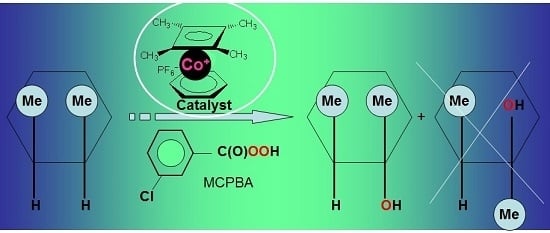
Stereoselective Alkane Oxidation with meta-Chloroperoxybenzoic Acid (MCPBA) Catalyzed by Organometallic Cobalt Complexes †
Abstract
:1. Introduction
2. Results and Discussion
2.1. Catalysts, Substrates and Oxidants
2.2. Syntheses of Catalysts
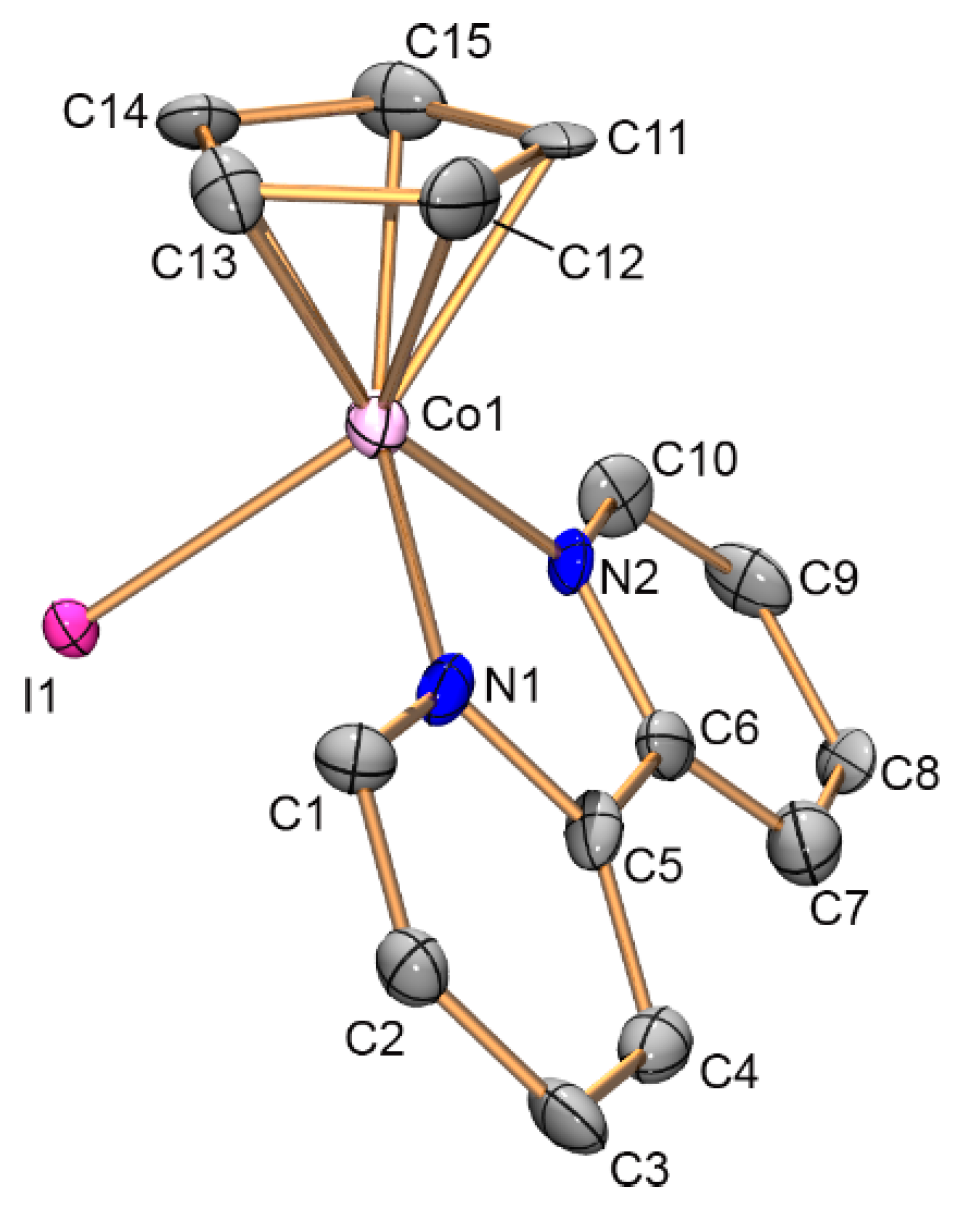
2.3. Structures of Catalysts
2.4. Determination of Alkyl Hydroperoxides by GC before and after Reduction with PPh3
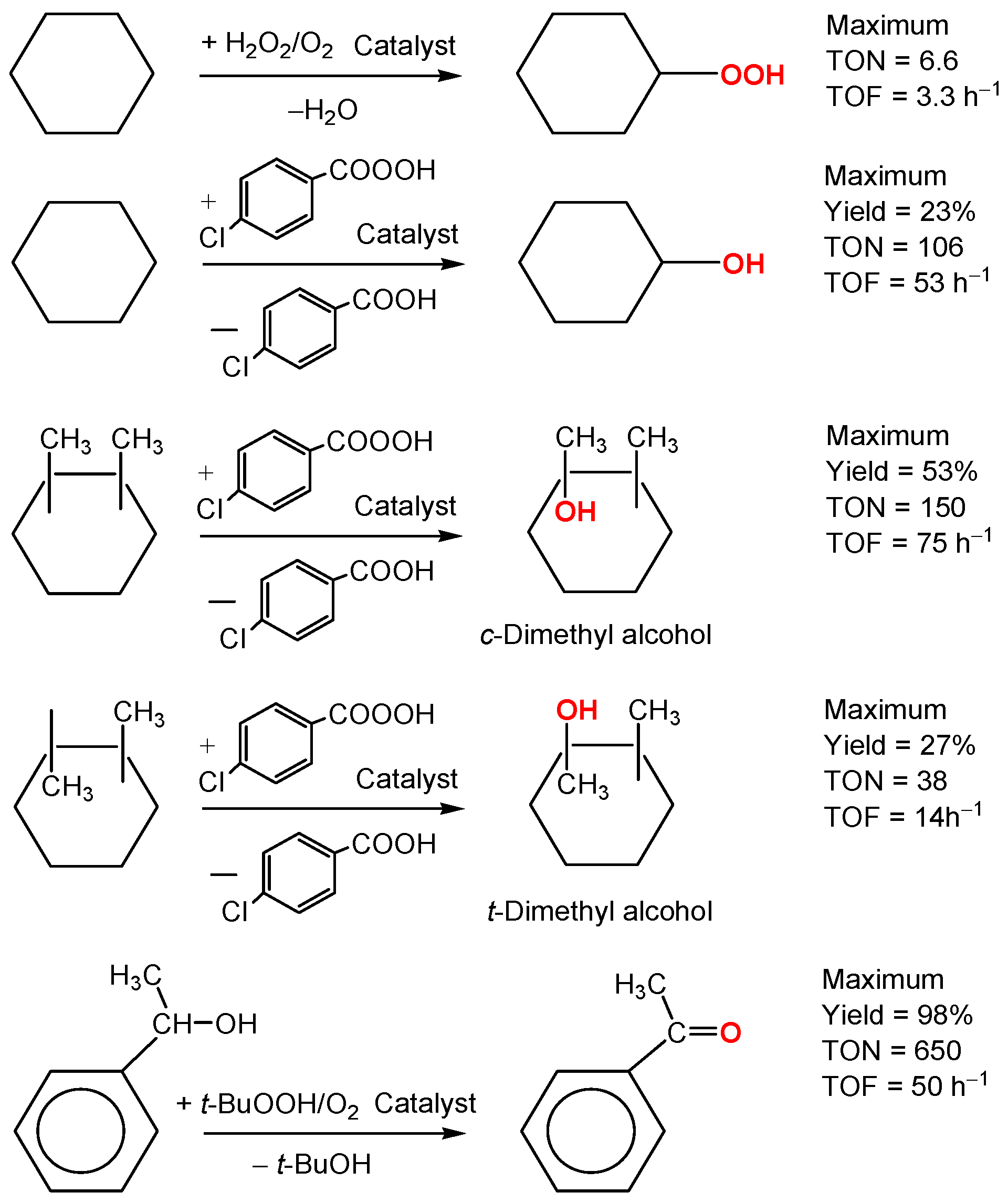
2.5. Oxidation of Alkanes and Alcohols with Peroxides
2.6. Stereoselective Oxidation with meta-Chloroperoxybenzoic Acid (MCPBA)
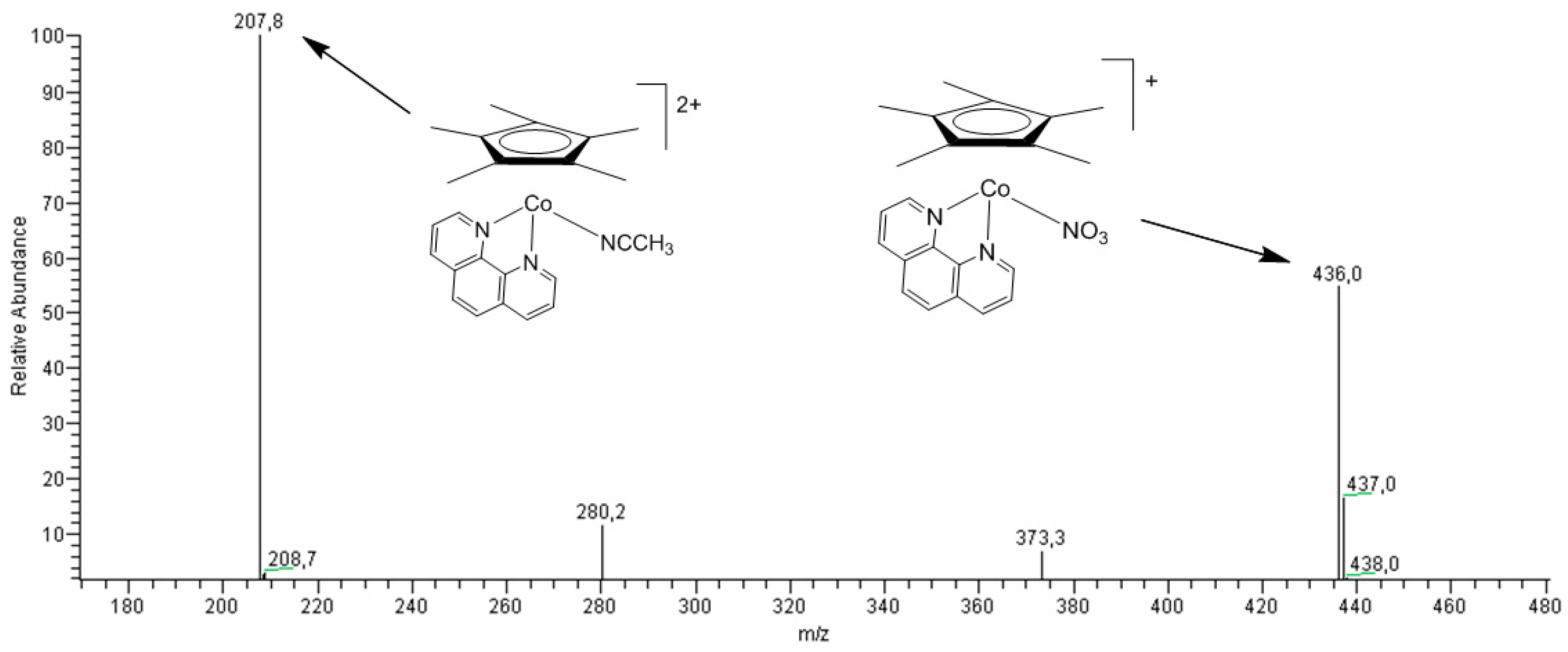
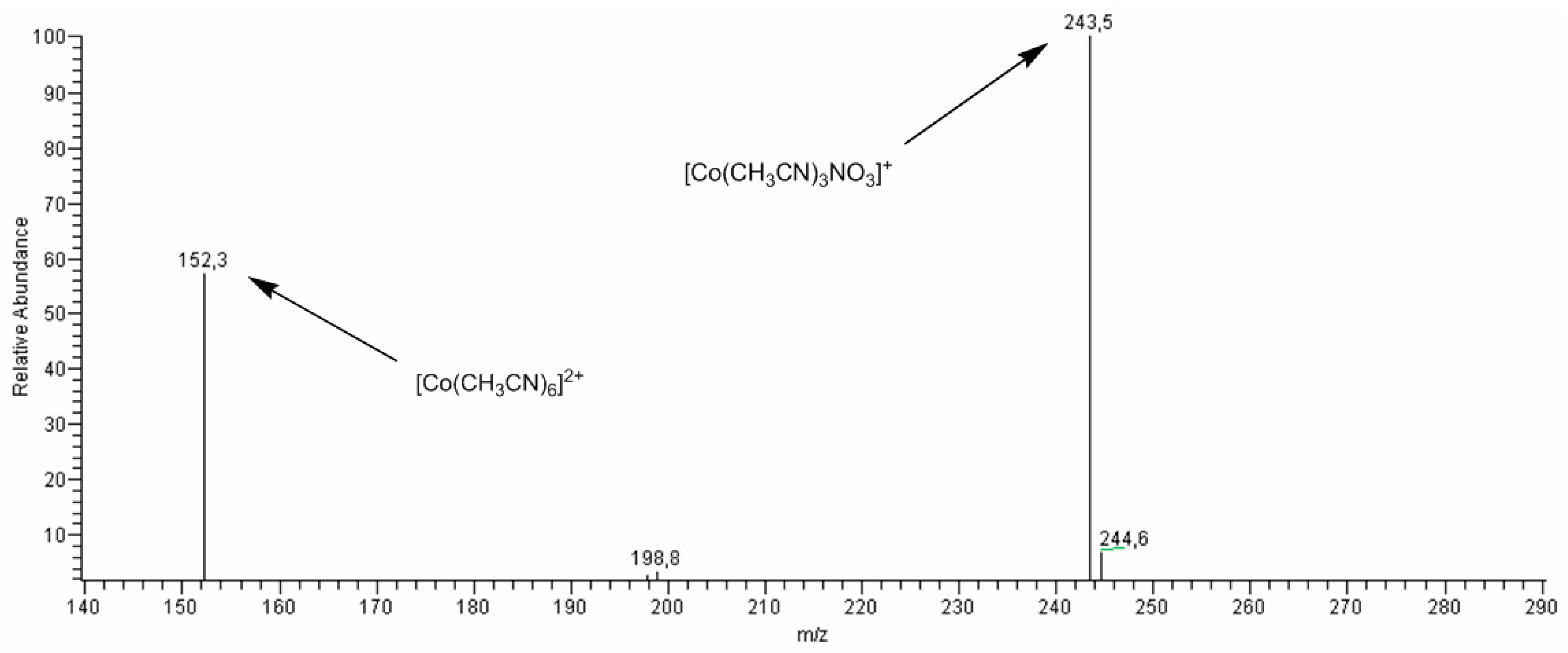
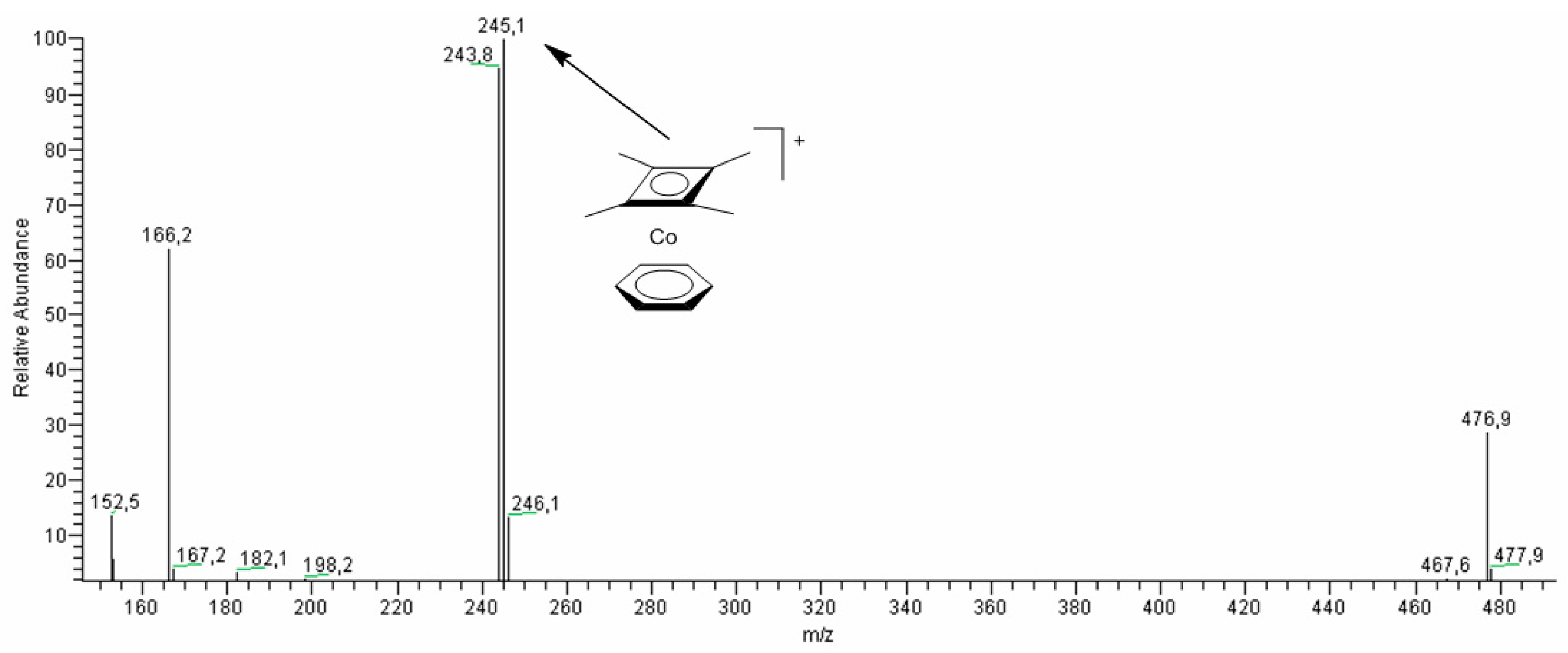
2.7. Studies of Obtained Complexes by Electrospray Ionization/Mass Spectrometry (ESI-MS) Method
2.8. On the Mechanism of Catalyzed C–H Bond Oxidation with MCPBA
3. Materials and Methods
3.1. General
3.2. Syntheses of Complexes
3.3. X-ray Diffraction Study
3.4. Catalytic Oxidation of Alkanes and 1-Phenylethanol
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Shilov, A.E.; Shul’pin, G.B. Activation and Catalytic Reactions of Saturated Hydrocarbons in the Presence of Metal Complexes; Kluwer Academic Publishers: New York, NY, USA; Boston, MA, USA; Dordrecht, The Netherlands; London, UK; Moscow, Russia, 2002. [Google Scholar]
- Denisov, E.T.; Afanas’ev, I.B. Oxidation and Antioxidants in Organic Chemistry and Biochemistry; Taylor & Francis: Boca Raton, FL, USA; London, UK; New York, NY, USA; Singapore, Singapore, 2005. [Google Scholar]
- Clerici, M.G.; Kholdeeva, O.A. Liquid Phase Oxidation via Heterogeneous Catalysis; Wiley: Hoboken, NJ, USA, 2013. [Google Scholar]
- Munz, D.; Strassner, T. Alkane C–H functionalization and oxidation with molecular oxygen. Inorg. Chem. 2015, 54, 5043–5052. [Google Scholar] [CrossRef] [PubMed]
- Shilov, A.E.; Shul’pin, G.B. Activation of C–H Bonds by Metal Complexes. Chem. Rev. 1997, 97, 2879–2932. [Google Scholar] [CrossRef]
- Chepaikin, E.G. Homogeneous catalysis in the oxidative functionalization of alkanes in protic media. Russ. Chem. Rev. 2011, 80, 363–396. [Google Scholar] [CrossRef]
- Costas, M. Selective C–H oxidation catalyzed by metalloporphyrins. Coord. Chem. Rev. 2011, 255, 2912–2932. [Google Scholar] [CrossRef]
- Pérez, P.J. Alkane C–H Activation by Single-Site Metal Catalysis; Springer: Heidelberg, Germany, 2012. [Google Scholar]
- Sivaramakrishna, A.; Suman, P.; Goud, E.V.; Janardan, S.; Sravani, C.; Sandep, T.; Vijayakrishna, K.; Clayton, H.S. Review: Active homogeneous reagents and catalysts in n-alkane activation reactions. J. Coord. Chem. 2013, 66, 2091–2109. [Google Scholar] [CrossRef]
- Sorokin, A.B. Phthalocyanine Metal Complexes in Catalysis. Chem. Rev. 2013, 113, 8152–8191. [Google Scholar] [CrossRef] [PubMed]
- Caballero, A.; Perez, P.J. Methane as raw material in synthetic chemistry: The final frontier. Chem. Soc. Rev. 2013, 42, 8809–8820. [Google Scholar] [CrossRef] [PubMed]
