Steric and Stereochemical Modulation in Pyridyl- and Quinolyl-Containing Ligands
Abstract
:1. Introduction
2. Steric and Stereochemical Modulation of Binding Affinity and Selectivity
2.1. Steric Control of Achiral Ligands’ Selectivity to Zn2+ and Cu2+
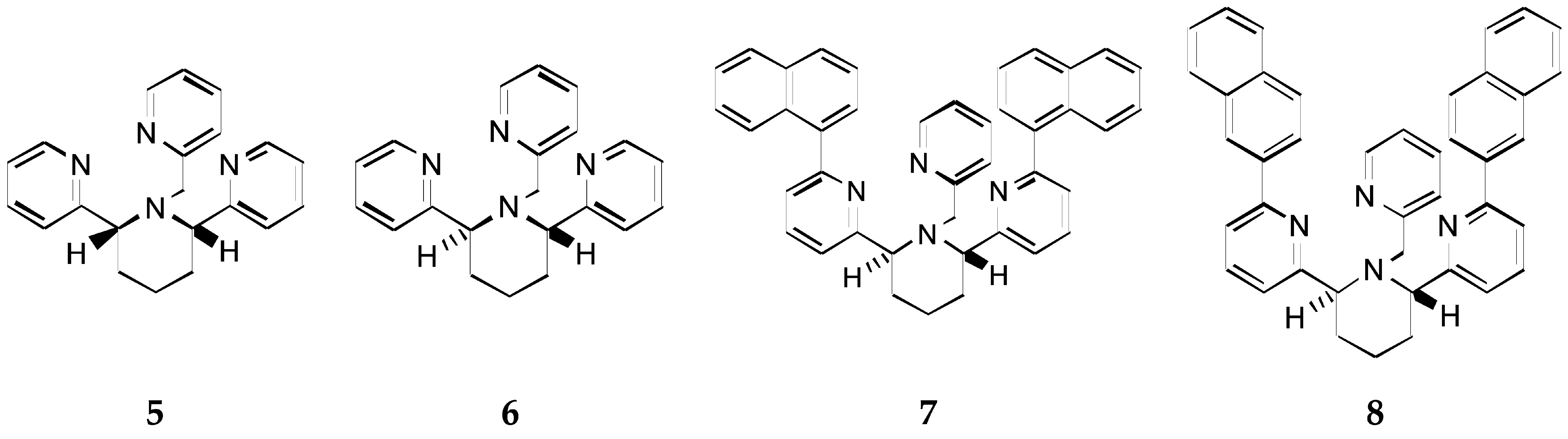
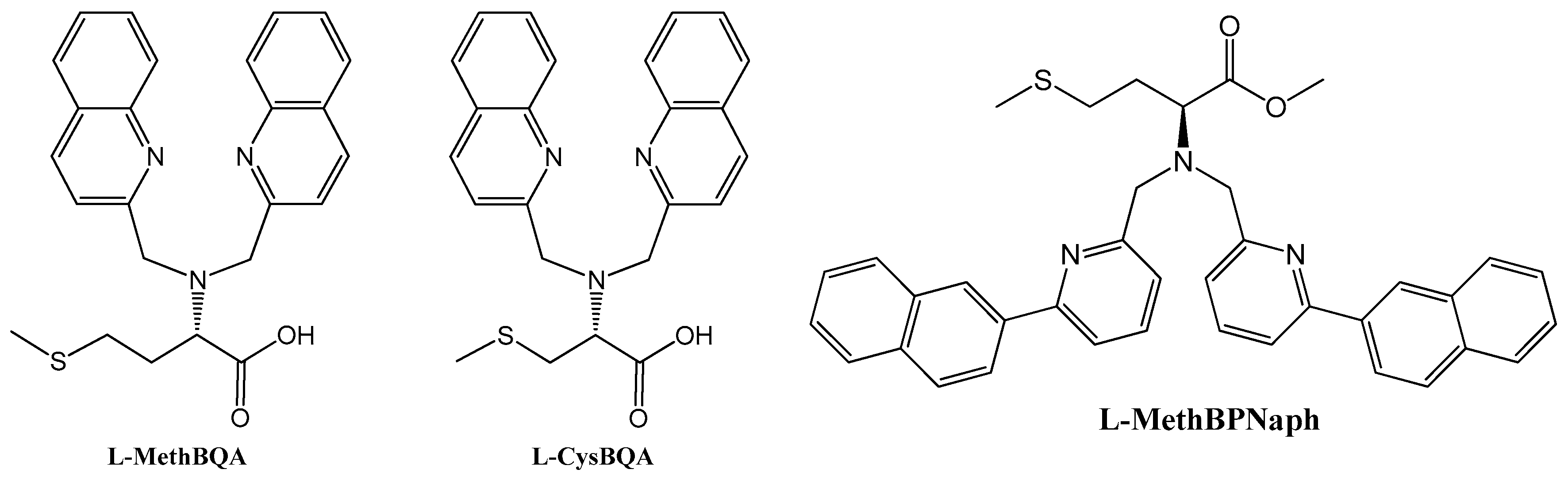
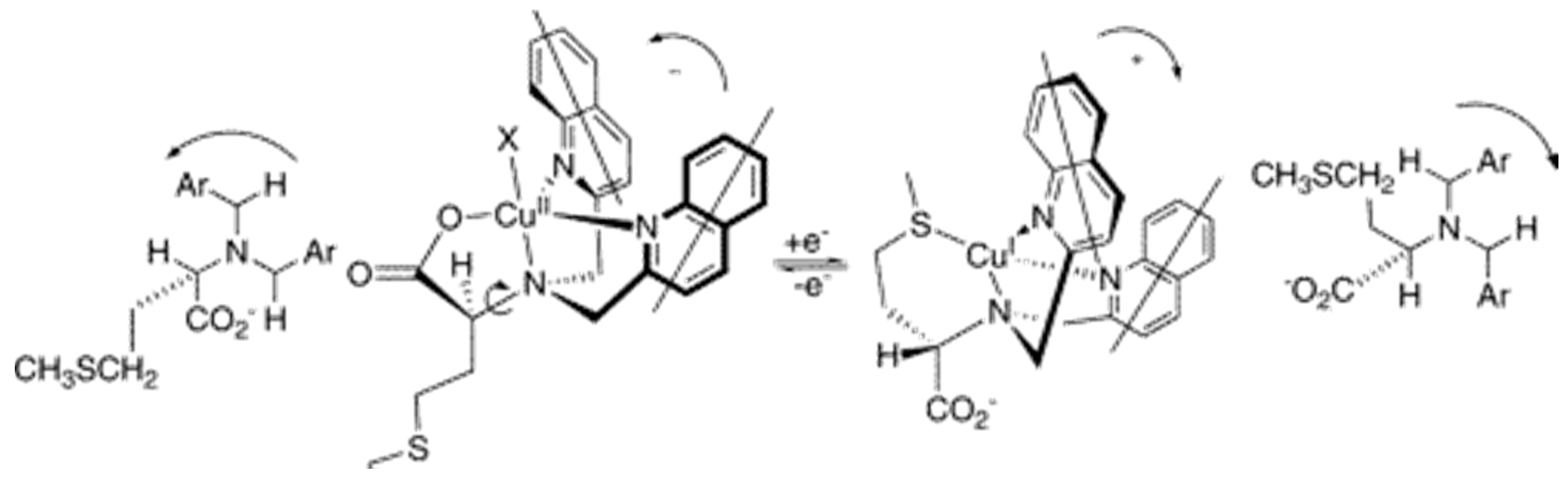
2.2. Stereochemical Control of Chiral Ligands’ Zn2+/Cu2+ Selectivity
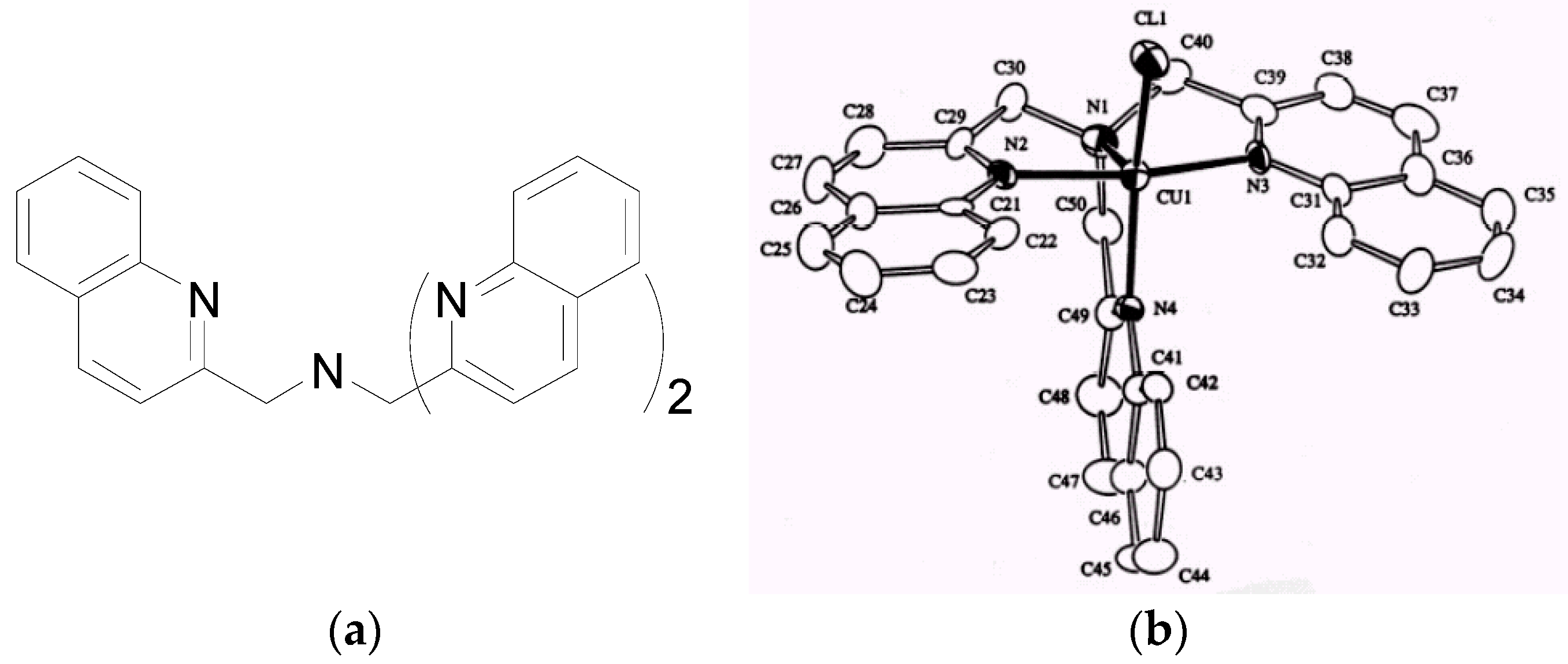
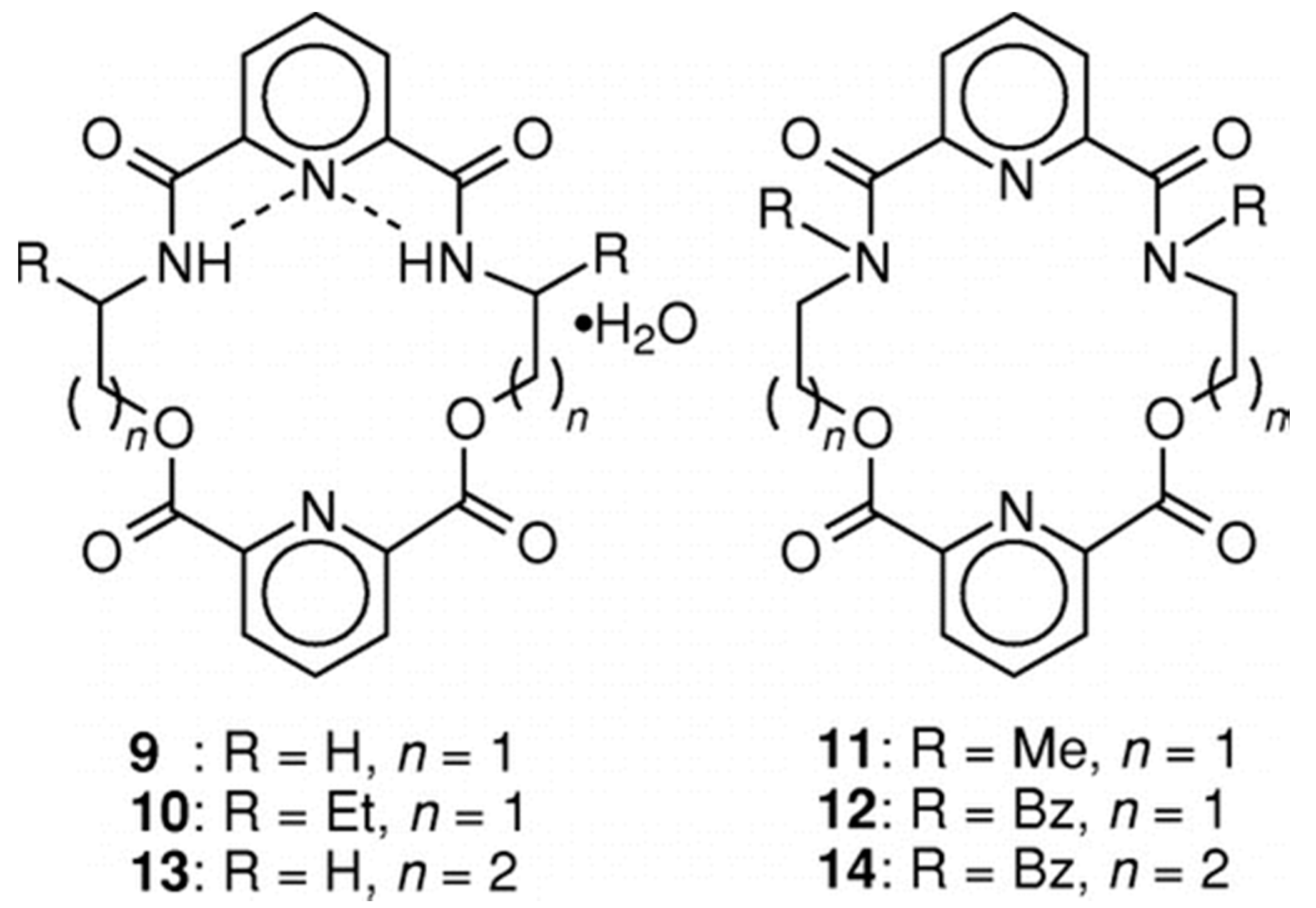
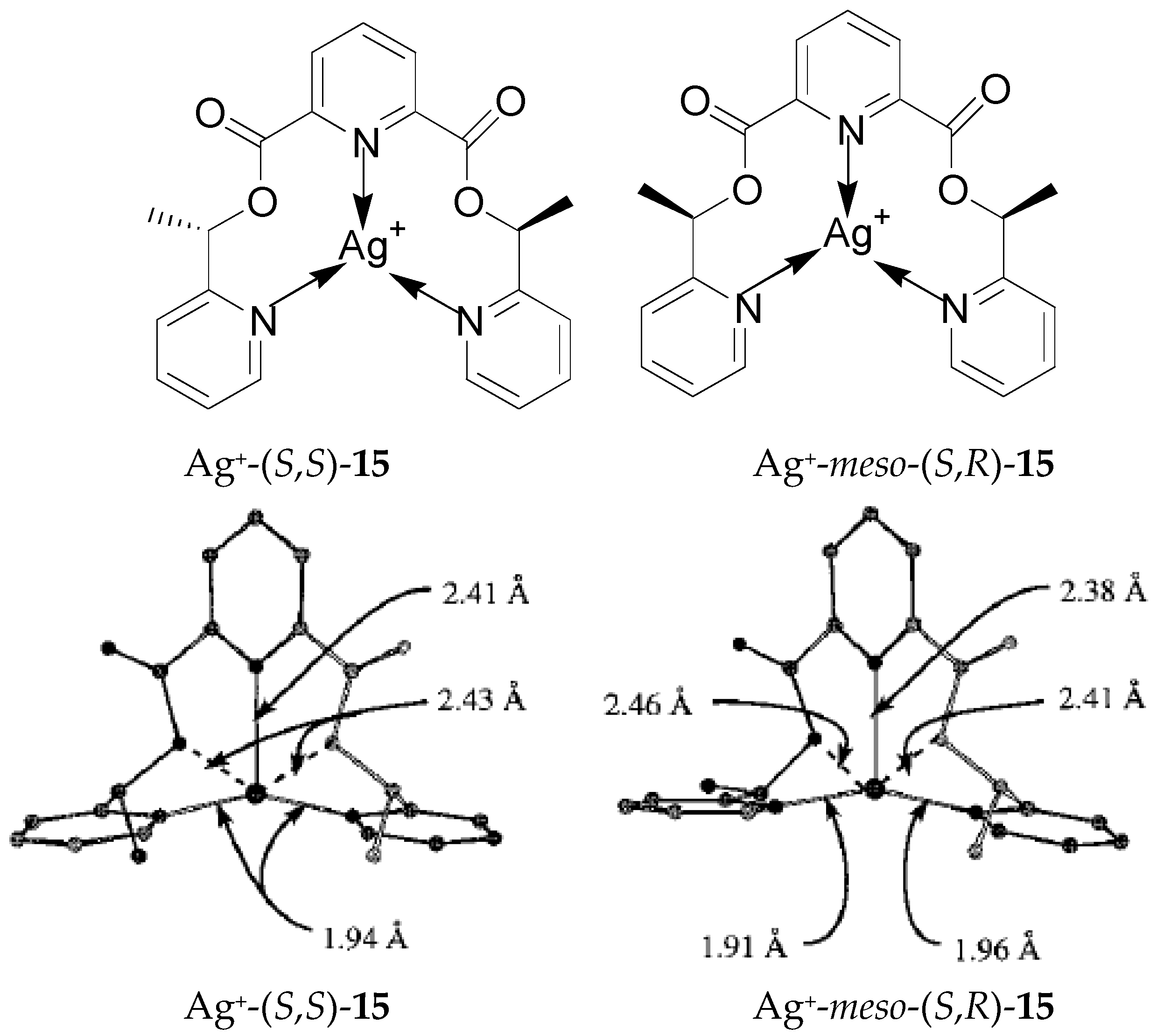
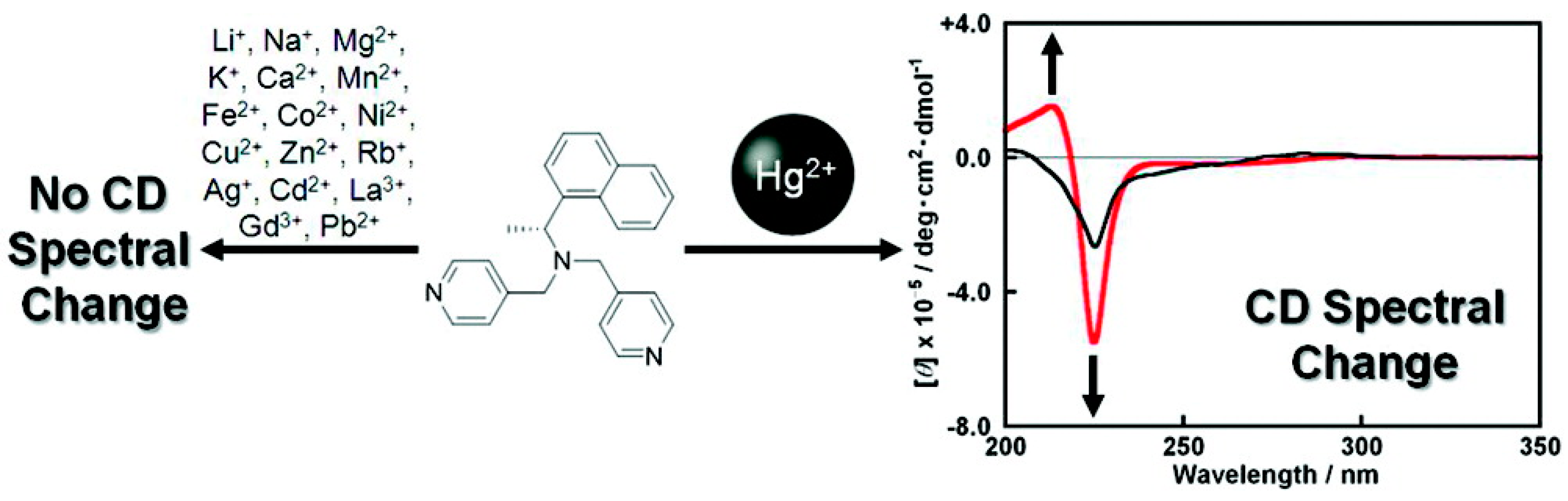
2.3. Steric and Stereochemical Control of Ligands’ Selectivity to Ag+
3. Steric and Stereochemical Modulation of Signal Transduction
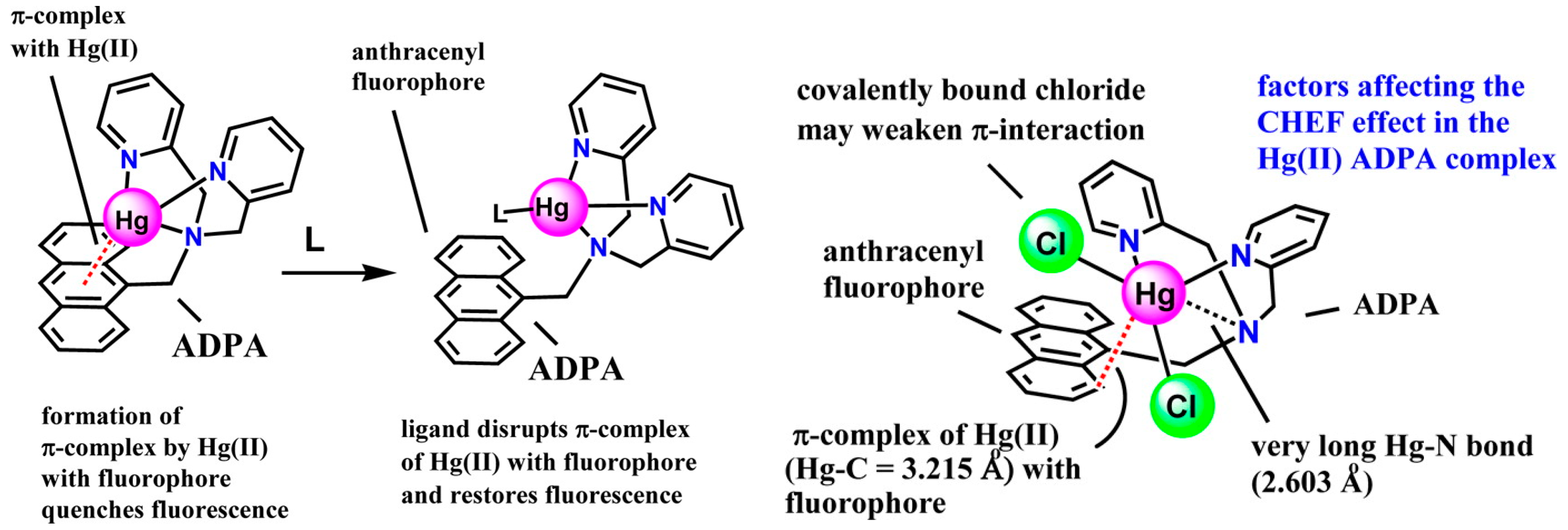
3.1. Modulating Fluorescence or Luminescence
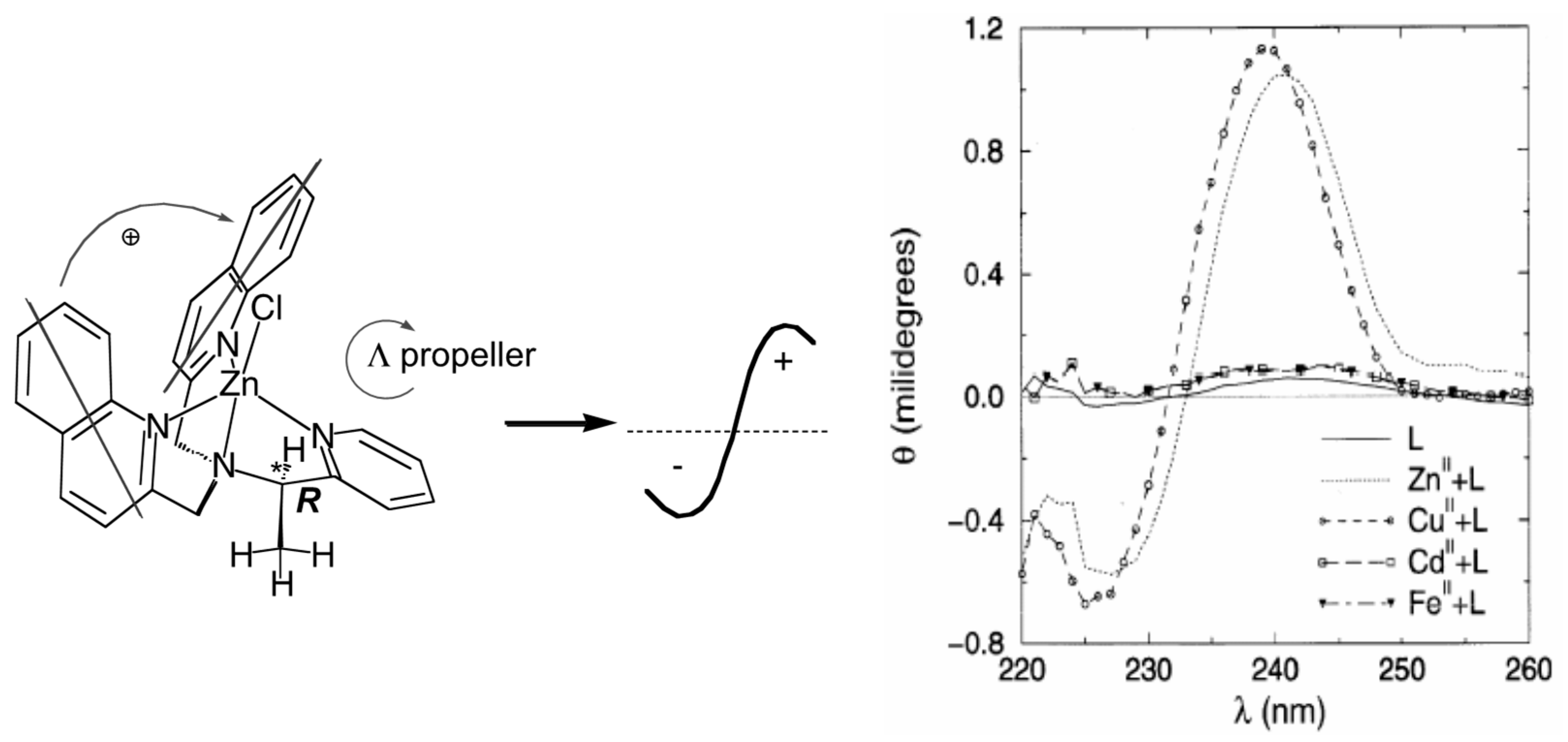
3.2. Modulating Chiroptical Signals
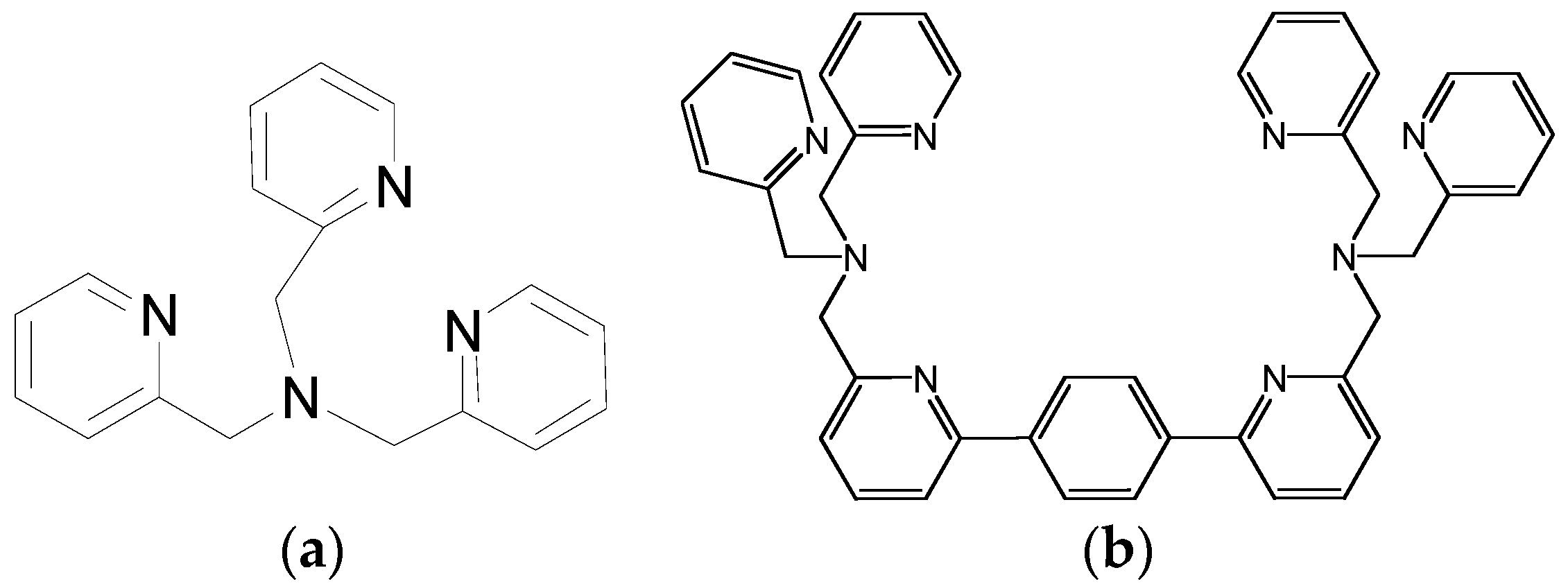
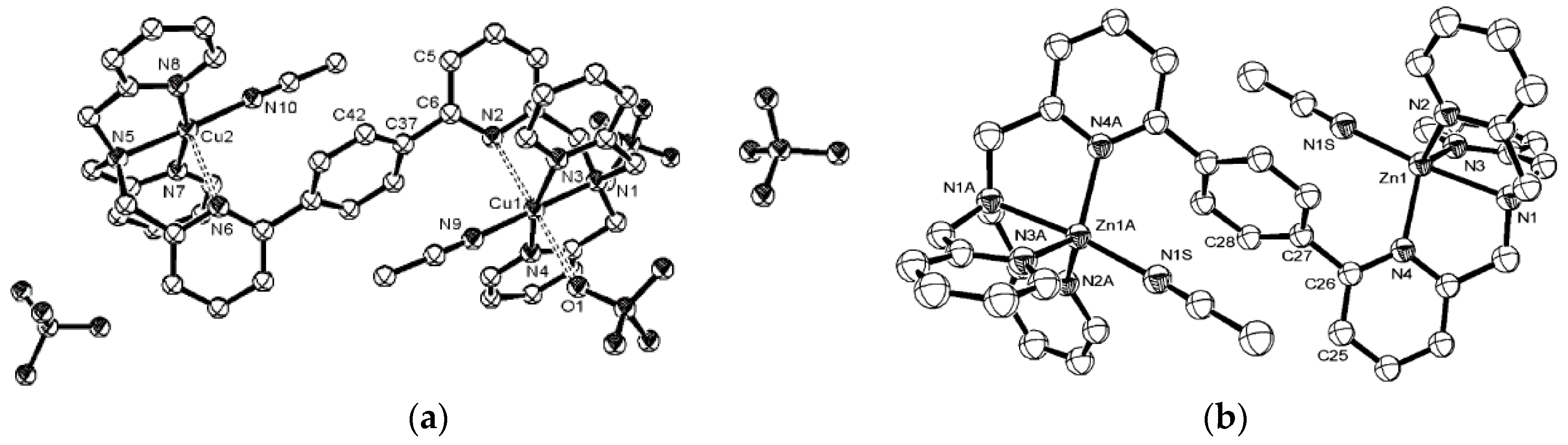
4. Modulating Two Binding Pockets in One Ligand
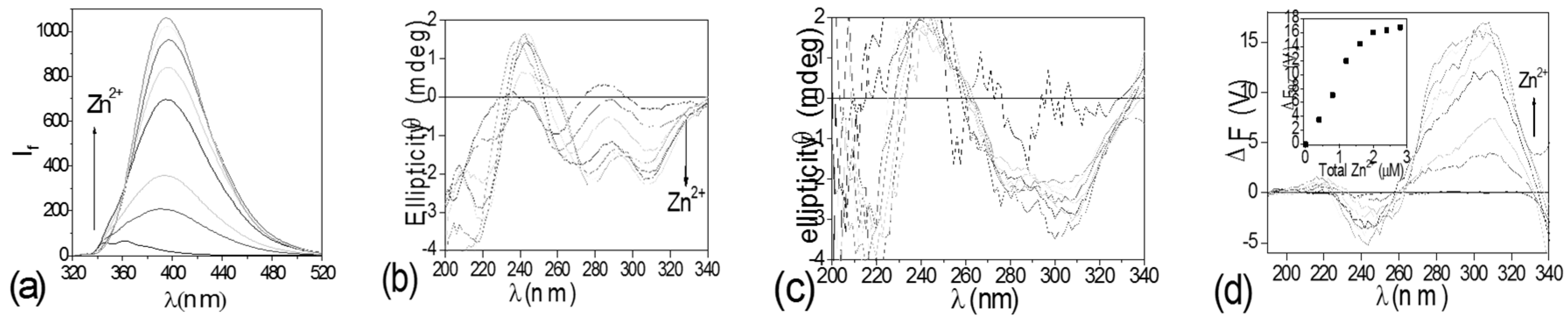
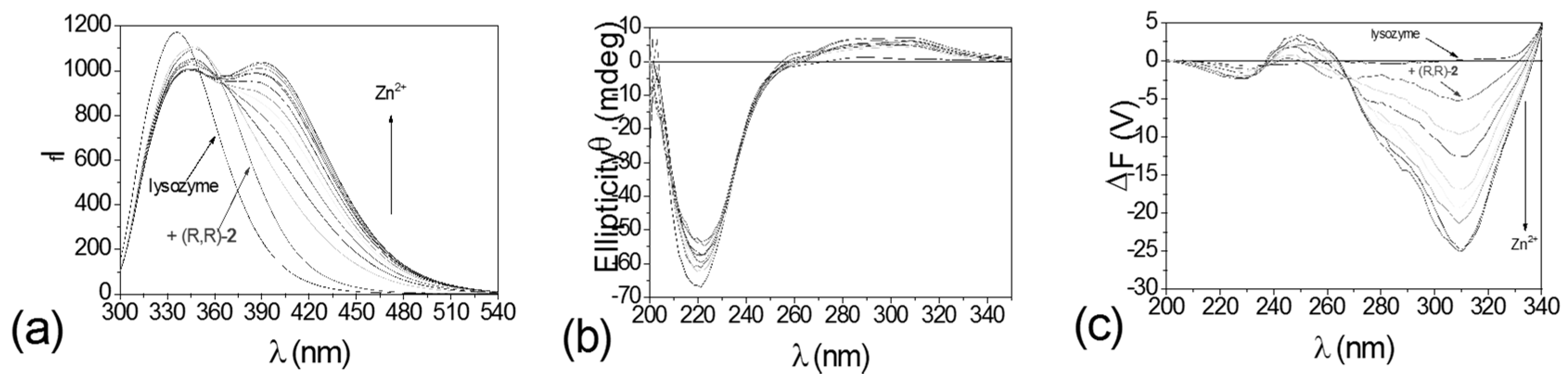
4.1. Modulating Ditoptic Ligands for Zn2+
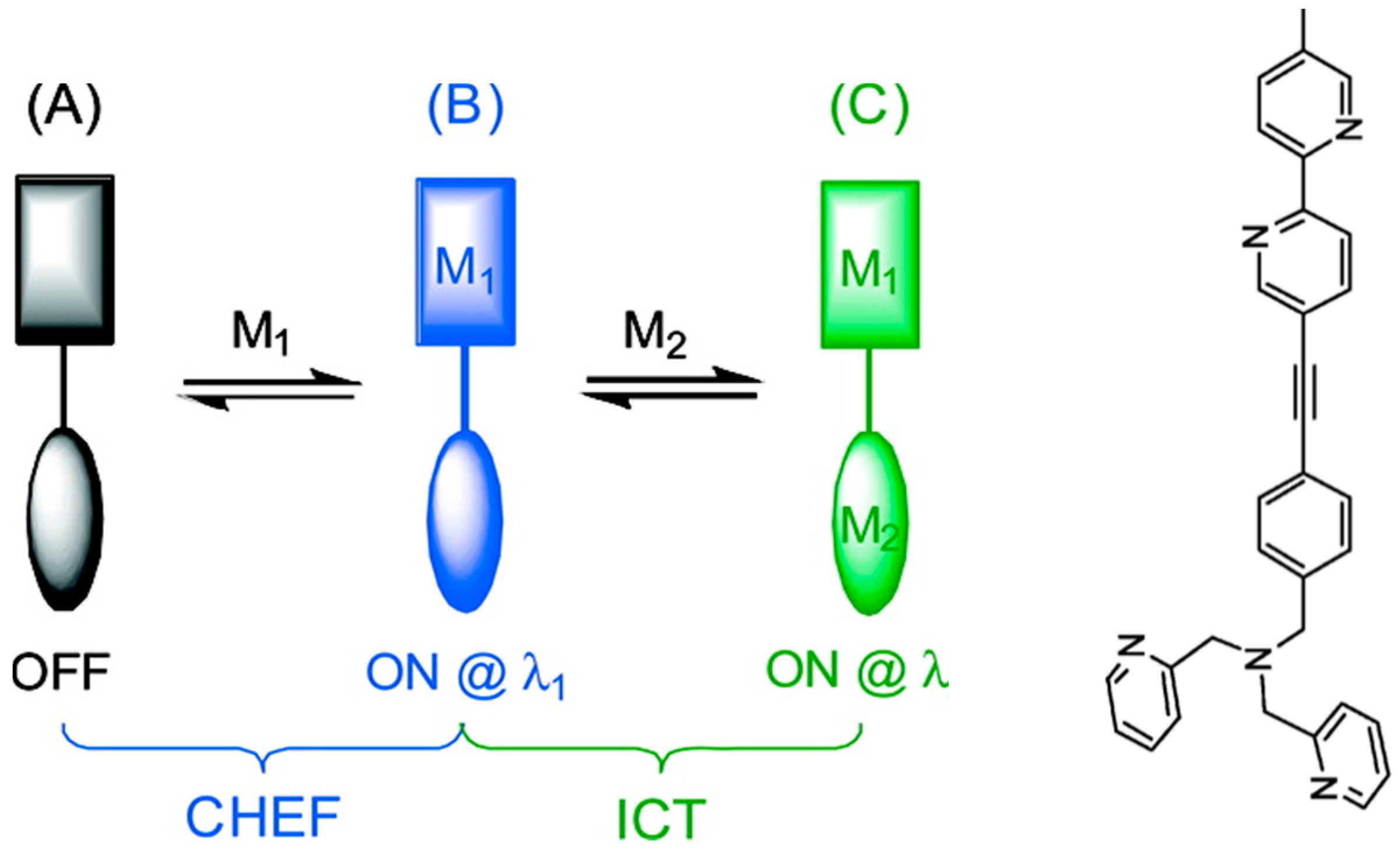
4.2. Modulating Switchable Binding Pockets in One Ligand
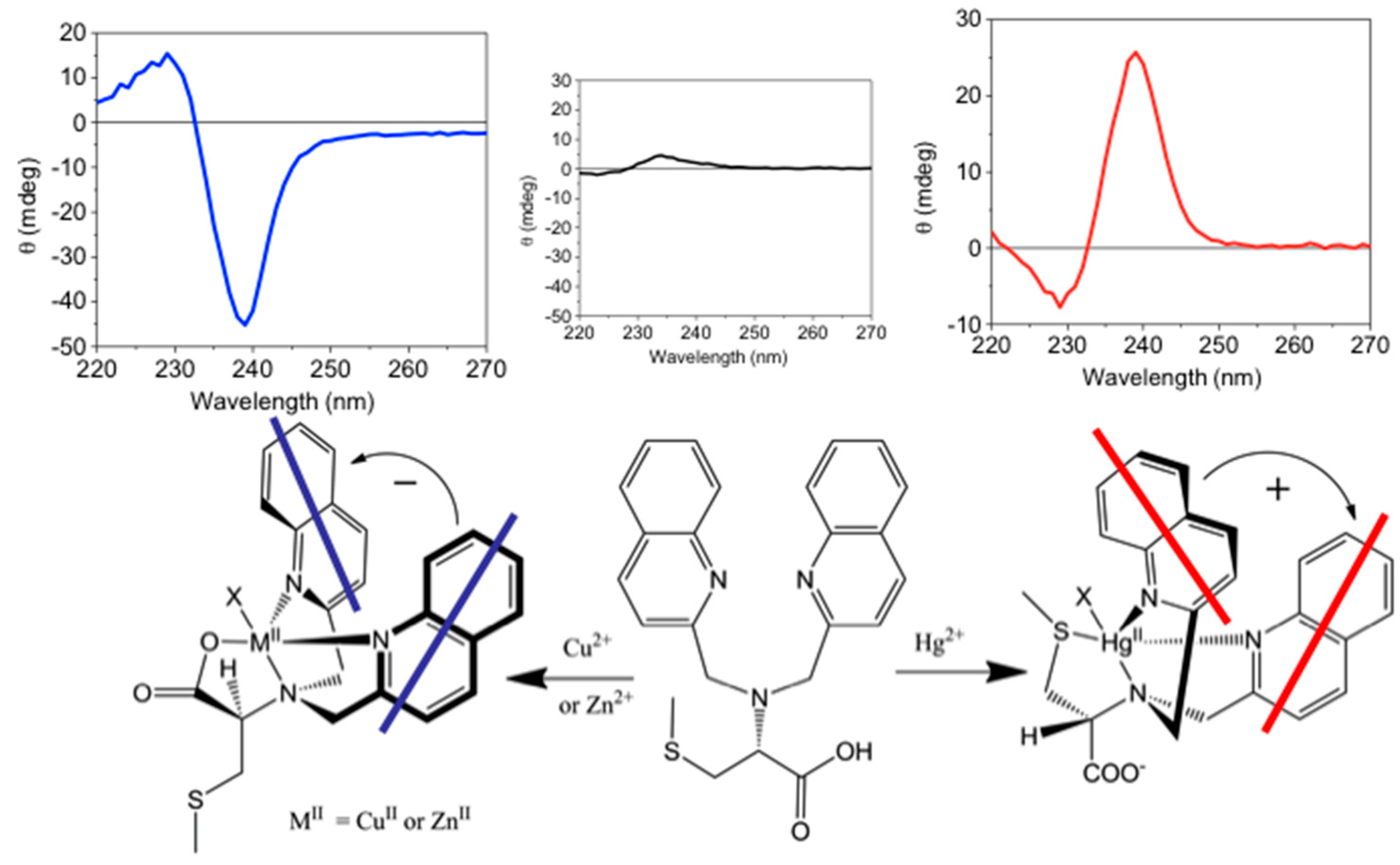
4.3. Modulating Switchable Chiroptical Sensors for Metal Oxidation States
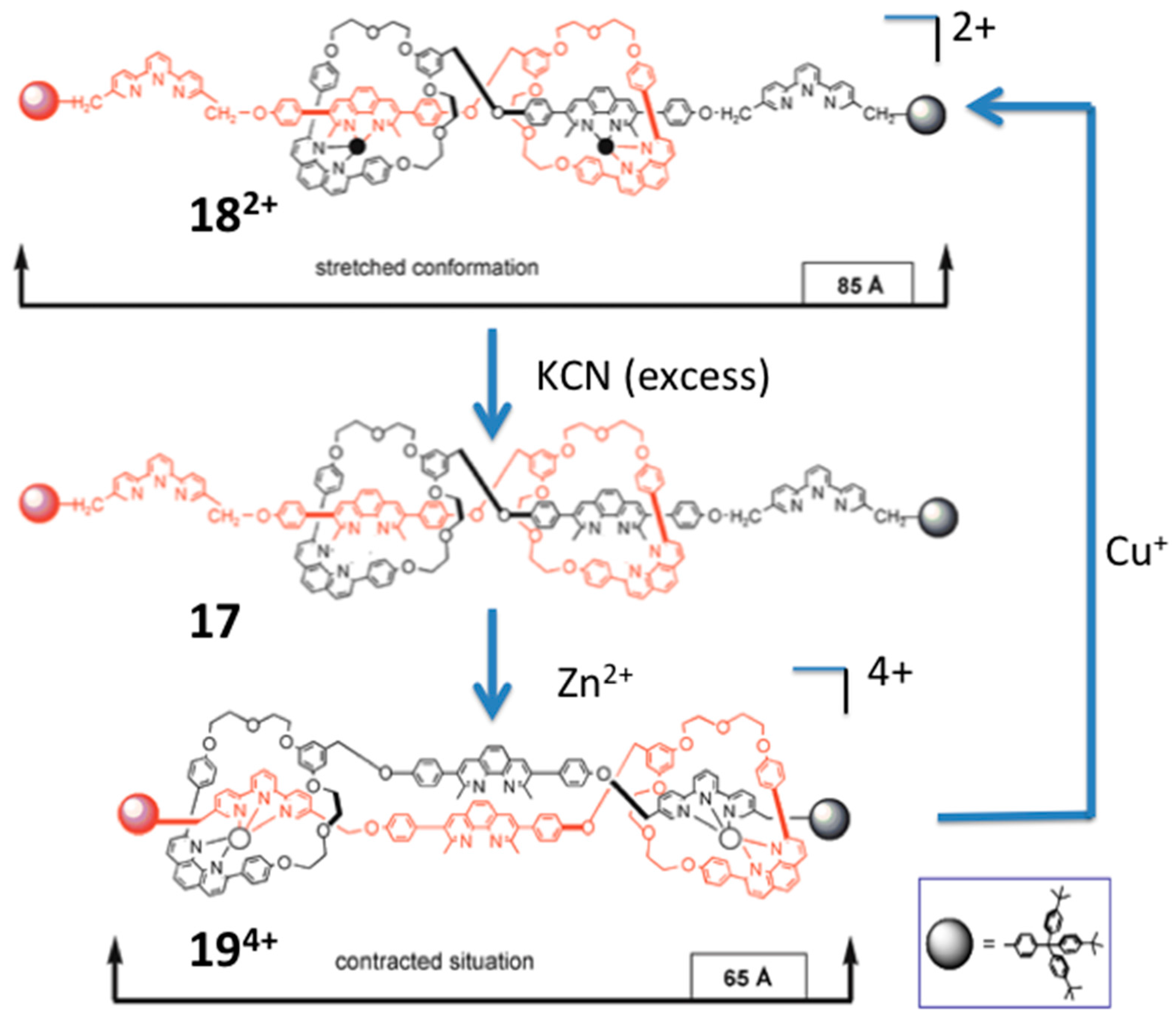
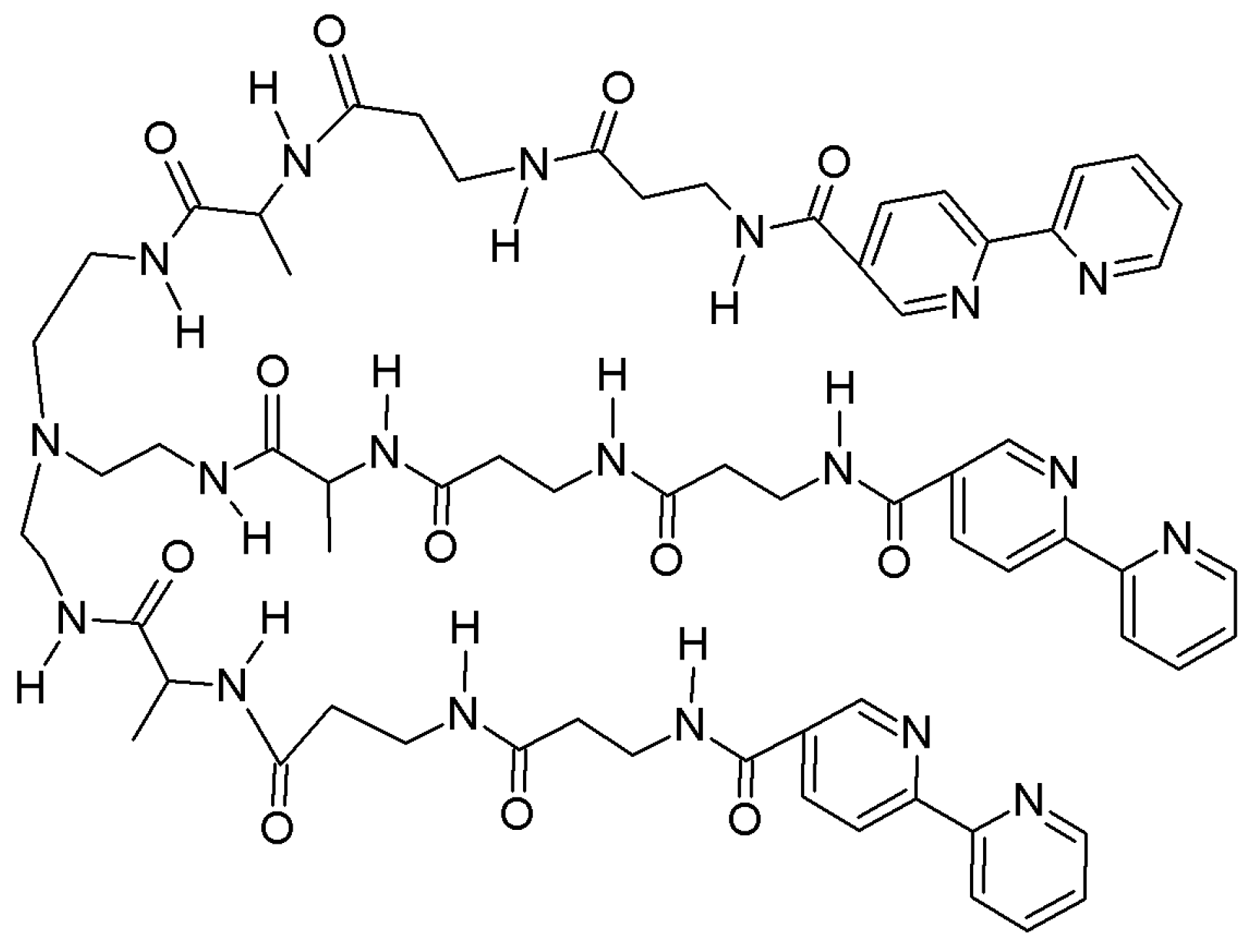
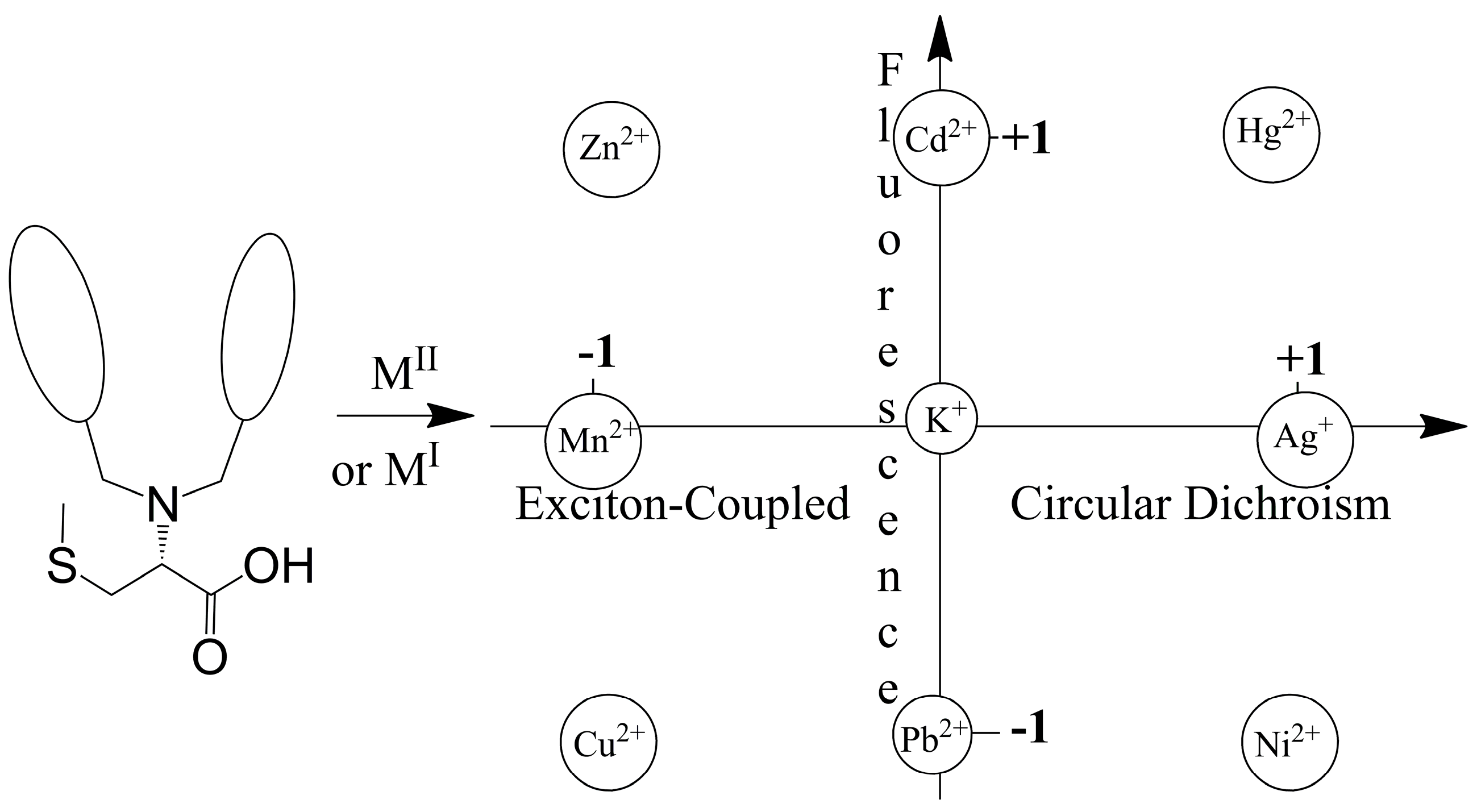
4.4. Modulating Switchable Chiroptical Sensors for Multiple Metal Ions
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Kwong, H.-L.; Yeung, H.-L.; Yeung, C.-T.; Lee, W.-S.; Lee, C.-S.; Wong, W.-L. Chiral pyridine-containing ligands in asymmetric catalysis. Coord. Chem. Rev. 2007, 251, 2188–2222. [Google Scholar] [CrossRef]
- Hancock, R.D. The pyridyl group in ligand design for selective metal ion complexation and sensing. Chem. Soc. Rev. 2013, 42, 1500–1524. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Younes, A.H.; Yuan, Z.; Clark, R.J. 5-Arylvinylene-2,2′-bipyridyls: Bright “push–pull” dyes as components in fluorescent indicators for zinc ions. J. Photochem. Photobiol. A Chem. 2015, 311, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Younes, A.H.; Allen, J.R.; Davidson, M.W.; Zhu, L. Enhancing the photostability of arylvinylenebipyridyl compounds as fluorescent indicators for intracellular Zinc(II) ions. J. Org. Chem. 2015, 80, 5600–5610. [Google Scholar] [CrossRef] [PubMed]
- Sreenath, K.; Yuan, Z.; Allen, J.R.; Davidson, M.W.; Zhu, L. A fluorescent indicator for imaging lysosomal Zinc(II) with Förster resonance energy transfer (FRET)-enhanced photostability and a narrow band of emission. Chem. Eur. J. 2015, 21, 867–874. [Google Scholar] [CrossRef] [PubMed]
- Younes, A.H.; Zhu, L. Tunable dual fluorescence of 3-(2,2′-bipyridyl)-substituted iminocoumarin. ChemPhysChem 2012, 13, 3827–3835. [Google Scholar] [CrossRef] [PubMed]
