836 |
| Complex 3 | ||||||||
| N4⋯H14 | 2.273 | 2.5969 | 0.0122 | 0.0382 | −0.0083 | 0.0089 | 0.0006 | 0.929 |
| N4⋯H14 | 2.273 | 2.6064 | 0.0122 | 0.0381 | −0.0083 | 0.0089 | 0.0006 | 0.932 |
| H8⋯O2 | 2.385 | 1.8904 | 0.0089 | 0.0313 | −0.0060 | 0.0069 | 0.0009 | 0.870 |
| H3⋯O2 | 2.422 | 1.7526 | 0.0085 | 0.0284 | −0.0056 | 0.0063 | 0.0008 | 0.881 |
| H2⋯O2 | 2.498 | 1.4290 | 0.0072 | 0.0250 | −0.0046 | 0.0054 | 0.0008 | 0.843 |
| Pt1⋯H16 | 2.650 | 2.2492 | 0.0114 | 0.0392 | −0.0072 | 0.0085 | 0.0013 | 0.845 |
| Pt1⋯H16 | 2.650 | 2.2517 | 0.0114 | 0.0392 | −0.0072 | 0.0085 | 0.0013 | 0.846 |
| Complex 4 | ||||||||
| C12⋯H9 | 2.708 | 0.7821 | 0.0057 | 0.0186 | −0.0025 | 0.0036 | 0.0011 | 0.699 |
| H16⋯O2 | 2.338 | 1.9276 | 0.0089 | 0.0303 | −0.0061 | 0.0069 | 0.0007 | 0.896 |
| H16⋯O2 | 2.338 | 1.9242 | 0.0088 | 0.0305 | −0.0061 | 0.0069 | 0.0007 | 0.891 |
| H14⋯O2 | 2.467 | 0.9670 | 0.0053 | 0.0218 | −0.0031 | 0.0043 | 0.0012 | 0.722 |
| H12⋯O1 | 2.511 | 1.3375 | 0.0068 | 0.0235 | −0.0043 | 0.0051 | 0.0008 | 0.842 |
| H12⋯O1 | 2.511 | 1.3392 | 0.0068 | 0.0237 | −0.0043 | 0.0051 | 0.0008 | 0.837 |
| H10⋯O1 | 2.507 | 1.6330 | 0.0081 | 0.0303 | −0.0052 | 0.0064 | 0.0012 | 0.814 |
| Complex 5 | ||||||||
| C17⋯H19 | 2.699 | 0.9949 | 0.0069 | 0.0240 | −0.0032 | 0.0046 | 0.0014 | 0.692 |
| H4⋯O3 | 2.396 | 1.6975 | 0.0080 | 0.0289 | −0.0054 | 0.0063 | 0.0009 | 0.856 |
| H4⋯O3 | 2.396 | 1.7129 | 0.0081 | 0.0287 | −0.0055 | 0.0063 | 0.0009 | 0.863 |
| H24⋯O1 | 2.461 | 1.5307 | 0.0075 | 0.0267 | −0.0049 | 0.0058 | 0.0009 | 0.844 |
| H25⋯O2 | 2.556 | 1.3488 | 0.0069 | 0.0270 | −0.0043 | 0.0055 | 0.0012 | 0.777 |
| H10⋯O5 | 2.324 | 2.2836 | 0.0103 | 0.0407 | −0.0073 | 0.0087 | 0.0015 | 0.834 |
| H23⋯O6 | 2.278 | 2.5966 | 0.0118 | 0.0373 | −0.0083 | 0.0088 | 0.0005 | 0.941 |
| H23⋯O6 | 2.278 | 2.6027 | 0.0118 | 0.0374 | −0.0083 | 0.0088 | 0.0005 | 0.940 |
| Complex 6 | ||||||||
| H9⋯F | 2.544 | 1.1047 | 0.0050 | 0.0260 | −0.0035 | 0.0050 | 0.0015 | 0.703 |
| H10⋯F | 2.526 | 1.1840 | 0.0054 | 0.0274 | −0.0038 | 0.0053 | 0.0015 | 0.711 |
| C16⋯H4 | 2.669 | 0.9030 | 0.0063 | 0.0212 | −0.0029 | 0.0041 | 0.0012 | 0.704 |
| H8⋯O3 | 2.360 | 1.8423 | 0.0087 | 0.0283 | −0.0059 | 0.0065 | 0.0006 | 0.907 |
| H8⋯O3 | 2.360 | 1.8405 | 0.0087 | 0.0282 | −0.0059 | 0.0065 | 0.0006 | 0.908 |
| H6⋯O3 | 2.503 | 0.5211 | 0.0031 | 0.0128 | −0.0017 | 0.0024 | 0.0008 | 0.683 |