- Shul’pin, G.B. Selectivity in C–H functionalizations. In Comprehensive Inorganic Chemistry II, 2nd ed.; Reedijk, J., Poeppelmeier, K., Casella, L., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; Volume 6, Chapter 6.04; pp. 79–104. [Google Scholar]
- Chepaikin, E.G. Oxidative functionalization of alkanes under dioxygen in the presence of homogeneous noble metal catalysts. J. Mol. Catal. A Chem. 2014, 385, 160–174. [Google Scholar] [CrossRef]
- Landaeta, V.R.; Rodríguez-Lugo, R.E. Catalytic oxygenation of organic substrates: Toward greener ways for incorporating oxygen. Inorg. Chim. Acta 2015, 431, 21–47. [Google Scholar] [CrossRef]
- Nam, W.; Kim, I.; Kim, Y.; Kim, C. Biomimetic alkane hydroxylation by cobalt(III) porphyrin complex and m-chloroperbenzoic acid. Chem. Commun. 2001, 1262–1263. [Google Scholar] [CrossRef]
- Nam, W.; Ryu, J.Y.; Kim, I.; Kim, C. Stereoselective alkane hydroxylations by metal salts and m-chloroperbenzoic acid. Tetrahedron Lett. 2002, 43, 5487–5490. [Google Scholar] [CrossRef]
- Strassner, T.; Ahrens, S.; Muehlhofer, M.; Munz, D.; Zeller, A. Cobalt-catalyzed oxidation of methane to methyl trifluoroacetate by dioxygen. Eur. J. Inorg. Chem. 2013, 3659–3663. [Google Scholar] [CrossRef]
- Tordin, E.; List, M.; Monkowius, U.; Schindler, S.; Knör, G. Synthesis and characterisation of cobalt, nickel and copper complexes with tripodal 4N ligands as novel catalysts for the homogeneous partial oxidation of alkanes. Inorg. Chim. Acta 2013, 402, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Silva, T.F.S.; Martins, L.M.D.R.S.; Guedes da Silva, M.F.C.; Kuznetsov, M.L.; Fernandes, A.R.; Silva, A.; Pan, C.-J.; Lee, J.-F.; Hwang, B.-J.; Pombeiro, A.J.L. X-ray Absorption Spectroscopy Studies and Biological Applications. Chem. Asian J. 2014, 9, 1132–1143. [Google Scholar] [CrossRef] [PubMed]
- Xing, N.; Xu, L.T.; Bai, F.Y.; Shan, H.; Xing, Y.H.; Shi, Z. Synthesis and characterization of novel transition metal complexes with indole acetic acid ligands: Evaluation of their catalytic activity for the oxidation of cyclohexane. Inorg. Chim. Acta 2014, 409, 360–366. [Google Scholar] [CrossRef]
- Bilyachenko, A.N.; Yalymov, A.I.; Levitsky, M.M.; Korlyukov, A.A.; Es’kova, M.A.; Long, J.; Larionova, J.; Guari, Y.; Shul’pina, L.S.; Ikonnikov, N.S.; et al. First cage-like pentanuclear Co(II)-silsesquioxane. Dalton Trans. 2016, 45, 13663–13666. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Yoshino, T.; Matsunaga, S.; Kanai, M. Air-Stable Carbonyl(pentamethylcyclopentadienyl)cobalt Diiodide Complex as a Precursor for Cationic (Pentamethylcyclopentadienyl)cobalt(III) Catalysis: Application for Directed C-2 Selective CH Amidation of Indoles. Adv. Synth. Catal. 2014, 356, 1491–1495. [Google Scholar] [CrossRef]
- Gandeepan, P.; Cheng, C.-H. Cobalt Catalysis Involving π Components in Organic Synthesis. Acc. Chem. Res. 2015, 48, 1194–1206. [Google Scholar] [CrossRef] [PubMed]
- Reinig, R.R.; Mukherjee, D.; Weinstein, Z.B.; Xie, W.; Albright, T.; Baird, B.; Gray, T.S.; Ellern, A.; Miller, G.J.; Winter, G.J.; et al. Synthesis and Oxidation Catalysis of [Tris(oxazolinyl)borato]cobalt(II) Scorpionates. Eur. J. Inorg. Chem. 2016, 2486–2494. [Google Scholar] [CrossRef]