- Comba, P. Metal ion selectivity and molecular modeling. Coord. Chem. Rev. 1999, 185–186, 81–98. [Google Scholar] [CrossRef]
- Erickson, S.D.; Still, W.C. On the stereochemical control of complexation in tetrahydropyranoid podand ionophores. Tetrahedron Lett. 1990, 31, 4253–4256. [Google Scholar] [CrossRef]
- Hirano, T.; Kikuchi, K.; Urano, Y.; Nagano, T. Improvement and biological applications of fluorescent probes for zinc, ZnAFs. J. Am. Chem. Soc. 2002, 124, 6555–6562. [Google Scholar] [CrossRef] [PubMed]
- Burdette, S.C.; Lippard, S.J. Meeting of the minds: Metalloneurochemistry. Proc. Natl. Acad. Sci. USA 2003, 100, 3605–3610. [Google Scholar] [CrossRef] [PubMed]
- Walkup, G.K.; Imperiali, B. Fluorescent chemosensors for divalent Zinc based on Zinc finger domains. Enhanced oxidative stability, metal binding affinity, and structural and functional characterization. J. Am. Chem. Soc. 1997, 119, 3443–3450. [Google Scholar] [CrossRef]
- Fahrni, C.J.; O’Halloran, T.V. Aqueous coordination chemistry of quinoline-based fluorescence probes for the biological chemistry of Zinc. J. Am. Chem. Soc. 1999, 121, 11448–11458. [Google Scholar] [CrossRef]
- Burdette, S.C.; Frederickson, C.J.; Bu, W.; Lippard, S.J. Zp4, an improved neuronal Zn2+ sensor of the Zinpyr family. J. Am. Chem. Soc. 2003, 125, 1778–1787. [Google Scholar] [CrossRef] [PubMed]
- Nolan, E.M.; Lippard, S.J. Small-molecule fluorescent sensors for investigating Zinc metalloneurochemistry. Acc. Chem. Res. 2009, 42, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Palmer, A.E.; Miranda, J.G.; Carter, K.P. Zinc: Fluorescent sensors. In Encyclopedia of Inorganic and Bioinorganic Chemistry; John Wiley & Sons, Ltd.: New York, NY, USA, 2011. [Google Scholar]
- Chyan, W.; Zhang, D.Y.; Lippard, S.J.; Radford, R.J. Reaction-based fluorescent sensor for investigating mobile Zn2+ in mitochondria of healthy versus cancerous prostate cells. Proc. Natl. Acad. Sci. USA 2014, 111, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Burdette, S.C.; Walkup, G.K.; Spingler, B.; Tsien, R.Y.; Lippard, S.J. Fluorescent sensors for Zn2+ based on a fluorescein platform: Synthesis, properties and intracellular distribution. J. Am. Chem. Soc. 2001, 123, 7831–7841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Radford, R.J.; Chyan, W.; Lippard, S.J. Peptide-based targeting of fluorescent zinc sensors to the plasma membrane of live cells. Chem. Sci. 2013, 4, 3080–3084. [Google Scholar] [CrossRef] [PubMed]
- Mikata, Y.; Yamashita, A.; Kawamura, A.; Konno, H.; Miyamoto, Y.; Tamotsu, S. Bisquinoline-based fluorescent Zinc sensors. Dalton Trans. 2009, 3800–3806. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Cao, M.; Li, J.; Wang, J. A sensitive ratiometric fluorescent sensor for Zinc(II) with high selectivity. Sensors 2013, 13, 3131–3134. [Google Scholar] [CrossRef] [PubMed]
- Kimura, E.; Koike, T. Recent development of Zinc-fluorophores. Chem. Soc. Rev. 1998, 27, 179–184. [Google Scholar] [CrossRef]
