Combined Use of Zoledronic Acid Augments Ursolic Acid-Induced Apoptosis in Human Osteosarcoma Cells through Enhanced Oxidative Stress and Autophagy
Abstract
:1. Introduction
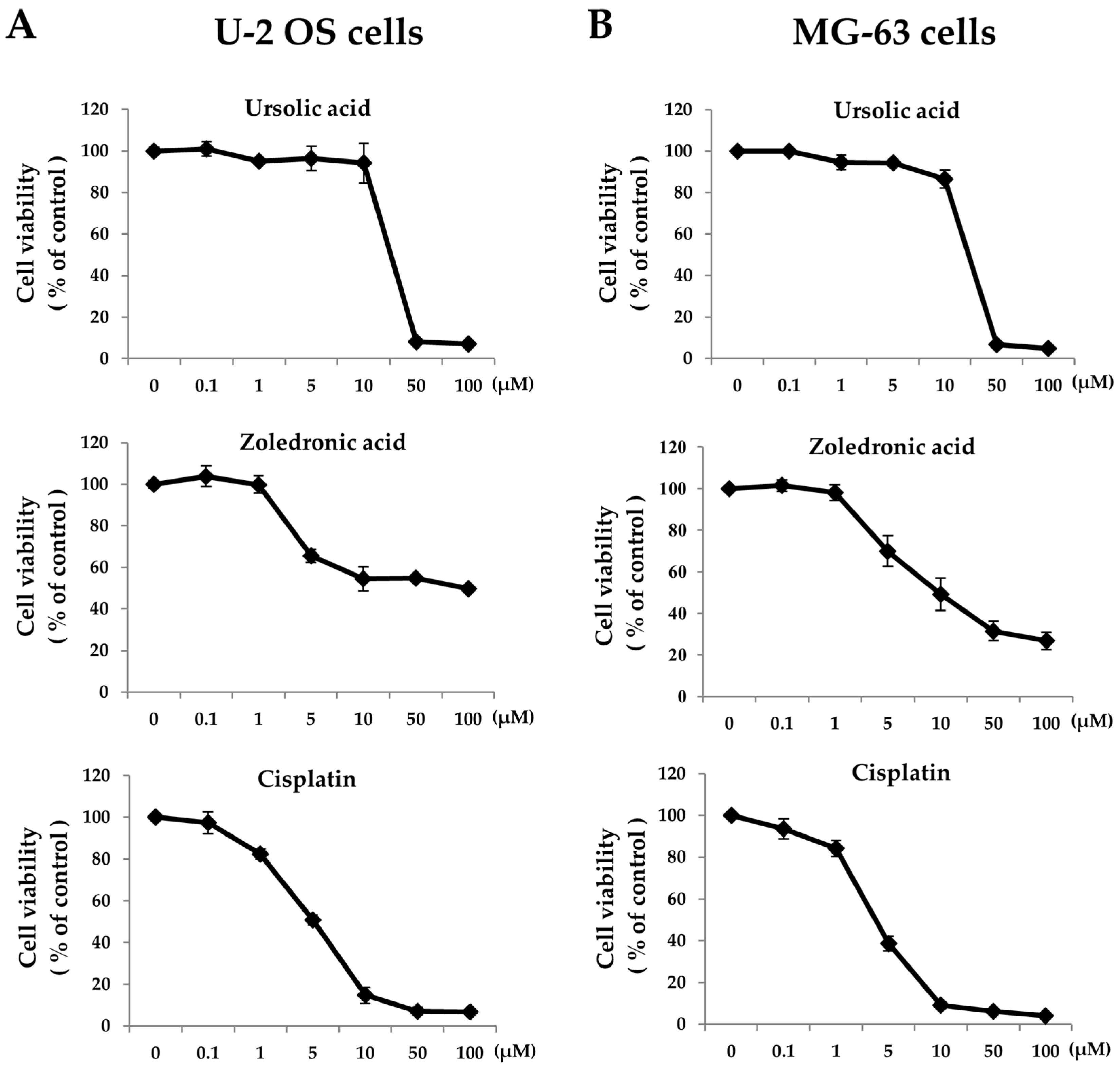
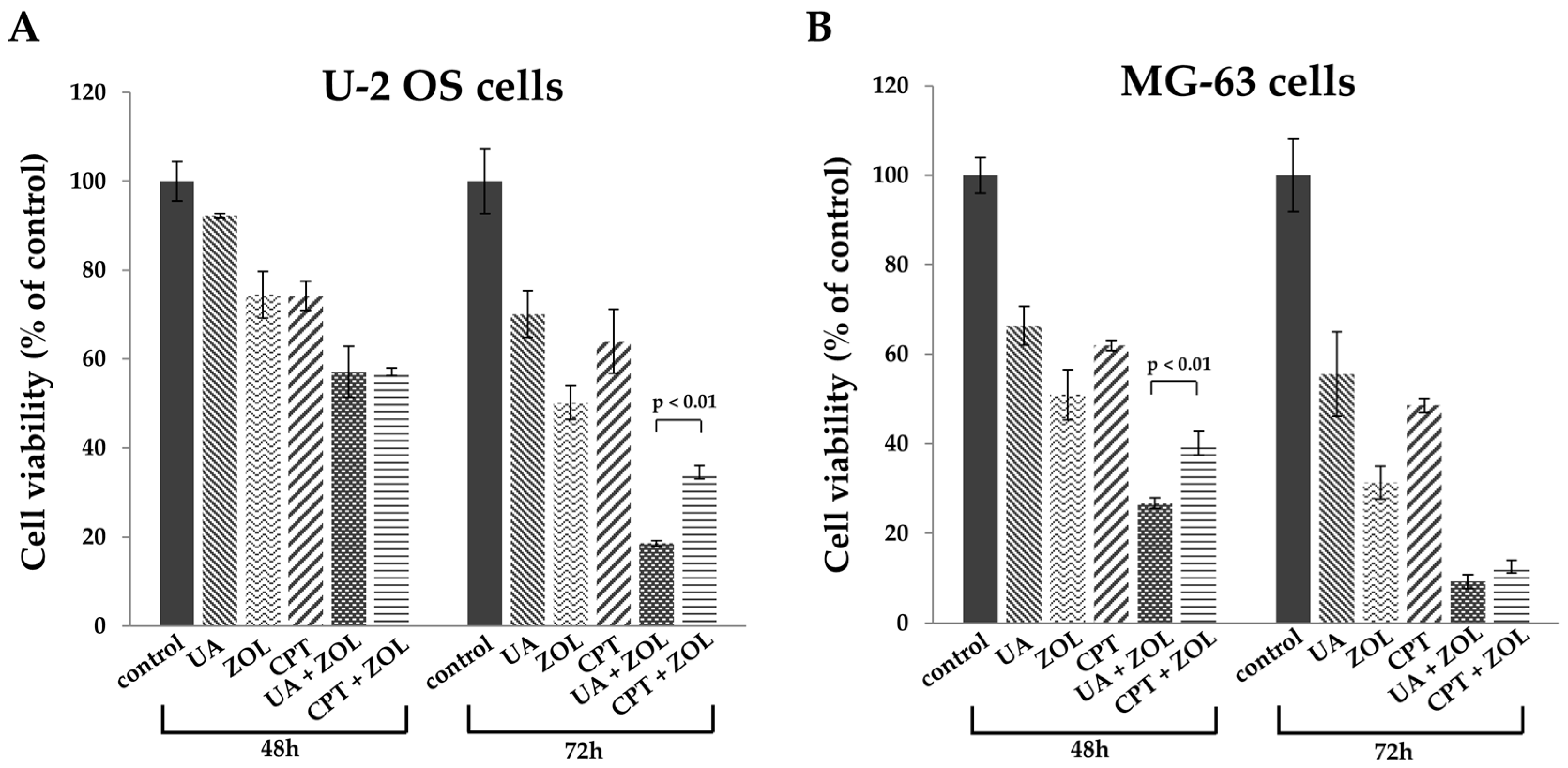
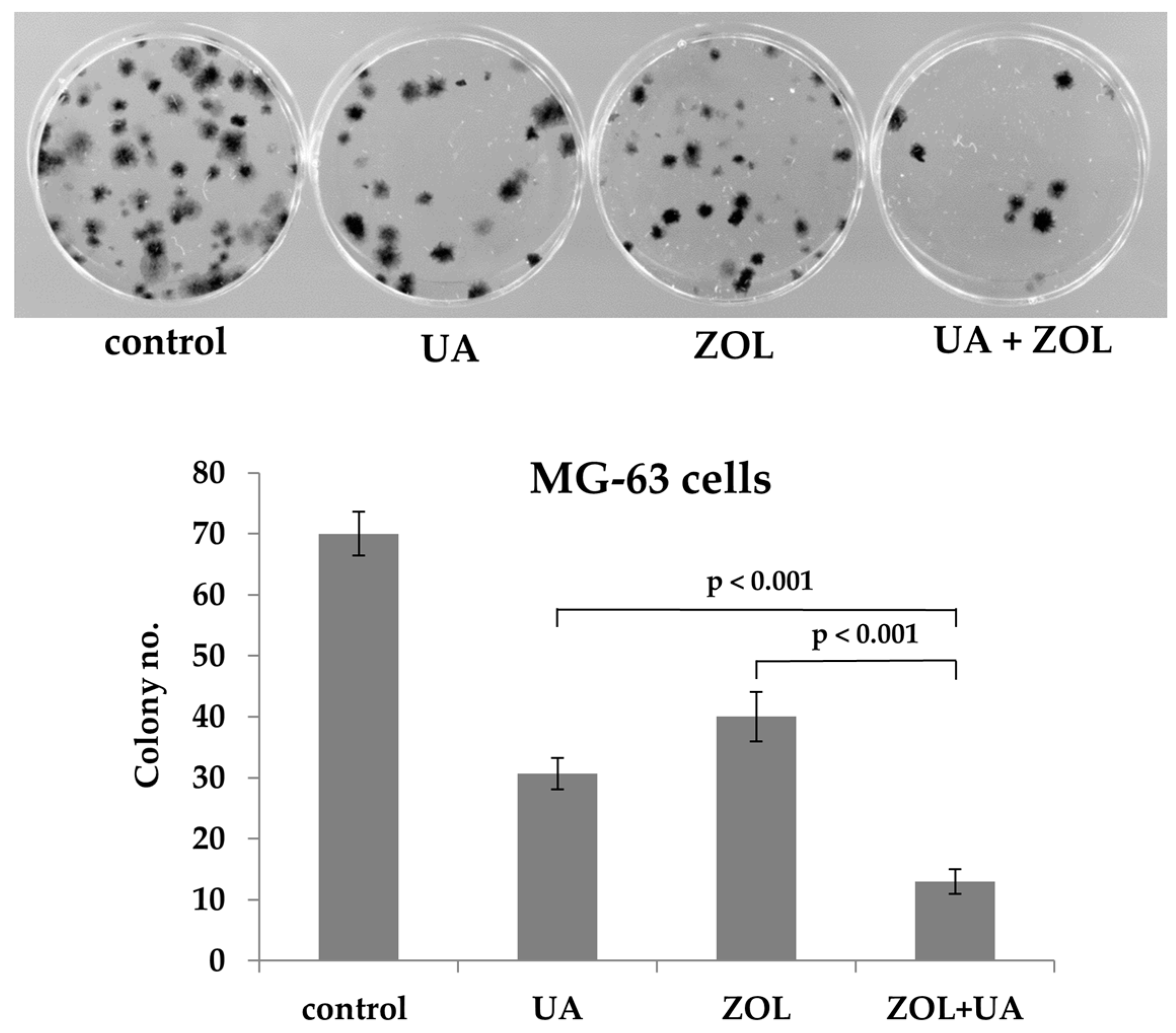
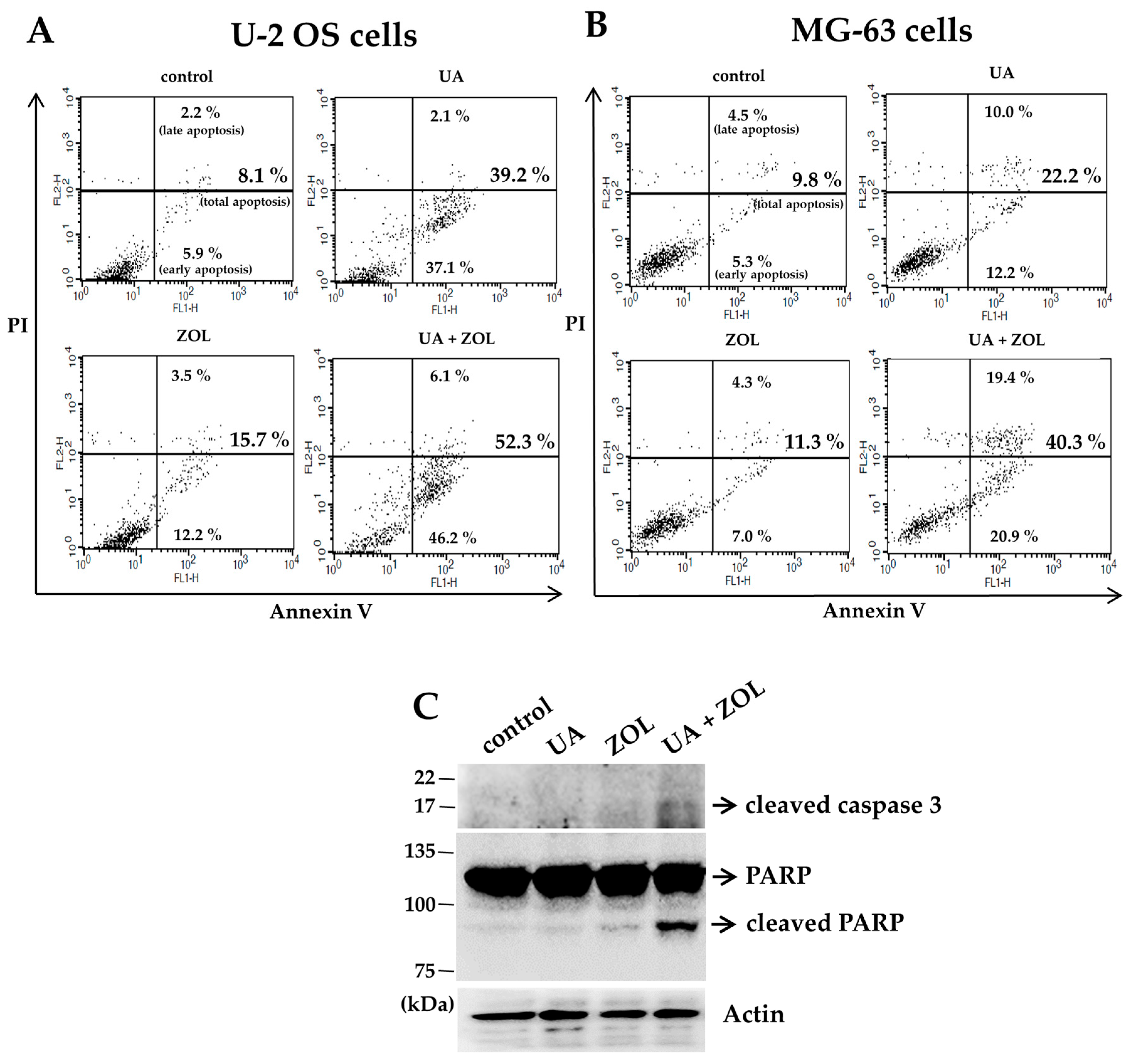
2. Results
2.1. Zoledronic Acid and Ursolic Acid, Alone or in Combination, Mediated Apoptosis in Osteosarcoma Cells
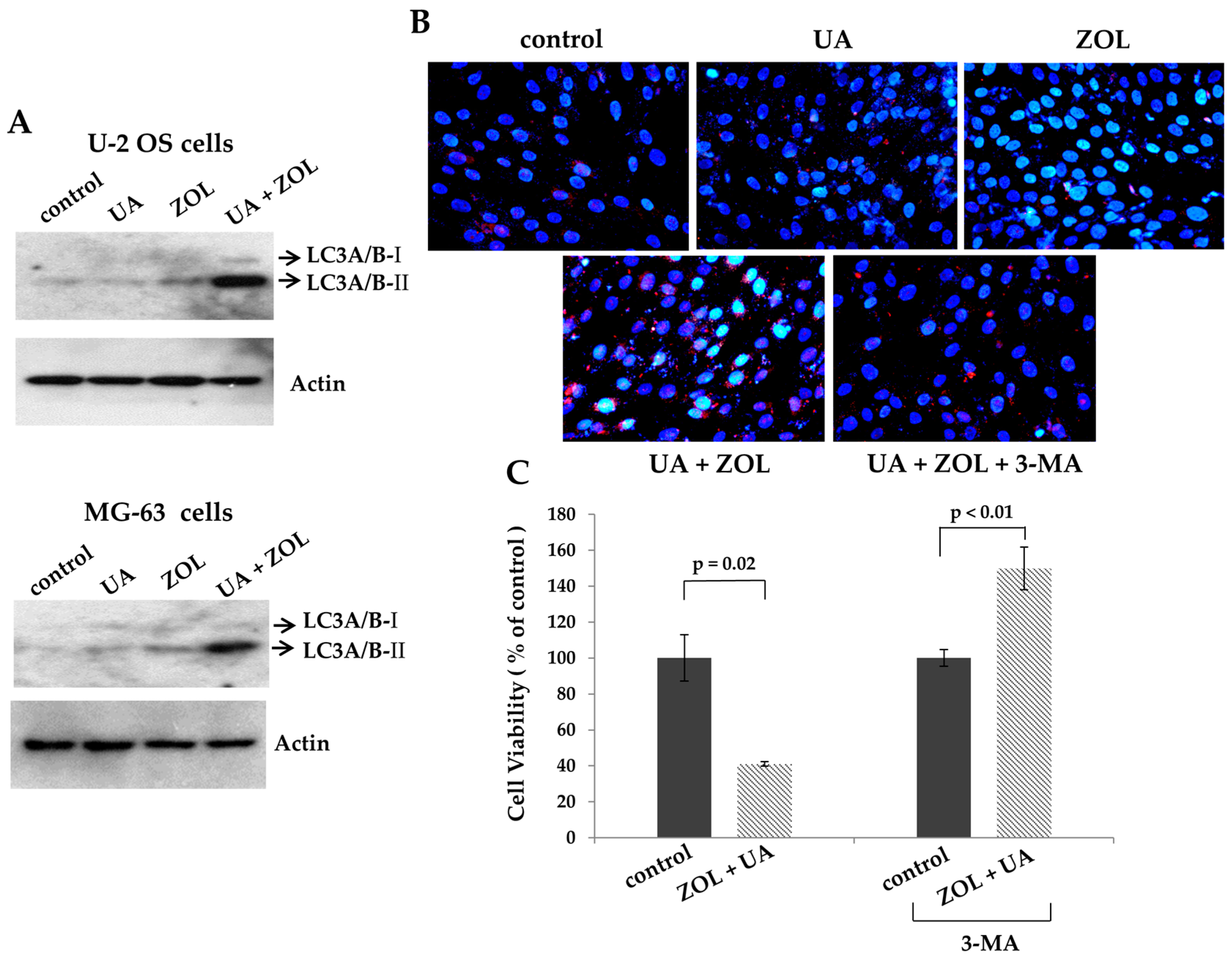
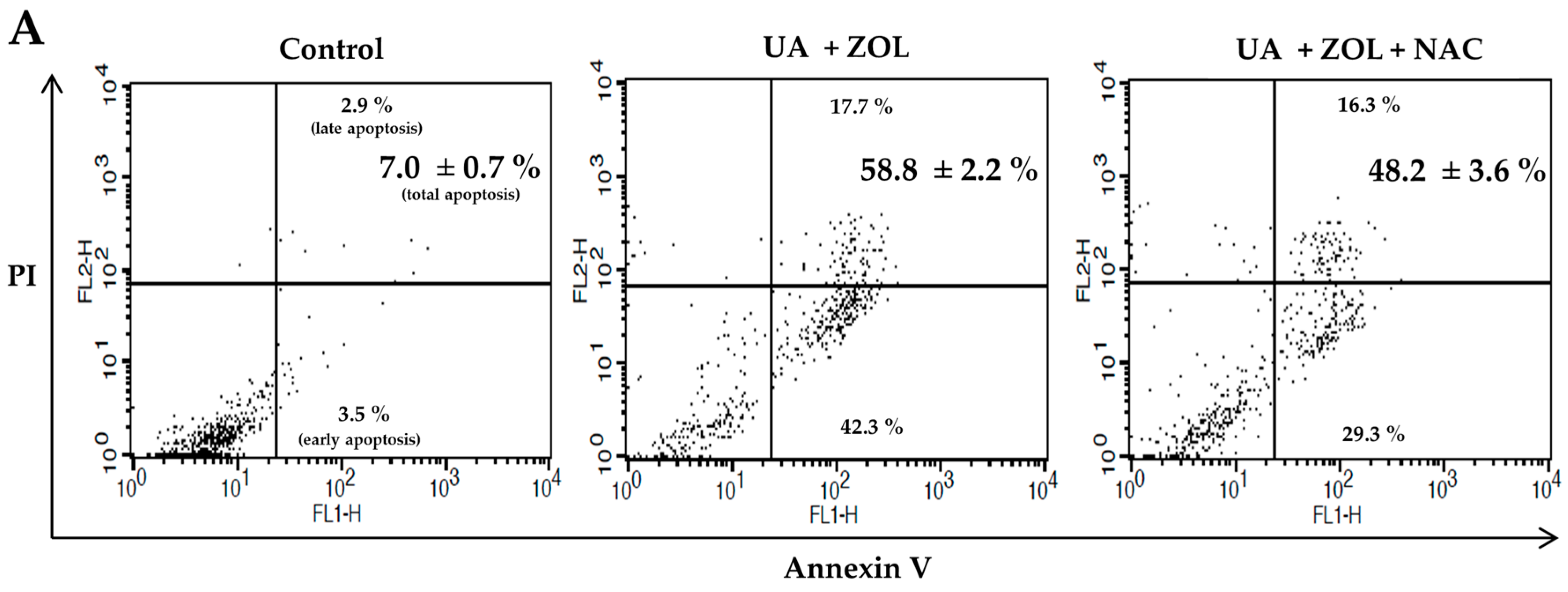
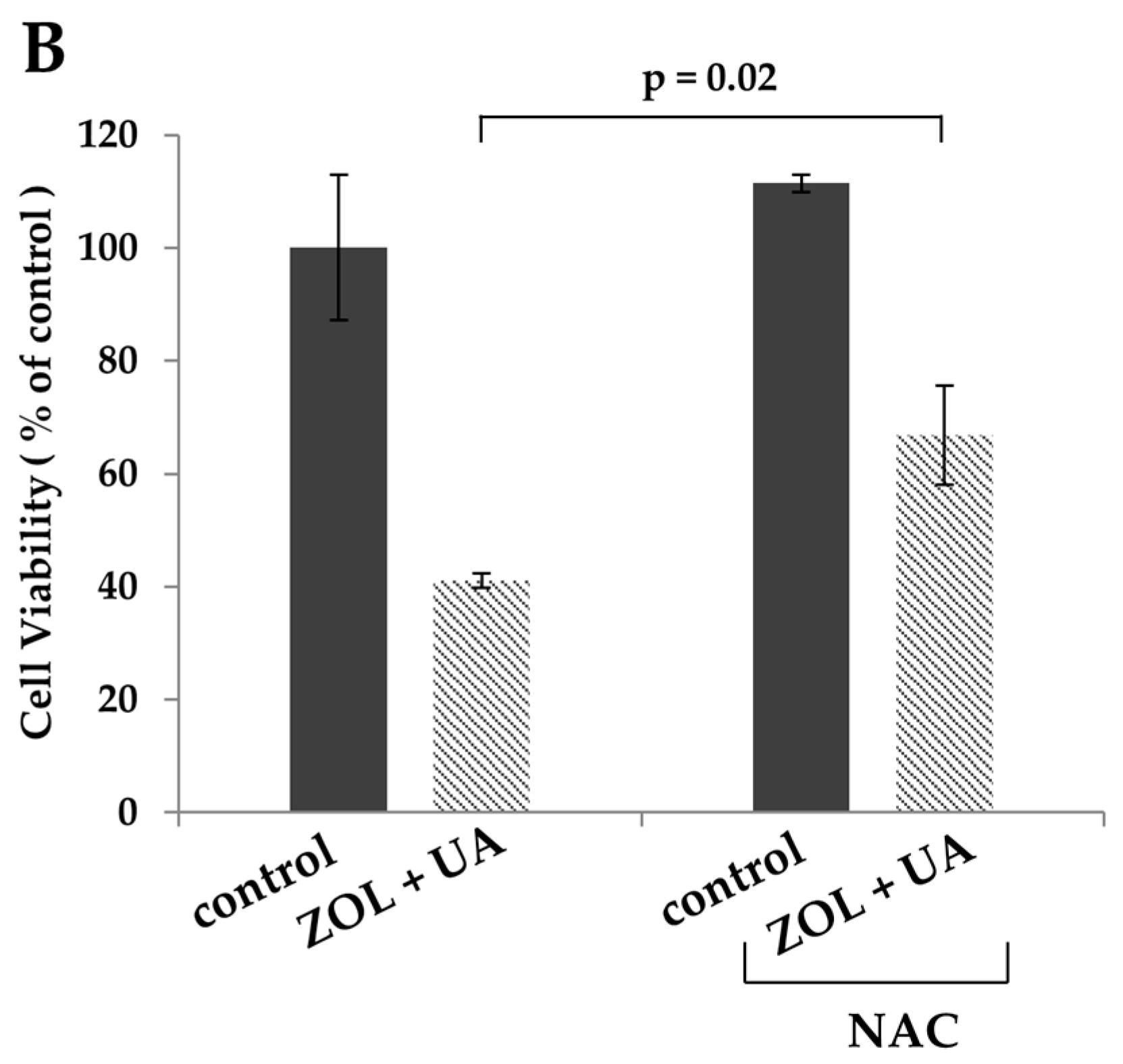
2.2. ROS Was Associated with Apoptosis Induced by Combination of Zoledronic Acid and Ursolic Acid
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Reagents
4.2. Cell Lysis and Immunoblotting
4.3. MTT Assay
4.4. Colony Formation Assay
4.5. Measurement of Oxidative Stress (ROS) Determination
4.6. Apoptosis Assay
4.7. Autophagy Determination
4.8. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Broadhead, M.L.; Clark, J.C.; Myers, D.E.; Dass, C.R.; Choong, P.F. The molecular pathogenesis of osteosarcoma: A review. Sarcoma 2011, 2011, 959248. [Google Scholar] [CrossRef] [PubMed]
- Jaffe, N. Osteosarcoma: Review of the past, impact on the future. The american experience. Cancer Treat. Res. 2009, 152, 239–262. [Google Scholar] [PubMed]
- Li, J.S.; Wang, W.J.; Sun, Y.; Zhang, Y.H.; Zheng, L. Ursolic acid inhibits the development of nonalcoholic fatty liver disease by attenuating endoplasmic reticulum stress. Food Funct. 2015, 6, 1643–1651. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.S.; Moon, A. Ursolic acid inhibits the invasive phenotype of snu-484 human gastric cancer cells. Oncol. Lett. 2015, 9, 897–902. [Google Scholar] [CrossRef] [PubMed]