- Fossey, J.; Lefort, D.; Massoudi, M.; Nedelec, J.-Y.; Sobra, J. Regiosélectivité et stéréosélectivité de l’hydroxylation homolytique des hydrocarbures par le peracide benzoïque. Can. J. Chem. 1985, 63, 678–680. [Google Scholar] [CrossRef]
- Lindsay Smith, J.R.; Shul’pin, G.B. Efficient stereoselective oxygenation of alkanes by peroxyacetic acid or hydrogen peroxide and acetic acid catalysed by a manganese(IV) 1,4,7-trimethyl-1,4,7-triazacyclononane complex. Tetrahedron Lett. 1998, 39, 4909–4912. [Google Scholar] [CrossRef]
- Shul’pin, G.B.; Lindsay-Smith, J.R. Oxidations by the reagent “H2O2—Manganese(IV) complex—Carboxylic acid”. Part 1. Oxidation of saturated hydrocarbons with peroxy acids and hydrogen peroxide. Russ. Chem. Bull. 1998, 47, 2379–2386. [Google Scholar] [CrossRef]
- Shul’pin, G.B.; Stoeckli-Evans, H.; Mandelli, D.; Kozlov, Y.N.; Tesouro Vallina, A.; Woitiski, C.B.; Jimenez, R.S.; Carvalho, W.A. Oxidation of alkanes with m-chloroperbenzoic acid catalyzed by iron(III) chloride and a polydentate amine. J. Mol. Catal. A Chem. 2004, 219, 255–264. [Google Scholar] [CrossRef]
- Shul’pina, L.S.; Kudinov, A.R.; Süss-Fink, G.; Loginov, D.A.; Shul’pin, G.B. Oxidation of saturated hydrocarbons with peroxides catalyzed by iridium and palladium complexes. Pet. Chem. 2005, 45, 309–311. [Google Scholar]
- Nagataki, T.; Tachi, Y.; Itoh, S. NiII(TPA) as an efficient catalyst for alkane hydroxylation with m-CPBA. Chem. Commun. 2006, 4016–4018. [Google Scholar] [CrossRef] [PubMed]
- Nagataki, T.; Itoh, S. Catalytic alkane hydroxylation reaction with nickel(II) complexes supported by di- and triphenol ligands. Chem. Lett. 2007, 36, 748–749. [Google Scholar] [CrossRef]
- Ray, K.; Lee, S.M.; Que, L., Jr. Iron-oxidation-state-dependent O–O bond cleavage of MCPBA to form an iron(IV)-oxo complex. Inorg. Chim. Acta 2008, 361, 1066–1069. [Google Scholar] [CrossRef] [PubMed]
- Nagataki, T.; Ishii, K.; Tachi, Y.; Itoh, S. Ligand effects on NiII-catalysed alkane-hydroxylation with m-CPBA. Dalton Trans. 2007, 1120–1128. [Google Scholar] [CrossRef] [PubMed]
- Kamijo, S.; Amaoka, Y.; Inoue, M. Unified Oxidation Protocol for the Synthesis of Carbonyl Compounds Using a Manganese Catalyst. Synthesis 2010, 2475–2489. [Google Scholar]
- Balamurugan, M.; Mayilmurugan, R.; Suresh, E.; Palaniandavar, M. Nickel(II) complexes of tripodal 4N ligands as catalysts for alkane oxidation using m-CPBA as oxidant: Ligand stereoelectronic effects on catalysis. Dalton Trans. 2011, 40, 9413–9424. [Google Scholar] [CrossRef] [PubMed]
- Kikunaga, T.; Matsumoto, T.; Ohta, T.; Nakai, H.; Naruta, Y.; Ahn, K.-H.; Watanabe, Y.; Ogo, S. Isolation of a MnIV acylperoxo complex and its monooxidation ability. Chem. Commun. 2013, 49, 8356–8358. [Google Scholar] [CrossRef] [PubMed]
- Hikichi, S.; Hanaue, K.; Fujimura, T.; Okuda, H.; Nakazawa, J.; Ohzu, Y.; Kobayashi, C.; Akita, M. Characterization of nickel(II)-acylperoxo species relevant to catalytic alkane hydroxylation by nickel complex with mCPBA. Dalton Trans. 2013, 42, 3346–3356. [Google Scholar] [CrossRef] [PubMed]