- Irving, H.; Williams, R.J.P. Order of stability of metal complexes. Nature 1948, 162, 746–747. [Google Scholar] [CrossRef]
- Martell, A.E.; Hancock, R.D. Metal Complexes in Aqueous Solutions; Plenum Press: New York, NY, USA, 1996. [Google Scholar]
- Koike, T.; Watanabe, T.; Aoki, S.; Kimura, E.; Shiro, M. A novel biomimetic Zinc(II)−fluorophore, dansylamidoethyl−pendant macrocyclic tetraamine 1,4,7,10-tetraazacyclododecane (cyclen). J. Am. Chem. Soc. 1996, 118, 12696–12703. [Google Scholar] [CrossRef]
- Jimenez-Sanchez, A.; Ortiz, B.; Ortiz Navarrete, V.; Farfan, N.; Santillan, R. Two fluorescent schiff base sensors for Zn2+: The Zn2+/Cu2+ ion interference. Analyst 2015, 140, 6031–6039. [Google Scholar] [CrossRef] [PubMed]
- Ambundo, E.A.; Deydier, M.-V.; Grall, A.J.; Aguera-Vega, N.; Dressel, L.T.; Cooper, T.H.; Heeg, M.J.; Ochrymowycz, L.A.; Rorabacher, D.B. Influence of coordination geometry upon copper(II/I) redox potentials. Physical parameters for twelve copper tripodal ligand complexes. Inorg. Chem. 1999, 38, 4233–4242. [Google Scholar] [CrossRef]
- Cotton, F.A.; Wilkinson, G.; Gaus, P. Basic Inorganic Chemistry, 3rd ed.; Wiley: New York, NY, USA, 1995. [Google Scholar]
- Zhu, L.; dos Santos, O.; Koo, C.W.; Rybstein, M.; Pape, L.; Canary, J.W. Geometry-dependent phosphodiester hydrolysis catalyzed by binuclear copper complexes. Inorg. Chem. 2003, 42, 7912–7920. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Holmes, A.E.; Sharma, A.; Brooks, N.R.; Rarig, R.S.; Zubieta, J.; Canary, J.W. Derivatization, complexation, and absolute configurational assignment of chiral primary amines: Application of exciton-coupled circular dichroism. Chirality 2003, 15, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Kojima, T.; Leising, R.A.; Yan, S.; Que, L. Alkane functionalization at nonheme iron centers. Stoichiometric transfer of metal-bound ligands to alkane. J. Am. Chem. Soc. 1993, 115, 11328–11335. [Google Scholar] [CrossRef]
- Toftlund, H.; Larsen, S.; Murray, K.S. Synthesis and spectroscopic and magnetic properties of a unique diamagnetic binuclear .Mu.-oxo vanadium(IV) complex. Crystal structure of [(tpa)VO(.Mu.-O)VO(tpa)](ClO4)2 (tpa = tris(2-pyridylmethyl)amine). Inorg. Chem. 1991, 30, 3964–3967. [Google Scholar] [CrossRef]
- Wei, N.; Murthy, N.N.; Karlin, K.D. Chemistry of pentacoordinate [LCuII-Cl]+ complexes with quinolyl containing tripodal tetradentate ligands L. Inorg. Chem. 1994, 33, 6093–6100. [Google Scholar] [CrossRef]
- Williams, N.J.; Gan, W.; Reibenspies, J.H.; Hancock, R.D. Possible steric control of the relative strength of chelation enhanced fluorescence for Zinc(II) compared to Cadmium(II): Metal ion complexing properties of tris(2-quinolylmethyl)amine, a crystallographic, UV-Visible, and fluorometric study. Inorg. Chem. 2009, 48, 1407–1415. [Google Scholar] [CrossRef] [PubMed]