| H5⋯O3 | 2.478 | 1.5297 | 0.0076 | 0.0260 | −0.0049 | 0.0057 | 0.0008 | 0.858 |
| H5⋯O3 | 2.478 | 1.5259 | 0.0076 | 0.0259 | −0.0049 | 0.0057 | 0.0008 | 0.857 |
| H14⋯O2 | 2.278 | 2.5177 | 0.0114 | 0.0360 | −0.0080 | 0.0085 | 0.0005 | 0.943 |
| H13⋯O2 | 2.346 | 2.3741 | 0.0108 | 0.0375 | −0.0076 | 0.0085 | 0.0009 | 0.893 |
| H11⋯O1 | 2.332 | 2.2869 | 0.0104 | 0.0369 | −0.0073 | 0.0083 | 0.0010 | 0.883 |
| Contact Type A⋯H-D | A⋯D Distance | Clusters | Monomer Complex | ΔN(H) | ΔN(D) | ΔN(A) | ΔNA⋯H | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| H | D | A | H | D | A | ||||||
| Complex 1 | |||||||||||
| O1⋯H8-C8 | 2.360 | 0.2028 | –0.0799 | –0.6605 | 0.1759 | –0.1090 | –0.6367 | 0.0269 | 0.0291 | –0.0238 | 0.8633 |
| O2⋯H13-C11 | 2.552 | 0.2086 | –0.6004 | –0.6842 | 0.1957 | –0.5904 | –0.6214 | 0.0129 | –0.01 | –0.0628 | 0.8928 |
| O2⋯H15-N3 | 2.083 | 0.3281 | –0.4735 | –0.6842 | 0.3075 | –0.4708 | –0.6214 | 0.0206 | –0.0027 | –0.0628 | 1.0123 |
| O2⋯H12-C11 | 2.450 | 0.2334 | –0.5976 | –0.6842 | 0.2063 | –0.5904 | –0.6214 | 0.0271 | –0.0072 | –0.0628 | 0.9176 |
| Complex 2 | |||||||||||
| C25⋯H3-C2 | 2.632 | 0.1977 | –0.6472 | –0.2038 | 0.2010 | –0.6467 | –0.2015 | –0.0033 | –0.0005 | –0.0023 | 0.4015 |
| C31⋯H6-C4 | 2.650 | 0.2074 | –0.6601 | –0.1517 | 0.2001 | –0.6522 | –0.1503 | 0.0073 | –0.0079 | –0.0014 | 0.3591 |
| C10⋯H32-C32 | 2.697 | 0.1847 | –0.1590 | 0.1273 | 0.1781 | –0.1622 | 0.1328 | 0.0066 | 0.0032 | –0.0055 | 0.0574 |
| O1⋯H34-N6 | 2.323 | 0.3398 | –0.4528 | –0.6923 | 0.3213 | –0.4543 | –0.6528 | 0.0185 | 0.0015 | –0.0395 | 1.0321 |
| O3⋯H16-N3 | 2.332 | 0.3363 | –0.4663 | –0.6757 | 0.3219 | –0.4670 | –0.6523 | 0.0144 | 0.0007 | –0.0234 | 1.0120 |
| O4⋯H12-C13 | 2.444 | 0.1911 | –0.1293 | –0.6149 | 0.1733 | –0.1497 | –0.6009 | 0.0178 | 0.0204 | –0.014 | 0.8060 |
| O2⋯H9-C9 | 2.486 | 0.1938 | –0.1778 | –0.6341 | 0.1813 | –0.1954 | –0.5979 | 0.0125 | 0.0176 | –0.0362 | 0.8279 |
| Complex 3 | |||||||||||
| N4⋯H14-N3 | 2.273 | 0.3205 | –0.4693 | –0.5312 | 0.3080 | –0.4800 | –0.4907 | 0.0125 | 0.0107 | –0.0405 | 0.8517 |
| N4⋯H14-N3 | 2.273 | 0.3198 | –0.4702 | –0.5326 | 0.3080 | –0.4800 | –0.4907 | 0.0118 | 0.0098 | –0.0419 | 0.8524 |
| O2⋯H8-C7 | 2.385 | 0.2367 | –0.6048 | –0.6671 | 0.2082 | –0.5996 | –0.6323 | 0.0285 | –0.0052 | –0.0348 | 0.9038 |
| O2⋯H3-C2 | 2.422 | 0.2102 | –0.6570 | –0.6671 | 0.2015 | –0.6499 | –0.6323 | 0.0087 | –0.0071 | –0.0348 | 0.8773 |