- Nakazawa, J.; Yata, A.; Hori, T.; Stack, T.D.P.; Naruta, Y.; Hikichi, S. Catalytic Alkane Oxidation by Homogeneous and Siica-supported Cobalt(III) Complex Catalysts with a Triazolyl Group-containing Tetradentate Ligand. Chem. Lett. 2013, 42, 1197–1199. [Google Scholar] [CrossRef]
- Nakazawa, J.; Hori, T.; Stack, T.D.P.; Hikichi, S. Alkane Oxidation by an Immobilized Nickel Complex Catalyst: Structural and Reactivity Differences Induced by Surface-Ligand Density on Mesoporous Silica. Chem. Asian J. 2013, 8, 1191–1199. [Google Scholar] [CrossRef] [PubMed]
- Silva, T.F.S.; Mac leod, T.C.O.; Martins, L.M.D.R.S.; Guedes da Silva, M.F.C.; Schiavon, M.A.; Pombeiro, A.J.L. Pyrazole or tris(pyrazolyl)ethanol oxo-vanadium(IV) complexes as homogeneous or supported catalysts for oxidation of cyclohexane under mild conditions. J. Mol. Catal. A Chem. 2013, 367, 52–60. [Google Scholar] [CrossRef]
- Hussain, H.; Al-Harrasi, A.; Gree, I.R.; Ahmed, I.; Abbas, G.; Rehman, N.U. meta-Chloroperbenzoic acid (mCPBA): A versatile reagent in organic synthesis. RSC Adv. 2014, 4, 12882–12917. [Google Scholar] [CrossRef]
- Sankaralingam, M.; Balamurugan, M.; Palaniandavar, M.; Vadivelu, P.; Suresh, C.H. Nickel(II) complexes of pentadentate N5 ligands as catalysts for alkane hydroxylation by using m-CPBA as oxidant: A combined experimental and computational study. Chem. Eur. J. 2014, 20, 11346–11361. [Google Scholar] [CrossRef] [PubMed]
- Bilyachenko, A.N.; Yalymov, A.I.; Shul’pina, L.S.; Mandelli, D.; Korlyukov, A.A.; Vologzhanina, A.V.; Es’kova, M.A.; Shubina, E.S.; Levitsky, M.M.; Shul’pin, G.B. Novel Cage-Like Hexanuclear Nickel(II) Silsesquioxane. Synthesis, Structure, and Catalytic Activity in Oxidations with Peroxides. Molecules 2016, 21, 665. [Google Scholar] [CrossRef] [PubMed]
- Shul’pin, G.B.; Nesterov, D.S.; Shul’pina, L.S.; Pombeiro, A.J.L. A hydroperoxo-rebound mechanism of alkane oxidation with hydrogen peroxide catalyzed by binuclear manganese (IV) complex in the presence of an acid with involvement of atmospheric dioxygen. Inorg. Chim. Acta 2016. [Google Scholar] [CrossRef]
- Badiei, Y.M.; Wang, W.-H.; Hull, J.F.; Szalda, D.J.; Muckerman, J.T.; Himeda, Y.; Fujita, E. Cp*Co(III) catalysts with proton-responsive ligands for carbon dioxide hydrogenation in aqueous media. Inorg. Chem. 2013, 52, 12576–12586. [Google Scholar] [CrossRef] [PubMed]
- Shul’pin, G.B.; Druzhinina, A.N. Hydroperoxidation of alkanes by atmospheric oxygen in the presence of hydroquinone or quinone catalyzed by copper(II) acetate under visible light irradiation. React. Kinet. Catal. Lett. 1992, 47, 207–211. [Google Scholar] [CrossRef]
- Shul’pin, G.B.; Nizova, G.V. Formation of alkyl peroxides in oxidation of alkanes by H2O2 catalyzed by transition metal complexes. React. Kinet. Catal. Lett. 1992, 48, 333–338. [Google Scholar] [CrossRef]
- Shul’pin, G.B. Metal-catalysed hydrocarbon oxygenations in solutions: The dramatic role of additives: A review. J. Mol. Catal. A Chem. 2002, 189, 39–66. [Google Scholar] [CrossRef]