- Frederickson, C.J.; Kasarskis, E.J.; Ringo, D.; Frederickson, R.E. A quinoline fluorescence method for visualizing and assaying the histochemically reactive zinc (bouton Zinc) in the brain. J. Neurosci. Methods 1987, 20, 91–103. [Google Scholar] [CrossRef]
- Koh, J.-Y.; Suh, S.W.; Gwag, B.J.; He, Y.Y.; Hsu, C.Y.; Choi, D.W. The role of zinc in selective neuronal death after transient global cerebral ischemia. Science 1996, 272, 1013–1016. [Google Scholar] [CrossRef] [PubMed]
- Snitsarev, V.; Budde, T.; Stricker, T.P.; Cox, J.M.; Krupa, D.J.; Geng, L.; Kay, A.R. Fluorescent detection of Zn(2+)-rich vesicles with zinquin: Mechanism of action in lipid environments. Biophys. J. 2001, 80, 1538–1546. [Google Scholar] [CrossRef]
- Nasir, S.M.; Fahrni, J.C.; Suhy, A.D.; Kolodsick, J.K.; Singer, P.C.; O’Halloran, V.T. The chemical cell biology of zinc: Structure and intracellular fluorescence of a Zinc-quinolinesulfonamide complex. J. Biol. Inorg. Chem. 1999, 4, 775–783. [Google Scholar] [CrossRef] [PubMed]
- Iimori, T.; Erickson, S.D.; Rheingold, A.L.; Still, W.C. Enantioselective complexation with a conformationally homogeneous C2 podand ionophore. Tetrahedron Lett. 1989, 30, 6947–6950. [Google Scholar] [CrossRef]
- Still, W.C.; Hauck, P.; Kempf, D. Stereochemical studies of lasalocid epimers. Ion-driven epimerizations. Tetrahedron Lett. 1987, 28, 2817–2820. [Google Scholar] [CrossRef]
- Smith, P.W.; Still, W.C. The effect of substitution and stereochemistry on ion binding in the polyether ionophore monensin. J. Am. Chem. Soc. 1988, 110, 7917–7919. [Google Scholar] [CrossRef]
- Sasaki, S.; Naito, H.; Maruta, K.; Kawahara, E.; Maeda, M. Novel calcium ionophores: Supramolecular complexation by the hydroxylated-bistetrahydrofuran skeleton of potent antitumor annonaceous acetogenins. Tetrahedron Lett. 1994, 35, 3337–3340. [Google Scholar] [CrossRef]
- Krizek, B.A.; Merkle, D.L.; Berg, J.M. Ligand variation and metal binding specificity in zinc finger peptides. Inorg. Chem. 1993, 32, 937–940. [Google Scholar] [CrossRef]
- Walkup, G.K.; Imperiali, B. Derivatives of 8-hydroxy-2-methylquinoline are powerful prototypes for Zinc sensors in biological systems. J. Org. Chem. 1998, 63, 6727–6731. [Google Scholar] [CrossRef]
- Shibutani, Y.; Mino, S.; Long, S.S.; Moriuchi-Kawakami, T.; Yakabe, K.; Shono, T. Chiral bis(12-crown-4)-based electrodes for sodium ion. Chem. Lett. 1997, 26, 49–50. [Google Scholar] [CrossRef]
- Choi, J.K.; Sargsyan, G.; Olive, A.M.; Balaz, M. Highly sensitive and selective spectroscopic detection of Mercury(II) in water by using pyridylporphyrin–DNA conjugates. Chem. Eur. J. 2013, 19, 2515–2522. [Google Scholar] [CrossRef] [PubMed]
- Castagnetto, J.M.; Canary, J. A chiroptically enhanced fluorescent chemosensor. Chem. Commun. 1998, 203–204. [Google Scholar] [CrossRef]
- Anderegg, G.; Wenk, F. Pyridine derivatives as complexing agents. VIII. Preparation of a new quadridentate and a new hexadentate ligand. Helv. Chim. Acta 1967, 50, 2330–2332. [Google Scholar] [CrossRef]