| O2⋯H2-C2 | 2.498 | 0.2231 | –0.6637 | –0.6671 | 0.2051 | –0.6499 | –0.6323 | 0.0180 | –0.0138 | –0.0348 | 0.8902 |
| Pt1⋯H16-N4 | 2.650 | 0.3253 | –0.4924 | 0.4082 | 0.3043 | –0.4907 | 0.4225 | 0.0210 | –0.0017 | –0.0143 | 0.0829 |
| Pt1⋯H16-N4 | 2.650 | 0.3199 | –0.4881 | 0.4136 | 0.3043 | –0.4907 | 0.4225 | 0.0156 | 0.0026 | –0.0089 | 0.0937 |
| Complex 4 | |||||||||||
| C12⋯H9-C8 | 2.708 | 0.1792 | –0.1077 | –0.2004 | 0.1749 | –0.1088 | 0.1817 | 0.0043 | 0.0011 | –0.3821 | 0.3796 |
| O2⋯H16-C16 | 2.338 | 0.1910 | –0.2396 | –0.6429 | 0.1742 | –0.2277 | –0.5938 | 0.0168 | –0.0119 | –0.0491 | 0.8339 |
| O2⋯H16-C16 | 2.338 | 0.1925 | –0.2367 | –0.6461 | 0.1742 | –0.2277 | –0.5938 | 0.0183 | –0.009 | –0.0523 | 0.8386 |
| O2⋯H14-C14 | 2.467 | 0.1817 | –0.1455 | –0.6429 | 0.1712 | –0.1799 | –0.5938 | 0.0105 | 0.0344 | –0.0491 | 0.8246 |
| O1⋯H12-C12 | 2.511 | 0.1933 | –0.1915 | –0.6827 | 0.1817 | –0.1856 | –0.6594 | 0.0116 | –0.0059 | –0.0233 | 0.8760 |
| O1⋯H12-C12 | 2.511 | 0.1959 | –0.2010 | –0.6797 | 0.1817 | –0.1856 | –0.6594 | 0.0142 | –0.0154 | –0.0203 | 0.8756 |
| O1⋯H10-C9 | 2.507 | 0.1895 | –0.1959 | –0.6827 | 0.1820 | –0.1986 | –0.6594 | 0.0075 | 0.0027 | –0.0233 | 0.8722 |
| Complex 5 | |||||||||||
| C17⋯H19-C16 | 2.699 | 0.2109 | –0.6558 | 0.0684 | 0.2040 | –0.6470 | 0.0832 | 0.0069 | –0.0088 | –0.0148 | 0.1425 |
| O3⋯H4-C4 | 2.396 | 0.2193 | –0.6544 | –0.6382 | 0.1982 | –0.6486 | –0.6272 | 0.02112 | –0.0058 | –0.01103 | 0.8576 |
| O3⋯H4-C4 | 2.396 | 0.2193 | –0.6544 | –0.6382 | 0.1982 | –0.6486 | –0.6272 | 0.02112 | –0.0058 | –0.01103 | 0.8576 |
| O1⋯H24-C20 | 2.461 | 0.1886 | –0.0996 | –0.6982 | 0.1777 | –0.1017 | –0.6724 | 0.0109 | 0.0021 | –0.0258 | 0.8868 |
| O2⋯H25-C21 | 2.556 | 0.1986 | –0.1619 | –0.6303 | 0.1825 | –0.1879 | –0.5975 | 0.0161 | 0.026 | –0.0328 | 0.8289 |
| O5⋯H10-C9 | 2.324 | 0.2069 | –0.1683 | –0.6463 | 0.1853 | –0.1912 | –0.5927 | 0.0216 | 0.0229 | –0.0536 | 0.8532 |
| O6⋯H23-C19 | 2.278 | 0.2010 | –0.1787 | –0.6460 | 0.1877 | –0.1816 | –0.6144 | 0.0133 | 0.0029 | –0.0316 | 0.8470 |
| O6⋯H23-C19 | 2.278 | 0.2034 | –0.1742 | –0.6452 | 0.1877 | –0.1816 | –0.6144 | 0.0157 | 0.0074 | –0.0308 | 0.8486 |
| Complex 6 | |||||||||||
| H9⋯F-C15 | 2.544 | 0.1890 | –0.1830 | –0.3625 | 0.1827 | –0.1849 | –0.3451 | 0.0063 | 0.0019 | –0.0174 | 0.5515 |