- Shul’pin, G.B. Metal-catalysed hydrocarbon oxidations. C. R. Chim. 2003, 6, 163–178. [Google Scholar] [CrossRef]
- Shul’pin, G.B. Hydrocarbon Oxygenations with Peroxides Catalyzed by Metal Compounds. Mini-Rev. Org. Chem. 2009, 6, 95–104. [Google Scholar] [CrossRef]
- Shul’pin, G.B.; Kozlov, Y.N.; Shul’pina, L.S.; Kudinov, A.R.; Mandelli, D. Extremely Efficient Alkane Oxidation by a New Catalytic Reagent H2O2/Os3(CO)12/Pyridine. Inorg. Chem. 2009, 48, 10480–10482. [Google Scholar] [CrossRef] [PubMed]
- Shul’pin, G.B.; Kozlov, Y.N.; Shul’pina, L.S.; Petrovskiy, P.V. Oxidation of alkanes and alcohols with hydrogen peroxide catalyzed by complex Os3(CO)10(μ-H)2. Appl. Organomet. Chem. 2010, 24, 464–472. [Google Scholar] [CrossRef]
- Shul’pin, G.B. Selectivity enhancement in functionalization of C–H bonds: A review. Org. Biomol. Chem. 2010, 8, 4217–4228. [Google Scholar] [CrossRef] [PubMed]
- Shul’pin, G.B. C–H functionalization: Thoroughly tuning ligands at a metal ion, a chemist can greatly enhance catalyst’s activity and selectivity. Perspective. Dalton Trans. 2013, 42, 12794–12818. [Google Scholar] [CrossRef] [PubMed]
- Shul’pin, G.B. New Trends in Oxidative Functionalization of Carbon–Hydrogen Bonds: A Review. Catalysts 2016, 6, 50. [Google Scholar] [CrossRef]
- Olivo, G.; Lanzalunga, O.; di Stefano, S. Non-Heme Imine-Based Iron Complexes as Catalysts for Oxidative Processes. Adv. Synth. Catal. 2016, 358, 843–863. [Google Scholar] [CrossRef]
- Hong, J.; Djernes, K.E.; Lee, I.; Hooley, R.J.; Zaera, F. Heterogeneous Catalyst for the Selective Oxidation of Unactivated Hydrocarbons Based on a Tethered Metal-Coordinated Cavitand. ACS Catal. 2013, 3, 2154–2157. [Google Scholar] [CrossRef]
- Zhou, W.-J.; Wisshert, R.; Xue, K.; Zheng, Y.-T.; Albela, B.; Bonneviot, L.; Clacens, J.-M.; Dea Campo, F.; Pera-Titus, M.; Wu, P. Highly Selective Liquid-Phase Oxidation of Cyclohexane to KA Oil over Ti-MWW Catalyst: Evidence of Formation of Oxyl Radicals. ACS Catal. 2014, 4, 53–62. [Google Scholar] [CrossRef]
- Mahdavi, V.; Hasheminasab, H.R. Liquid-phase efficient oxidation of cyclohexane over cobalt promoted VPO catalyst using tert-butylhydroperoxide. J. Taiwan Inst. Chem. Eng. 2015, 51, 53–62. [Google Scholar] [CrossRef]
- Palomas, D.; Kalamaras, C.; Haycock, P.; White, A.J.P.; Hellgardst, K.; Horton, A.; Crimmin, M.R. Re-evaluating selectivity as a determining factor in peroxidative methane oxidation by multimetallic copper complexes. Catal. Sci. Technol. 2015, 5, 4108–4115. [Google Scholar] [CrossRef]
- Wang, B.; Lee, Y.-M.; Clémancey, M.; Seo, M.S.; Sarangi, R.; Latour, J.-M.; Nam, W. Mononuclear Nonheme High-Spin Iron(III)-Acylperoxo Complexes in Olefin Epoxidation and Alkane Hydroxylation Reactions. J. Am. Chem. Soc. 2016, 138, 2426–2436. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Cheng, H.; Sun, L.; Liang, F.; Zhang, C.; Ying, Z.; Lin, W.; Zhao, F. Synthesis of acetophenone from aerobic catalytic oxidation of ethylbenzene over Ti–Zr–Co alloy catalyst: Influence of annealing conditions. Appl. Catal. A Gen. 2016, 512, 9–14. [Google Scholar] [CrossRef]