- Xiang, L.; Chi, T.; Tang, Q.; Yang, X.; Ou, M.; Chen, X.; Yu, X.; Chen, J.; Ho, R.J.; Shao, J.; et al. A pentacyclic triterpene natural product, ursolic acid and its prodrug US597 inhibit targets within cell adhesion pathway and prevent cancer metastasis. Oncotarget 2015, 6, 9295–9312. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, W.; Qian, L.; Zhang, Q.; Lai, D.; Qi, C. Ursolic acid inhibits the proliferation of human ovarian cancer stem-like cells through epithelial-mesenchymal transition. Oncol. Rep. 2015, 34, 2375–2384. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Kim, Y.H.; Song, G.Y.; Kim, D.E.; Jeong, Y.J.; Liu, K.H.; Chung, Y.H.; Oh, S. Ursolic acid and its natural derivative corosolic acid suppress the proliferation of APC-mutated colon cancer cells through promotion of beta-catenin degradation. Food Chem. Toxicol. 2014, 67, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Saraswati, S.; Agrawal, S.S.; Alhaider, A.A. Ursolic acid inhibits tumor angiogenesis and induces apoptosis through mitochondrial-dependent pathway in ehrlich ascites carcinoma tumor. Chem. Biol. Interact. 2013, 206, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.C.; Cheng, C.H.; Lee, Y.H.; Chang, I.L.; Chen, H.Y.; Hsieh, C.P.; Chueh, P.J. Ursolic acid triggers apoptosis in human osteosarcoma cells via caspase activation and the ERK1/2 MAPK pathway. J. Agric. Food Chem. 2016, 64, 4220–4226. [Google Scholar] [CrossRef] [PubMed]