| H10⋯F-C15 | 2.526 | 0.1824 | –0.0970 | –0.3625 | 0.1774 | –0.1078 | –0.3451 | 0.0050 | 0.0108 | –0.0174 | 0.5449 |
| C16⋯H4-C4 | 2.669 | 0.1960 | –0.6500 | –0.2518 | 0.1910 | –0.6513 | –0.2459 | 0.0050 | 0.0013 | –0.0059 | 0.4478 |
| O3⋯H8-C7 | 2.360 | 0.2067 | –0.1633 | –0.6540 | 0.1862 | –0.1730 | –0.6268 | 0.0205 | 0.0097 | –0.0272 | 0.8607 |
| O3⋯H8-C7 | 2.360 | 0.2028 | –0.1665 | –0.6450 | 0.1862 | –0.1730 | –0.6268 | 0.0166 | 0.0065 | –0.0182 | 0.8478 |
| O3⋯H6-C4 | 2.503 | 0.2195 | –0.6698 | –0.6336 | 0.2052 | –0.6513 | –0.6268 | 0.0143 | –0.0185 | –0.0068 | 0.8531 |
| O3⋯H5-C4 | 2.478 | 0.2030 | –0.6528 | –0.6301 | 0.1938 | –0.6513 | –0.6268 | 0.0092 | –0.0015 | –0.0033 | 0.8331 |
| O3⋯H5-C4 | 2.478 | 0.2055 | –0.6528 | –0.6331 | 0.1938 | –0.6513 | –0.6268 | 0.0117 | –0.0015 | –0.0063 | 0.8386 |
| O2⋯H14-C16 | 2.278 | 0.2137 | –0.2478 | –0.6088 | 0.1917 | –0.2459 | –0.5928 | 0.0220 | –0.0019 | –0.016 | 0.8225 |
| O2⋯H13-C14 | 2.346 | 0.2167 | –0.2310 | –0.6240 | 0.1930 | –0.2377 | –0.5928 | 0.0237 | 0.0067 | –0.0312 | 0.8407 |
| O1⋯H11-C10 | 2.332 | 0.1852 | 0.1362 | –0.6918 | 0.1715 | 0.1394 | –0.6786 | 0.0137 | –0.0032 | –0.0132 | 0.8770 |
| Contact type A⋯H-D | A⋯D Dist. | (NBO) i | (Occupancy) i | Ei | (NBO) j | (Occupancy) j | Ej | E(2) |
|---|---|---|---|---|---|---|---|---|
| Complex 1 | ||||||||
| O1⋯H8-C8 | 2.360 | LP(1)O1 | 1.97137 (1.97169) | –0.66824 (–0.64828) | σ*(C8-H8) | 0.01397 (0.01265) | 0.71211 (0.66653) | 0.628 |
| LP(2)O1 | 1.86311 (1.85818) | –0.26356 (–0.24078) | σ*(C8-H8) | 0.01397 (0.01265) | 0.71211 (0.66653) | 2.261 | ||
| O2⋯H13-C11 | 2.552 | π(C3-O2) | 1.99011 (0.53040) | –0.36890 (–0.33956) | σ*(C11-H13) | 0.00788 (0.00670) | 0.55924 (0.53040) | 1.005 |
| O2⋯H15-N3 | 2.083 | LP(1)O2 | 1.97123 (0.73470) | –0.67080 (0.73470) | σ*(N3-H15) | 0.01347 (0.73470) | 0.78719 (0.73470) | 2.386 |
| LP(2)O2 | 1.87487 (1.86076) | –0.26693 (–0.23237) | σ*(N3-H15) | 0.01347 (0.00912) | 0.78719 (0.73470) | 6.155 | ||
| π(C3=O2) | 1.99011 (1.98993) | –0.36890 (–0.33956) | σ*(N3-H15) | 0.01347 (0.00912) | 0.78719 (0.73470) | 0.544 | ||
| O2⋯H12-C11 | 2.450 | LP(1)O2 | 1.97123 (1.97351) | –0.67080 (–0.64215) | σ*(C11-H12) | 0.01168 (0.00886) | 0.57514 (0.52857) | 0.628 |
| LP(2)O2 | 1.87487 (1.86076) | –0.26693 (–0.23237) | σ*(C11-H12) | 0.01168 (0.00886) | 0.57514 (0.52857) | 2.680 | ||
| Complex 2 | ||||||||