- Martins, L.M.D.R.S.; de Almeida, M.P.; Carabineiro, S.A.C.; Figueiredo, J.L.; Pombeiro, A.J.L. Heterogenisation of a C-Scorpionate FeII Complex on Carbon Materials for Cyclohexane Oxidation with Hydrogen Peroxide. ChemCatChem 2013, 5, 3847–3856. [Google Scholar] [CrossRef]
- Das, B.; Al-Hunaiti, A.; Haukka, M.; Demeshko, S.; Meyer, S.; Shteinman, A.A.; Meyer, F.; Repo, T.; Norlander, E. Catalytic Oxidation of Alkanes and Alkenes by H2O2 with a μ-Oxido Diiron(III) Complex as Catalyst/Catalyst Precursor. Eur. J. Inorg. Chem. 2015, 3590–3601. [Google Scholar] [CrossRef]
- Dias, S.S.P.; Kirillova, M.V.; André, V.; Kłak, J.; Kirillov, A.M. New tricopper(II) cores self-assembled fromaminoalcohol biobuffers and homophthalic acid: Synthesis, structural and topological features, magnetic properties and mild catalytic oxidation of cyclic and linear C5–C8 alkanes. Inorg. Chem. Front. 2015, 2, 525–537. [Google Scholar] [CrossRef]
- Olivo, G.; Nardi, M.; Vìdal, D.; Barbieri, A.; Lapi, A.; Gómez, L.; Lanzalunga, O.; Costas, M.; di Stefano, S. C–H Bond Oxidation Catalyzed by an Imine-Based Iron Complex: A Mechanistic Insight. Inorg. Chem. 2015, 54, 10141–10152. [Google Scholar] [CrossRef] [PubMed]
- Martins, L.; Nasani, R.; Saha, M.; Mobin, S.; Mukhopadhyay, S.; Pombeiro, A.J.L. Greener Selective Cycloalkane Oxidations with Hydrogen Peroxide Catalyzed by Copper-5-(4-pyridyl)tetrazolate Metal-Organic Frameworks. Molecules 2015, 20, 19203–19220. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.P.C.; Martins, L.M.D.R.S.; Hazra, S.; Pombeiro, A.J.L. Catalytic oxidation of cyclohexane with hydrogen peroxide and a tetracopper(II) complex in an ionic liquid. C. R. Chim. 2015, 18, 758–765. [Google Scholar] [CrossRef]
- Al-Hunaiti, A.; Räisänen, M.; Repo, T. From DNA to Catalysis: Thymine-acetate ligated non-heme iron(III) catalyst for oxidative activation of aliphatic C–H bonds. Chem. Commun. 2016, 52, 2043–2046. [Google Scholar] [CrossRef] [PubMed]
- Szymańska, A.; Nitck, W.; Oszajaca, M.; Łasocha, W.; Pamin, K.; Pońtowicz, J. Molybdenum Complexes as Catalysts for the oxidation of Cycloalkanes with Molecular Oxygen. Catal. Lett. 2016, 146, 998–1010. [Google Scholar] [CrossRef]
- Simaioforidou, A.; Papastergiou, M.; Margellou, A.; Petrakis, D.; Louloudi, M. Activated vs. Pyrolytic Carbon as Support Matrix for Chemical Functionalization: Efficient Heterogeneous non-Heme Mn(II) Catalysts for Alkene Oxidation with H2O2. J. Mol. Catal. A Chem. 2016. [Google Scholar] [CrossRef]
- Zhan, B-Z.; Modén, B.; Dakka, J.; Santiesteban, J.G.; Iglesia, E. Catalytic oxidation of n-hexane on Mn-exchanged zeolites: Turnover rates, regioselectivity, and spatial constraints. J. Catal. 2007, 245, 316–325. [Google Scholar]
- Aprile, C.; Corma, A.; Domine, M.E.; Garcia, H.; Mitchell, C. A cascade aerobic epoxidation of alkenes over Au/CeO2 and Ti-mesoporous material by “in situ” formed peroxides. J. Catal. 2009, 264, 44–53. [Google Scholar] [CrossRef]