- Clemons, M.J.; Dranitsaris, G.; Ooi, W.S.; Yogendran, G.; Sukovic, T.; Wong, B.Y.; Verma, S.; Pritchard, K.I.; Trudeau, M.; Cole, D.E. Phase II trial evaluating the palliative benefit of second-line zoledronic acid in breast cancer patients with either a skeletal-related event or progressive bone metastases despite first-line bisphosphonate therapy. J. Clin. Oncol. 2006, 24, 4895–4900. [Google Scholar] [CrossRef] [PubMed]
- Kohno, N.; Aogi, K.; Minami, H.; Nakamura, S.; Asaga, T.; Iino, Y.; Watanabe, T.; Goessl, C.; Ohashi, Y.; Takashima, S. Zoledronic acid significantly reduces skeletal complications compared with placebo in japanese women with bone metastases from breast cancer: A randomized, placebo-controlled trial. J. Clin. Oncol. 2005, 23, 3314–3321. [Google Scholar] [CrossRef] [PubMed]
- Rosen, L.S.; Gordon, D.; Kaminski, M.; Howell, A.; Belch, A.; Mackey, J.; Apffelstaedt, J.; Hussein, M.; Coleman, R.E.; Reitsma, D.J.; et al. Zoledronic acid versus pamidronate in the treatment of skeletal metastases in patients with breast cancer or osteolytic lesions of multiple myeloma: A phase III, double-blind, comparative trial. Cancer J. 2001, 7, 377–387. [Google Scholar] [PubMed]
- Rosen, L.S.; Gordon, D.; Kaminski, M.; Howell, A.; Belch, A.; Mackey, J.; Apffelstaedt, J.; Hussein, M.A.; Coleman, R.E.; Reitsma, D.J.; et al. Long-term efficacy and safety of zoledronic acid compared with pamidronate disodium in the treatment of skeletal complications in patients with advanced multiple myeloma or breast carcinoma: A randomized, double-blind, multicenter, comparative trial. Cancer 2003, 98, 1735–1744. [Google Scholar] [CrossRef] [PubMed]
- Polascik, T.J.; Given, R.W.; Metzger, C.; Julian, S.R.; Vestal, J.C.; Karlin, G.S.; Barkley, C.S.; Bilhartz, D.L.; McWhorter, L.T.; Lacerna, L.V. Open-label trial evaluating the safety and efficacy of zoledronic acid in preventing bone loss in patients with hormone-sensitive prostate cancer and bone metastases. Urology 2005, 66, 1054–1059. [Google Scholar] [CrossRef] [PubMed]