| C25⋯H3-C2 | 2.632 | π(C25-C26) | 1.63140 (1.63245) | –0.27948 (–0.29051) | σ*(C2-H3) | 0.00867 (0.00810) | 0.59404 (0.59837) | 1.214 |
| C31⋯H6-C4 | 2.650 | π(C31-C32) | 1.64661 (1.65943) | –0.26358 (–0.27596) | σ*(C4-H6) | 0.00826 (0.00734) | 0.59477 (0.58464) | 1.382 |
| C10⋯H32-C32 | 2.697 | σ(C32-H32) | 1.97739 (1.97819) | –0.57490 (–0.58925) | π*(C10-N2) | 0.43750 (0.43608) | –0.03118 (–0.04048) | 0.586 |
| π(C10-N2) | 1.76913 (1.75890) | –0.33656 (–0.34197) | σ*(C32-H32) | 0.01374 (0.01350) | 0.69918 (0.67576) | 0.586 | ||
| O1⋯H34-N6 | 2.323 | LP(1)O1 | 1.97191 (1.97185) | –0.66711 (–0.65175) | σ*(N6-H34) | 0.01218 (0.01182) | 0.80595 (0.77963) | 0.209 |
| LP(2)O1 | 1.88227 (1.87315) | –0.25176 (–0.23319) | σ*(N6-H34) | 0.01218 (0.01182) | 0.80595 (0.77963) | 0.879 | ||
| O3⋯H16-N3 | 2.332 | π(C17-O3) | 1.98910 (1.98983) | –0.35705 (–0.33828) | σ*(N3-H17) | 0.01256 (0.01108) | 0.74628 (0.71888) | 0.795 |
| LP(2)O3 | 1.87408 (1.86995) | –0.25616 (–0.23664) | σ*(N3-H17 ) | 0.01256 (0.01108) | 0.74628 (0.71888) | 0.963 | ||
| O4⋯H12-C13 | 2.444 | LP(2)O4 | 1.85563 (1.85073) | –0.24363 (–0.23150) | σ*(C13-H12) | 0.01286 (0.01281) | 0.72157 (0.68308) | 0.461 |
| O2⋯H9-C9 | 2.486 | LP(2)O2 | 1.84626 (1.84547) | –0.23524 (–0.23060) | σ*(C9-H9) | 0.01214 (0.01129) | 0.65796 (0.66930) | 0.712 |
| Complex 3 | ||||||||
| N4⋯H14-N3 | 2.273 | LP(1)N4 | 1.85986 (1.84172) | –0.36581 (–0.34055) | σ*(N3-H14) | 0.01164 (0.00884) | 0.81339 (0.78729) | 5.192 |
| N4⋯H14-N3 | 2.273 | LP (1)N4 | 1.86168 (1.84172) | –0.35759 (–0.34055) | σ*(N3-H14) | 0.01161 (0.00884) | 0.82344 (0.78729) | 5.192 |
| O2⋯H8-C7 | 2.385 | LP(1)O2 | 1.97169 (1.97261) | –0.66099 (–0.64978) | σ*(C7-H8) | 0.01186 (0.01044) | 0.58379 (0.52851) | 1.675 |
| LP(2)O2 | 1.86505 (1.85902) | –0.25512 (–0.24097) | σ*(C7-H8) | 0.01186 (0.01044) | 0.58379 (0.52851) | 0.963 | ||
| O2⋯H3-C2 | 2.422 | LP(1)O2 | 1.97169 (1.97261) | –0.66099 (–0.64978) | σ*(C2-H3) | 0.01035 (0.00768) | 0.62219 (0.60008) | 0.293 |
| LP(2)O2 | 1.86505 (1.85902) | –0.25512 (–0.24097) | σ*(C2-H3) | 0.01035 (0.00768) | 0.62219 (0.60008) | 1.005 | ||
| π(C3-O2) | 1.99010 (1.99023) | –0.36459 (–0.35580) | σ*(C2-H3) | 0.01035 (0.00768) | 0.62219 (0.60008) | 1.424 | ||
| O2⋯H2-C2 | 2.498 | LP(1)O2 | 1.97169 (1.97261) | –0.66099 (–0.64978) | σ*(C2-H2) | 0.00979 (0.00743) | 0.61830 (0.58366) | 1.005 |
| LP(2)O2 | 1.86505 (1.85902) | –0.25512 (–0.24097) | σ*(C2-H2) | 0.00979 (0.00743) | 0.61830 (0.58366) | 1.633 | ||