- Mahmudov, K.T.; Kopylovich, M.N.; Guedes da Silva, M.F.C.; Figiel, P.J.; Karabach, Y.Y.; Pombeiro, A.J.L. New copper(II) dimer with 3-(2-hydroxy-4-nitrophenylhydrazo)pentane-2,4-dione and its catalytic activity in cyclohexane and benzyl alcohol oxidations. J. Mol. Catal. A Chem. 2010, 318, 44–50. [Google Scholar] [CrossRef]
- Estrada, A.C.; Simões, M.M.Q.; Santos, I.C.M.S.; Graca, M.; Neves, P.M.S.; Cavaleiro, J.A.S.; Cavaleiro, A.M.V. Catalytic activity of iron-substituted polyoxotungstates in the oxidation of aromatic compounds with hydrogen peroxide. Monatsh. Chem. 2010, 141, 1223–1235. [Google Scholar] [CrossRef]
- Jiang, D.; Mallat, T.; Meier, D.M.; Urakawa, A.; Baiker, A. Copper metal–organic framework: Structure and activity in the allylic oxidation of cyclohexene with molecular oxygen. J. Catal. 2010, 270, 26–33. [Google Scholar] [CrossRef]
- Lloyd, R.; Jenkins, R.L.; Piccinini, M.; He, Q.; Kiely, C.J.; Carley, A.F.; Golunski, S.E.; Bethell, D.; Bartley, J.K.; Hutchings, G.J. Low-temperature aerobic oxidation of decane using an oxygen-free radical initiator. J. Catal. 2011, 283, 161–167. [Google Scholar] [CrossRef]
- Maksimchuk, N.V.; Kolenko, K.A.; Fedin, V.P.; Kholdeeva, O.A. Cyclohexane selective oxidation over metal–organic frameworks of MIL-101 family: Superior catalytic activity and selectivity. Chem. Commun. 2012, 48, 6812–6814. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Yu, H.; Peng, F.; Wang, H. Selective Allylic Oxidation of Cyclohexene Catalyzed by Nitrogen-Doped Carbon Nanotubes. ACS Catal. 2014, 4, 1617–1625. [Google Scholar] [CrossRef]
- Garcia-Bosch, I.; Siegler, M.A. Copper-Catalyzed Oxidation of Alkanes with H2O2 under a Fenton-like Regime. Angew. Chem. Int. Ed. 2016, 55, 12873–12876. [Google Scholar] [CrossRef] [PubMed]
- Hazra, S.; Ribeiro, A.P.C.; Guedes da Silva, M.F.C.; de Castro, C.A.N.; Pombeiro, A.J.L. Syntheses and crystal structures of benzene-sulfonate and -carboxylate copper polymers and their application in the oxidation of cyclohexane in ionic liquid under mild conditions. Dalton Trans. 2016. [Google Scholar] [CrossRef]
- Haslinger, S.; Raba, A.; Cokoja, M.; Pöthig, A.; Kühn, F.E. Iron-catalyzed oxidation of unreactive C-H bonds: Utilizing bio-inspired axial ligand modification to increase catalyst stability. J. Catal. 2015, 331, 147–153. [Google Scholar] [CrossRef]
- Silva, T.F.S.; Rocha, B.G.M.; Guedes da Silva, M.F.C.; Martins, L.M.D.R.S.; Pombeiro, A.J.L. V(IV), Fe(II), Ni(II) and Cu(II) complexes bearing 2,2,2-tris(pyrazol-1-yl)ethyl methanesulfonate: Application as catalysts for the cyclooctane oxidation. New J. Chem. 2016, 40, 528–537. [Google Scholar] [CrossRef]
- Gupta, S.; Kirillova, M.V.; Guedes da Silva, M.F.C.; Pombeiro, A.J.L.; Kirillov, A.M. Alkali Metal Directed Assembly of Heterometallic Vv/M (M = Na, K, Cs) Coordination Polymers: Structures, Topological Analysis, and Oxidation Catalytic Properties. Inorg. Chem. 2013, 52, 8601–8611. [Google Scholar] [CrossRef] [PubMed]