- Zahn, S.; Canary, J.W. Cu(I/II) redox control of molecular conformation and shape in chiral tripodal ligands: Binary exciton-coupled circular dichroic states. J. Am. Chem. Soc. 2002, 124, 9204–9211. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Xu, X.; Canary, J.W. Stereochemical control of Zn(II)/Cu(II) selectivity in piperidine tripod ligands. Chem. Commun. 2002, 1414–1415. [Google Scholar] [CrossRef]
- Xu, X. Design and Synthesis of Enantiosensors Based on Metal-Ligand Complexes. Ph.D. Thesis, New York University, New York, NY, USA, 2000. [Google Scholar]
- Dai, Z. Chiral Tripodal Ligands in Zinc Sensing. Ph.D. Thesis, New York University, New York, NY, USA, 2004. [Google Scholar]
- Dai, Z.; Proni, G.; Mancheno, D.; Karimi, S.; Berova, N.; Canary, J.W. Detection of zinc ion by differential circularly polarized fluorescence excitation. J. Am. Chem. Soc. 2004, 126, 11760–11761. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Hundal, M.S.; Kaur, N.; Singh, R.; Singh, H.; Hundal nee Sood, G.; Ripoll, M.M.; Aparicio, J.S. Synthetic ionophores. 13. Pyridine–diamide–diester receptors: Remarkable effect of amide substituents on molecular organization and Ag+ selectivity. J. Org. Chem. 1996, 61, 7819–7825. [Google Scholar] [CrossRef] [PubMed]
- Tsukube, H.; Shinoda, S.; Uenishi, J.i.; Hiraoka, T.; Imakoga, T.; Yonemitsu, O. Ag+-specific pyridine podands: Effects of ligand geometry and stereochemically controlled substitution on cation complexation and transport functions. J. Org. Chem. 1998, 63, 3884–3894. [Google Scholar] [CrossRef]
- Tsukube, H.; Yamada, T.; Shinoda, S. Chirality technology in metal separation: Stereochemical design of Ag+ ion-specific ionophores for practical membrane separation. Ind. Eng. Chem. Res. 2000, 39, 3412–3418. [Google Scholar] [CrossRef]
- Nolan, E.M.; Lippard, S.J. A “turn-on” fluorescent sensor for the selective detection of mercuric ion in aqueous media. J. Am. Chem. Soc 2003, 125, 14270–14271. [Google Scholar] [CrossRef] [PubMed]
- Nolan, E.M.; Racine, M.E.; Lippard, S.J. Selective Hg(II) detection in aqueous solution with thiol derivatized fluoresceins. Inorg. Chem. 2006, 45, 2742–2749. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.; Albers, A.E.; Wong, A.P.; Chang, C.J. Screening mercury levels in fish with a selective fluorescent chemosensor. J. Am. Chem. Soc. 2005, 127, 16030–16031. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Qian, X.; Jia, L. A highly selective and sensitive fluorescent chemosensor for Hg2+ in neutral buffer aqueous solution. J. Am. Chem. Soc. 2004, 126, 2272–2273. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Lee, H.-S.; Reibenspies, J.H.; Hancock, R.D. Mechanism of “turn-on” fluorescent sensors for mercury(II) in solution and its implications for ligand design. Inorg. Chem. 2012, 51, 10904–10915. [Google Scholar] [CrossRef] [PubMed]
- Carney, P.; Lopez, S.; Mickley, A.; Grinberg, K.; Zhang, W.; Dai, Z. Multimode selective detection of mercury by chiroptical fluorescent sensors based on methionine/cysteine. Chirality 2011, 23, 916–920. [Google Scholar] [CrossRef] [PubMed]