- Saad, F.; Gleason, D.M.; Murray, R.; Tchekmedyian, S.; Venner, P.; Lacombe, L.; Chin, J.L.; Vinholes, J.J.; Goas, J.A.; Chen, B.; et al. A randomized, placebo-controlled trial of zoledronic acid in patients with hormone-refractory metastatic prostate carcinoma. J. Natl. Cancer Inst. 2002, 94, 1458–1468. [Google Scholar] [CrossRef] [PubMed]
- Saad, F.; Gleason, D.M.; Murray, R.; Tchekmedyian, S.; Venner, P.; Lacombe, L.; Chin, J.L.; Vinholes, J.J.; Goas, J.A.; Zheng, M.; et al. Long-term efficacy of zoledronic acid for the prevention of skeletal complications in patients with metastatic hormone-refractory prostate cancer. J. Natl. Cancer Inst. 2004, 96, 879–882. [Google Scholar] [CrossRef] [PubMed]
- Rosen, L.S.; Gordon, D.; Tchekmedyian, N.S.; Yanagihara, R.; Hirsh, V.; Krzakowski, M.; Pawlicki, M.; De Souza, P.; Zheng, M.; Urbanowitz, G.; et al. Long-term efficacy and safety of zoledronic acid in the treatment of skeletal metastases in patients with nonsmall cell lung carcinoma and other solid tumors: A randomized, phase III, double-blind, placebo-controlled trial. Cancer 2004, 100, 2613–2621. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Wang, W.; Zhang, H.; Hu, Y.; Yin, Z. Bisphosphonates regulate cell proliferation, apoptosis and pro-osteoclastic expression in MG-63 human osteosarcoma cells. Oncol. Lett. 2012, 4, 299–304. [Google Scholar] [PubMed]