| Pt1⋯H16-N4 | 2.650 | LP(1)Pt1 | 1.87880 (1.99158) | –0.26130 (–0.24303) | σ*(N4-H16) | 0.01058 (0.00839) | 0.77742 (0.74610) | 4.061 |
| Pt1⋯H16-N4 | 2.650 | LP(1)Pt1 | 1.91600 (1.91561) | –0.46068 (–0.45392) | σ*(N4-H16) | 0.01057 (0.00839) | 0.76746 (0.74610) | 3.894 |
| Complex 4 | ||||||||
| C12⋯H9-C8 | 2.708 | σ(C8-H9) | 1.97880 (1.97943) | –0.59280 (–0.60808) | π*(C12-C13) | 0.01734 (0.30861) | 0.57893 (0.02913) | 0.461 |
| π(C12-C13) | 1.97514 (1.97564) | –0.71226 (–0.69807) | σ*(C8-H9) | 0.01350 (0.01312) | 0.69110 (0.66991) | 0.419 | ||
| O2⋯H16–C16 | 2.338 | π(C3-O2) | 1.98923 (1.98983) | –0.38610 (–0.35347) | σ*(C16-H16) | 0.01424 (0.01304) | 0.69995 (0.67898) | 1.549 |
| LP(1)O2 | 1.97068 (1.97264) | –0.67192 (–0.63955) | σ*(C16-H16) | 0.01424 (0.01304) | 0.69995 (0.67898) | 1.089 | ||
| O2⋯H16-C16 | 2.338 | π(C3-O2) | 1.98947 (1.98983) | –0.37977 (–0.35347) | σ*(C16-H16) | 0.01423 (0.01304) | 0.69933 (0.67898) | 1.591 |
| LP(1)O2 | 1.97110 (1.97264) | –0.66751 (–0.63955) | σ*(C16-H16) | 0.01423 (0.01304) | 0.69933 (0.67898) | 1.089 | ||
| O2⋯H14-C14 | 2.467 | LP(1)O2 | 1.97068 (1.97264) | –0.67192 (–0.63955) | σ*(C14-H14) | 0.01283 (0.01256) | 0.72291 (0.69606) | 0.419 |
| O1⋯H12-C12 | 2.511 | LP(1)O1 | 1.96440 (1.99026) | –0.69115 (–0.35247) | σ*(C12-H12) | 0.01253 (0.01158) | 0.70138 (0.68580) | 0.544 |
| LP(2)O1 | 1.86902 (1.86203) | –0.28550 (–0.25502) | σ*(C12-H12) | 0.01253 (0.01158) | 0.70138 (0.68580) | 0.502 | ||
| O1⋯H12-C12 | 2.511 | LP(1)O1 | 1.96527 (1.99026) | –0.68160 (–0.35247) | σ*(C12-H12) | 0.01253 (0.01158) | 0.70098 (0.68580) | 0.544 |
| LP(2)O1 | 1.86794 (1.86203) | –0.27517 (–0.25502) | σ*(C12-H12) | 0.01253 (0.01158) | 0.70098 (0.68580) | 0.544 | ||
| O1⋯H10-C9 | 2.507 | LP(2)O1 | 1.86902 (1.86203) | –0.28550 (–0.25502) | σ*(C9-H10) | 0.01279 (0.01193) | 0.68180 (0.66621) | 0.419 |
| Complex 5 | ||||||||
| C17⋯H19-C16 | 2.699 | σ(C16-H19) | 1.96707 (1.96868) | –0.54074 (–0.53534) | π*(C17-N4) | 0.01548 (0.25607) | 0.54744 (−0.03872) | 1.130 |
| π(C17-N4) | 1.98824 (1.92080) | –0.91673 (–0.37610) | σ*(C16-H19) | 0.00922 (0.00877) | 0.57426 (0.57142) | 0.377 | ||
| O3⋯H4-C4 | 2.396 | π(C11-O3) | 1.98337 (1.99523) | (–0.39427) (–1.06665) | σ*(C4-H4) | 0.00963 (0.00835) | 0.59373 (0.56896) | 0.7534 |