- Yamada, T.; Shinoda, S.; Sugimoto, H.; Uenishi, J.-I.; Tsukube, H. Luminescent lanthanide complexes with stereocontrolled tris(2-pyridylmethyl)amine ligands: Chirality effects on lanthanide complexation and luminescence properties. Inorg. Chem. 2003, 42, 7932–7937. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K.; Suzuki, K.; Tsukuda, T.; Tsubomura, T. A chiral 2,6-bis(oxazolinyl)pyridine ligand with amide groups to form isomorphous complexes through all the lanthanoid series. Inorg. Chem. 2010, 49, 4717–4719. [Google Scholar] [CrossRef] [PubMed]
- Masaki, M.E.; Paul, D.; Nakamura, R.; Kataoka, Y.; Shinoda, S.; Tsukube, H. Chiral tripode approach toward multiple anion sensing with lanthanide complexes. Tetrahedron 2009, 65, 2525–2530. [Google Scholar] [CrossRef]
- Canary, J.W.; Mortezaei, S.; Liang, J. Transition metal-based chiroptical switches for nanoscale electronics and sensors. Coord. Chem. Rev. 2010, 254, 2249–2266. [Google Scholar] [CrossRef]
- Ikeda, M.; Matsu-ura, A.; Kuwahara, S.; Lee, S.S.; Habata, Y. Hg2+-sensing system based on structures of complexes. Org. Lett. 2012, 14, 1564–1567. [Google Scholar] [CrossRef] [PubMed]
- Du, G.; Liu, K.; Liu, B.; Ye, L.; Jiang, L. A chiroptical chemodosimeter for fast and specific detection of Mercury(II) ions in aqueous media. Anal. Methods 2015, 7, 8550–8553. [Google Scholar] [CrossRef]
- Caricato, M.; Leza, N.J.; Roy, K.; Dondi, D.; Gattuso, G.; Shimizu, L.S.; Vander Griend, D.A.; Pasini, D. A chiroptical probe for sensing metal ions in water. Eur. J. Org. Chem. 2013, 2013, 6078–6083. [Google Scholar] [CrossRef]
- Zhu, Y.; Xu, L.; Ma, W.; Xu, Z.; Kuang, H.; Wang, L.; Xu, C. A one-step homogeneous plasmonic circular dichroism detection of aqueous mercury ions using nucleic acid functionalized gold nanorods. Chem. Commun. 2012, 48, 11889–11891. [Google Scholar] [CrossRef] [PubMed]
- Nehira, T.; Parish, C.A.; Jockusch, S.; Turro, N.J.; Nakanishi, K.; Berova, N. Fluoresence-detected exciton-coupled circular dichroism: Scope and limitation in structural studies of organic molecules. J. Am. Chem. Soc. 1999, 121, 8681–8691. [Google Scholar] [CrossRef]
- Zhang, L.; Clark, R.J.; Zhu, L. A heteroditopic fluoroionophoric platform for constructing fluorescent probes with large dynamic ranges for zinc ions. Chem. Eur. J. 2008, 14, 2894–2903. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhu, L. Photochemically stable fluorescent heteroditopic ligands for Zinc ion. J. Org. Chem. 2008, 73, 8321–8330. [Google Scholar] [CrossRef] [PubMed]
- Wandell, R.J.; Younes, A.H.; Zhu, L. Metal-coordination-mediated sequential chelation-enhanced fluorescence (CHEF) and fluorescence resonance energy transfer (FRET) in a heteroditopic ligand system. New. J. Chem. 2010, 34, 2176–2182. [Google Scholar] [CrossRef]