- Ory, B.; Blanchard, F.; Battaglia, S.; Gouin, F.; Redini, F.; Heymann, D. Zoledronic acid activates the DNA s-phase checkpoint and induces osteosarcoma cell death characterized by apoptosis-inducing factor and endonuclease-g translocation independently of p53 and retinoblastoma status. Mol. Pharmacol. 2007, 71, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Hirbe, A.C.; Roelofs, A.J.; Floyd, D.H.; Deng, H.; Becker, S.N.; Lanigan, L.G.; Apicelli, A.J.; Xu, Z.; Prior, J.L.; Eagleton, M.C.; et al. The bisphosphonate zoledronic acid decreases tumor growth in bone in mice with defective osteoclasts. Bone 2009, 44, 908–916. [Google Scholar] [CrossRef] [PubMed]
- Moriceau, G.; Ory, B.; Mitrofan, L.; Riganti, C.; Blanchard, F.; Brion, R.; Charrier, C.; Battaglia, S.; Pilet, P.; Denis, M.G.; et al. Zoledronic acid potentiates mTOR inhibition and abolishes the resistance of osteosarcoma cells to RAD001 (everolimus): Pivotal role of the prenylation process. Cancer Res. 2010, 70, 10329–10339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dass, C.R.; Choong, P.F. Zoledronic acid inhibits osteosarcoma growth in an orthotopic model. Mol. Cancer Ther. 2007, 6, 3263–3270. [Google Scholar] [CrossRef] [PubMed]
- Labrinidis, A.; Hay, S.; Liapis, V.; Ponomarev, V.; Findlay, D.M.; Evdokiou, A. Zoledronic acid inhibits both the osteolytic and osteoblastic components of osteosarcoma lesions in a mouse model. Clin. Cancer Res. 2009, 15, 3451–3461. [Google Scholar] [CrossRef] [PubMed]
- Ory, B.; Heymann, M.F.; Kamijo, A.; Gouin, F.; Heymann, D.; Redini, F. Zoledronic acid suppresses lung metastases and prolongs overall survival of osteosarcoma-bearing mice. Cancer 2005, 104, 2522–2529. [Google Scholar] [CrossRef] [PubMed]