| LP(1)O3 | 1.97713 (1.97866) | (–0.70347) (–0.69621) | σ*(C4-H4) | 0.00963 (0.00835) | 0.59373 (0.56896) | 1.6324 | ||
| O3⋯H4-C4 | 2.396 | π(C11-O3) | 1.98337 (1.99523) | (–0.39427) (–1.06665) | σ*(C4-H4) | 0.00963 (0.00835) | 0.59373 (0.56896) | 0.7534 |
| LP(1)O3 | 1.97713 (1.97866) | –0.70347 (–0.69621) | σ*(C4-H4) | 0.00963 (0.00835) | 0.59373 (0.56896) | 1.6324 | ||
| O1⋯H24-C20 | 2.461 | LP(1)O1 | 1.96648 (1.96689) | –0.68368 (–0.67330) | σ*(C20-H24) | 0.01276 (0.01209) | 0.70137 (0.66118) | 0.7540 |
| LP(1)O1 | 1.87419 (1.86803) | –0.26999 (–0.25705) | σ*(C20-H24) | 0.01276 (0.01209) | 0.70137 (0.66118) | 0.2930 | ||
| O2⋯H25-C21 | 2.556 | LP(2)O2 | 1.97269 (1.86803) | –0.65652 (–0.25705) | σ*(C21-H25) | 0.01146 (0.01137) | 0.70641 (0.65909) | 0.209 |
| O5⋯H10-C9 | 2.324 | LP(1)O5 | 1.97273 (1.97354) | –0.66258 (–0.65175) | σ*(C9-H10) | 0.01165 (0.01184) | 0.69823 (0.65601) | 0.628 |
| O6⋯H23-C19 | 2.278 | LP(1)O6 | 1.97702 (1.97845) | –0.69553 (–0.69126) | σ*(C19-H23) | 0.01490 (0.01292) | 0.68884 (0.64597) | 0.586 |
| LP(2)O6 | 1.87205 (1.86984) | –0.27847 (–0.27097) | σ*(C19-H23) | 0.01490 (0.01292) | 0.68884 (0.64597) | 2.721 | ||
| O6⋯H23-C19 | 2.278 | LP(1)O6 | 1.97712 (1.97845) | –0.69886 (–0.69126) | σ*(C19-H23) | 0.01473 (0.01292) | 0.68329 (0.64597) | 0.586 |
| LP(2)O6 | 1.87194 (1.86984) | –0.28146 (–0.27097) | σ*(C19-H23) | 0.01473 (0.01292) | 0.68329 (0.64597) | 2.680 | ||
| Complex 6 | ||||||||
| C16⋯H4-C4 | 2.669 | σ(C4-H4) | 1.98252 (1.98376) | –0.53852 (–0.53308) | π*(C16-C17) | 0.29616 (0.30335) | 0.02878 (0.01810) | 0.544 |
| π(C16-C17) | 1.66193 (1.66922) | –0.26125 (–0.27086) | σ*(C4-H4) | 0.01050 (0.01030) | 0.58775 (0.58738) | 1.005 | ||
| O3⋯H8-C7 | 2.360 | LP(1)O3 | 1.97802 (1.97901) | –0.71166 (–0.70195) | σ*(C7-H8) | 0.01316 (0.01180) | 0.67217 (0.64842) | 1.256 |
| LP(2)O3 | 1.87851 (1.87585) | –0.28994 (–0.27736) | σ*(C7-H8) | 0.01316 (0.01180) | 0.67217 (0.64842) | 1.758 | ||
| O3⋯H8-C7 | 2.360 | LP(1)O3 | 1.97816 (1.97901) | –0.71673 (–0.70195) | σ*(C7-H8) | 0.01316 (0.01180) | 0.67815 (0.64842) | 1.256 |
| LP(2)O3 | 1.87761 (1.87585) | –0.29446 (–0.27736) | σ*(C7-H8) | 0.01316 (0.01180) | 0.67815 (0.64842) | 1.675 | ||
| O3⋯H6-C4 | 2.503 | LP(1)O3 | 1.97816 (1.97901) | –0.69908 (–0.70195) | σ*(C4-H6) | 0.01029 (0.00859) | 0.58979 (0.57290) | 0.670 |
| LP(2)O3 | 1.87657 (1.87585) | –0.27608 (–0.27736) | σ*(C4-H6) | 0.01029 (0.00859) | 0.58979 (0.5729) | 0.502 | ||