- Younes, A.H.; Zhang, L.; Clark, R.J.; Davidson, M.W.; Zhu, L. Electronic structural dependence of the photophysical properties of fluorescent heteroditopic ligands—Implications in designing molecular fluorescent indicators. Org. Biomol. Chem. 2010, 8, 5431–5441. [Google Scholar] [CrossRef] [PubMed]
- Kuang, G.-C.; Allen, J.R.; Baird, M.A.; Nguyen, B.T.; Zhang, L.; Morgan, T.J.; Levenson, C.W.; Davidson, M.W.; Zhu, L. Balance between fluorescence enhancement and association affinity in fluorescent heteroditopic indicators for imaging zinc ion in living cells. Inorg. Chem. 2011, 50, 10493–10504. [Google Scholar] [CrossRef] [PubMed]
- Sreenath, K.; Clark, R.J.; Zhu, L. Tricolor emission of a fluorescent heteroditopic ligand over a concentration gradient of Zinc(II) ions. J. Org. Chem. 2012, 77, 8268–8279. [Google Scholar] [CrossRef] [PubMed]
- Sreenath, K.; Yi, C.; Knappenberger, K.L.; Zhu, L. Distinguishing forster resonance energy transfer and solvent-mediated charge-transfer relaxation dynamics in a Zinc(II) indicator: A femtosecond time-resolved transient absorption spectroscopic study. Phys. Chem. Chem. Phys. 2014, 16, 5088–5092. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Yuan, Z.; Simmons, J.T.; Sreenath, K. Zn(II)-coordination modulated ligand photophysical processes—The development of fluorescent indicators for imaging biological Zn(II) ions. RSC Adv. 2014, 4, 20398–20440. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Murphy, C.S.; Kuang, G.-C.; Hazelwood, K.L.; Constantino, M.H.; Davidson, M.W.; Zhu, L. A fluorescent heteroditopic ligand responding to free Zinc ion over six orders of magnitude concentration range. Chem. Commun. 2009, 7408–7410. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, M.C.; Dietrich-Buchecker, C.; Sauvage, J.-P. Towards synthetic molecular muscles: Contraction and stretching of a linear rotaxane dimer. Angew. Chem. Int. Ed. 2000, 39, 3284–3287. [Google Scholar] [CrossRef]
- Jimenez-Molero, M.C.; Dietrich-Buchecker, C.; Sauvage, J.-P. Towards artificial muscles at the nanometric level. Chem. Commun. 2003, 1613–1616. [Google Scholar] [CrossRef]
- Livoreil, A.; Dietrich-Buchecker, C.O.; Sauvage, J.-P. Electrochemically triggered swinging of a [2]-catenate. J. Am. Chem. Soc. 1994, 116, 9399–9400. [Google Scholar] [CrossRef] [PubMed]
- Armaroli, N.; Balzani, V.; Collin, J.-P.; Gaviña, P.; Sauvage, J.-P.; Ventura, B. Rotaxanes incorporating two different coordinating units in their thread: Synthesis and electrochemically and photochemically induced molecular motions. J. Am. Chem. Soc. 1999, 121, 4397–4408. [Google Scholar] [CrossRef]
- Kern, J.-M.; Raehm, L.; Sauvage, J.-P.; Divisia-Blohorn, B.; Vidal, P.-L. Controlled molecular motions in copper-complexed rotaxanes: An XAS study. Inorg. Chem. 2000, 39, 1555–1560. [Google Scholar] [CrossRef] [PubMed]
- Zelikovich, L.; Libman, J.; Shanzer, A. Molecular redox switches based on chemical triggering of iron translocation in triple-stranded helical complexes. Nature 1995, 374, 790–792. [Google Scholar] [CrossRef]