- Conry, R.M.; Rodriguez, M.G.; Pressey, J.G. Zoledronic acid in metastatic osteosarcoma: Encouraging progression free survival in four consecutive patients. Clin. Sarcoma Res. 2016, 6, 6. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, H.; Zhou, H.J.; Ji, W.; Min, W. Mitochondrial redox signaling and tumor progression. Cancers 2016, 8, 40. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, T.; Liverani, C.; Mercatali, L.; Sacanna, E.; Zanoni, M.; Fabbri, F.; Zoli, W.; Amadori, D. Cisplatin in combination with zoledronic acid: A synergistic effect in triple-negative breast cancer cell lines. Int. J. Oncol. 2013, 42, 1263–1270. [Google Scholar] [CrossRef] [PubMed]
- Ozturk, O.H.; Bozcuk, H.; Burgucu, D.; Ekinci, D.; Ozdogan, M.; Akca, S.; Yildiz, M. Cisplatin cytotoxicity is enhanced with zoledronic acid in A549 lung cancer cell line: Preliminary results of an in vitro study. Cell Biol. Int. 2007, 31, 1069–1071. [Google Scholar] [CrossRef] [PubMed]
- Koto, K.; Murata, H.; Kimura, S.; Horie, N.; Matsui, T.; Nishigaki, Y.; Ryu, K.; Sakabe, T.; Itoi, M.; Ashihara, E.; et al. Zoledronic acid inhibits proliferation of human fibrosarcoma cells with induction of apoptosis, and shows combined effects with other anticancer agents. Oncol. Rep. 2010, 24, 233–239. [Google Scholar] [PubMed]
- Kashyap, D.; Tuli, H.S.; Sharma, A.K. Ursolic acid (UA): A metabolite with promising therapeutic potential. Life Sci. 2016, 146, 201–213. [Google Scholar] [CrossRef] [PubMed]
- Chuang, W.L.; Lin, P.Y.; Lin, H.C.; Chen, Y.L. The apoptotic effect of ursolic acid on SK-Hep-1 cells is regulated by the PI3K/Akt, p38 and JNK MAPK signaling pathways. Molecules 2016, 21, 460. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Baehrecke, E.H. Autophagy, cell death, and cancer. Mol. Cell. Oncol. 2015, 2, e985913. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Klionsky, D.J. Eaten alive: A history of macroautophagy. Nat. Cell Biol. 2010, 12, 814–822. [Google Scholar] [CrossRef] [PubMed]