| O3⋯H5-C4 | 2.478 | π(C11-O3) | 1.97576 (1.97564) | –0.39637 (–0.39003) | σ*(C4-H5) | 0.00951 (0.0082) | 0.58267 (0.56896) | 1.256 |
| LP(1)O3 | 1.97818 (1.97901) | –0.70613 (–0.70195) | σ*(C4-H5) | 0.00951 (0.0082) | 0.58267 (0.56896) | 0.025 | ||
| O3⋯H5-C4 | 2.478 | π(C3-O3) | 1.97554 (1.97564) | –0.39514 (–0.39003) | σ*(C4-H5) | 0.00972 (0.0082) | 0.57705 (0.56896) | 1.298 |
| LP(1)O3 | 1.97828 (1.97901) | –0.70568 (–0.70195) | σ*(C4-H5) | 0.00972 (0.00820) | 0.57705 (0.56896) | 0.251 | ||
| O2⋯H14-C16 | 2.278 | LP(1)O2 | 1.97197 (1.97301) | –0.66209 (–0.64902) | σ*(C16-H14) | 0.01430 (0.01217) | 0.70710 (0.67024) | 0.921 |
| LP(2)O2 | 1.85410 (1.85107) | –0.25874 (–0.24082) | σ*(C16-H14) | 0.01430 (0.01217) | 0.70710 (0.67024) | 2.763 | ||
| O2⋯H13-C14 | 2.346 | LP(1)O2 | 1.97245 (1.97301) | –0.65870 (–0.64902) | σ*(C14-H13) | 0.01325 (0.01153) | 0.70642 (0.67422) | 0.322 |
| LP(2)O2 | 1.85944 (1.85107) | –0.25185 (–0.24082) | σ*(C14-H13) | 0.01325 (0.01153) | 0.70642 (0.67422) | 2.010 | ||
| O1⋯H11-C10 | 2.332 | π(C1-O1) | 1.98826 (1.98947) | –0.37137 (–0.35014) | σ*(C10-H11) | 0.01643 (0.01559) | 0.67607 (0.65302) | 0.502 |
| LP(1)O1 | 1.96970 (1.97111) | –0.69047 (–0.67364) | σ*(C10-H11) | 0.01643 (0.01559) | 0.67607 (0.65302) | 0.335 | ||
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soliman, S.M.; Barakat, A. Decomposition of Intermolecular Interactions in the Crystal Structure of Some Diacetyl Platinum(II) Complexes: Combined Hirshfeld, AIM, and NBO Analyses. Molecules 2016, 21, 1669. https://doi.org/10.3390/molecules21121669
Soliman SM, Barakat A. Decomposition of Intermolecular Interactions in the Crystal Structure of Some Diacetyl Platinum(II) Complexes: Combined Hirshfeld, AIM, and NBO Analyses. Molecules. 2016; 21(12):1669. https://doi.org/10.3390/molecules21121669
Chicago/Turabian StyleSoliman, Saied M., and Assem Barakat. 2016. "Decomposition of Intermolecular Interactions in the Crystal Structure of Some Diacetyl Platinum(II) Complexes: Combined Hirshfeld, AIM, and NBO Analyses" Molecules 21, no. 12: 1669. https://doi.org/10.3390/molecules21121669
APA StyleSoliman, S. M., & Barakat, A. (2016). Decomposition of Intermolecular Interactions in the Crystal Structure of Some Diacetyl Platinum(II) Complexes: Combined Hirshfeld, AIM, and NBO Analyses. Molecules, 21(12), 1669. https://doi.org/10.3390/molecules21121669