- Zahn, S.; Canary, J.W. Electron-induced inversion of helical chirality in copper complexes of N,N-dialkylmethionines. Science 2000, 288, 1404–1407. [Google Scholar] [CrossRef] [PubMed]
- Canary, J.W. Redox-triggered chiroptical molecular switches. Chem. Soc. Rev. 2009, 38, 747–756. [Google Scholar] [CrossRef] [PubMed]
- Barcena, H.S.; Liu, B.; Mirkin, M.V.; Canary, J.W. An electrochiroptical molecular switch: Mechanistic and kinetic studies. Inorg. Chem. 2005, 44, 7652–7660. [Google Scholar] [CrossRef] [PubMed]
- Das, D.; Dai, Z.; Holmes, A.E.; Canary, J.W. Exploring the scope of redox-triggered chiroptical switches: Syntheses, X-ray structures and circular dichroism of cobalt and nickel complexes of N,N-bis(arylmethyl)methionine derivatives. Chirality 2008, 20, 585–591. [Google Scholar] [CrossRef] [PubMed]
- Zahn, S.; Das, D.; Canary, J.W. Redox-induced ligand reorganization and helicity inversion in copper complexes of N,N-dialkylmethionine derivatives. Inorg. Chem. 2006, 45, 6056–6063. [Google Scholar] [CrossRef] [PubMed]
- Holmes, A.E.; Zahn, S.; Canary, J.W. Synthesis and circular dichroism studies of N,N-bis(2-quinolylmethyl)amino acid Cu(II) complexes: Determination of absolute configuration and enantiomeric excess by the exciton coupling method. Chirality 2002, 14, 471–477. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Dai, Z.; Pace University, New York, NY, USA. Unpublished work. 2016.
- Dai, Z.; Lee, J.; Zhang, W. Chiroptical switches: Applications in sensing and catalysis. Molecules 2012, 17, 1247–1277. [Google Scholar] [CrossRef] [PubMed]
- Meudtner, R.M.; Hecht, S. Helicity inversion in responsive foldamers induced by achiral halide ion guests. Angew. Chem. Int. Ed. 2008, 47, 4926–4930. [Google Scholar] [CrossRef] [PubMed]
- Muller, G. Luminescent chiral Lanthanide(III) complexes as potential molecular probes. Dalton Trans. 2009, 9692–9707. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Mortezaei, S.; Canary, J.W. Spectroscopic analysis: Chiroptical sensors. In Comprehensive Chirality; Carreira, E.M., Yamamoto, H., Eds.; Elsevier: Amsterdam, The Netherlands, 2012; Volume 8, pp. 600–624. [Google Scholar]
- Agnes, M.; Nitti, A.; Vander Griend, D.A.; Dondi, D.; Merli, D.; Pasini, D. A chiroptical molecular sensor for ferrocene. Chem. Comm. 2016, 52, 11492–11495. [Google Scholar] [CrossRef] [PubMed]





© 2016 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, Z. Steric and Stereochemical Modulation in Pyridyl- and Quinolyl-Containing Ligands. Molecules 2016, 21, 1647. https://doi.org/10.3390/molecules21121647
Dai Z. Steric and Stereochemical Modulation in Pyridyl- and Quinolyl-Containing Ligands. Molecules. 2016; 21(12):1647. https://doi.org/10.3390/molecules21121647
Chicago/Turabian StyleDai, Zhaohua. 2016. "Steric and Stereochemical Modulation in Pyridyl- and Quinolyl-Containing Ligands" Molecules 21, no. 12: 1647. https://doi.org/10.3390/molecules21121647