- White, E. Deconvoluting the context-dependent role for autophagy in cancer. Nat. Rev. Cancer 2012, 12, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.F.; Lin, Y.C.; Lin, Y.H.; Tsai, T.F.; Chou, K.Y.; Chen, H.E.; Hwang, T.I. Zoledronic acid induces autophagic cell death in human prostate cancer cells. J. Urol. 2011, 185, 1490–1496. [Google Scholar] [CrossRef] [PubMed]
- Wang, I.T.; Chou, S.C.; Lin, Y.C. Zoledronic acid induces apoptosis and autophagy in cervical cancer cells. Tumour Biol. 2014, 35, 11913–11920. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.Y.; Yang, L.Q.; Jiang, Y.; Yang, W.W.; Fu, J.; Li, S.L. Reactive oxygen species and autophagy associated apoptosis and limitation of clonogenic survival induced by zoledronic acid in salivary adenoid cystic carcinoma cell line SACC-83. PLoS ONE 2014, 9, e101207. [Google Scholar] [CrossRef] [PubMed]
- Schumacker, P.T. Reactive oxygen species in cancer: A dance with the devil. Cancer Cell 2015, 27, 156–157. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Jiang, M.; Hu, J.; Lv, X.; Yu, L.; Qian, X.; Liu, B. Enhancement of radiation effects by ursolic acid in bgc-823 human adenocarcinoma gastric cancer cell line. PLoS ONE 2015, 10, e0133169. [Google Scholar] [CrossRef] [PubMed]
- De Colli, M.; Zara, S.; di Giacomo, V.; Patruno, A.; Marconi, G.D.; Gallorini, M.; Zizzari, V.L.; Tete, G.; Cataldi, A. Nitric oxide-mediated cytotoxic effect induced by zoledronic acid treatment on human gingival fibroblasts. Clin. Oral Investig. 2015, 19, 1269–1277. [Google Scholar] [CrossRef] [PubMed]
- Bruzzese, F.; Pucci, B.; Milone, M.R.; Ciardiello, C.; Franco, R.; Chianese, M.I.; Rocco, M.; Di Gennaro, E.; Leone, A.; Luciano, A.; et al. Panobinostat synergizes with zoledronic acid in prostate cancer and multiple myeloma models by increasing ros and modulating mevalonate and p38-MAPK pathways. Cell Death Dis. 2013, 4, e878. [Google Scholar] [CrossRef] [PubMed]
- Koto, K.; Murata, H.; Kimura, S.; Sawai, Y.; Horie, N.; Matsui, T.; Ryu, K.; Ashihara, E.; Maekawa, T.; Kubo, T.; et al. Zoledronic acid significantly enhances radiationinduced apoptosis against human fibrosarcoma cells by inhibiting radioadaptive signaling. Int. J. Oncol. 2013, 42, 525–534. [Google Scholar] [PubMed]
- Sample Availability: Samples of the compounds are not available from the authors.

© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, C.-C.; Huang, Y.-F.; Hsieh, C.-P.; Chueh, P.-J.; Chen, Y.-L. Combined Use of Zoledronic Acid Augments Ursolic Acid-Induced Apoptosis in Human Osteosarcoma Cells through Enhanced Oxidative Stress and Autophagy. Molecules 2016, 21, 1640. https://doi.org/10.3390/molecules21121640
Wu C-C, Huang Y-F, Hsieh C-P, Chueh P-J, Chen Y-L. Combined Use of Zoledronic Acid Augments Ursolic Acid-Induced Apoptosis in Human Osteosarcoma Cells through Enhanced Oxidative Stress and Autophagy. Molecules. 2016; 21(12):1640. https://doi.org/10.3390/molecules21121640
Chicago/Turabian StyleWu, Chia-Chieh, Yi-Fu Huang, Chen-Pu Hsieh, Pin-Ju Chueh, and Yao-Li Chen. 2016. "Combined Use of Zoledronic Acid Augments Ursolic Acid-Induced Apoptosis in Human Osteosarcoma Cells through Enhanced Oxidative Stress and Autophagy" Molecules 21, no. 12: 1640. https://doi.org/10.3390/molecules21121640