- Naicker, D.; Friedrich, H.; Omondi, B. Cobalt aminodiphosphine complexes as catalysts in the oxidation of n-octane. RSC Adv. 2015, 5, 63123–63129. [Google Scholar] [CrossRef]
- Xue, S.; Chen, G.; Long, Z.; Zhou, Y.; Wang, J. Efficient and recyclable multi-cationic polyoxometalate-based hybrid catalyst for heterogeneous cyclohexane oxidation with H2O2. RSC Adv. 2015, 5, 19306–19314. [Google Scholar] [CrossRef]
- Wu, M.; Zhan, W.; Guo, Y.; Guo, Y.; Wang, Y.; Wang, L.; Lu, G. An effective Mn-Co mixed oxide catalyst for the solvent-free selective oxidation of cyclohexane with molecular oxygen. Appl. Catal. A: General 2016, 523, 97–106. [Google Scholar] [CrossRef]
- Mncube, S.G.; Bala, M.D. Recoverable aqueous-ionic liquid biphasic catalyst system for the oxidation of n-octane. J. Mol. Liq. 2016, 215, 396–401. [Google Scholar] [CrossRef]
- Dias, S.S.P.; Kirillova, M.V.; André, V.; Klak, J.; Kirillov, A.M. New Tetracopper(II) Cubane Cores Driven by a Diamino Alcohol: Selfassembly Synthesis, Structural and Topological Features, and Magnetic and Catalytic Oxidation Properties. Inorg. Chem. 2015, 54, 5204–5212. [Google Scholar] [CrossRef] [PubMed]
- Sutradhar, M.; Martins, L.M.D.R.S.; Guedes da Silva, M.F.C.; Mahmudov, K.T.; Liu, C.-M.; Pombeiro, A.J.L. Trinuclear CuII Structural Isomers: Coordination, Magnetism, Electrochemistry and Catalytic Activity towards the Oxidation of Alkanes. Eur. J. Inorg. Chem. 2015, 3959–3969. [Google Scholar] [CrossRef]
- Fernandes, T.A.; Santos, C.I.M.; André, V.; Dias, S.S.P.; Kirillova, M.V.; Kirillov, A.M. New aqua-soluble dicopper(II) aminoalcoholate cores for mild and water-assisted catalytic oxidation of alkanes. Catal. Sci. Technol. 2016, 6, 4584–4593. [Google Scholar] [CrossRef]
- Vikse, K.L.; Ahmadi, Z.; McIndoe, J.S. The application of electrospray ionization mass spectrometry to homogeneous catalysis. Coord. Chem. Rev. 2014, 279, 96–114. [Google Scholar] [CrossRef]
- Mutseneck, E.V.; Loginov, D.A.; Perekalin, D.S.; Starikova, Z.A.; Golovanov, D.G.; Petrovskii, P.V.; Zanello, P.; Corsini, M.; Laschi, F.; Kudinov, A.R. (Tetramethylcyclobutadiene)cobalt Complexes with Five-Electron Carbo- and Heterocyclic Ligands. Organometallics 2004, 23, 5944–5957. [Google Scholar] [CrossRef]
- Kudinov, A.R.; Mutseneck, E.V.; Loginov, D.A. (Tetramethylcyclobutadiene)cobalt chemistry. Coord. Chem. Rev. 2004, 248, 571–585. [Google Scholar] [CrossRef]
- King, R.B. Organometallic chemistry of the transition metals. XI. Some new cyclopentadienyl derivatives of cobalt and rhodium. Inorg. Chem. 1966, 5, 82–87. [Google Scholar] [CrossRef]
- Kudinov, A.R.; Loginov, D.A.; Muratov, D.V.; Petrovskii, P.V. Synthesis of dicationic triple-decker complexes with a central B-cyclohexyl-substituted borole ligand. Russ. Chem. Bull. 2001, 50, 1332–1333. [Google Scholar] [CrossRef]
- Roe, D.M.; Maitlis, P.M. Disproportionation of Dihalogenocyclopentadienylcobalt Complexes to Cobaltocenium Salts. J. Chem. Soc. A 1971, 3173–3175. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. 2008, A64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds 1–10 are available from the authors.



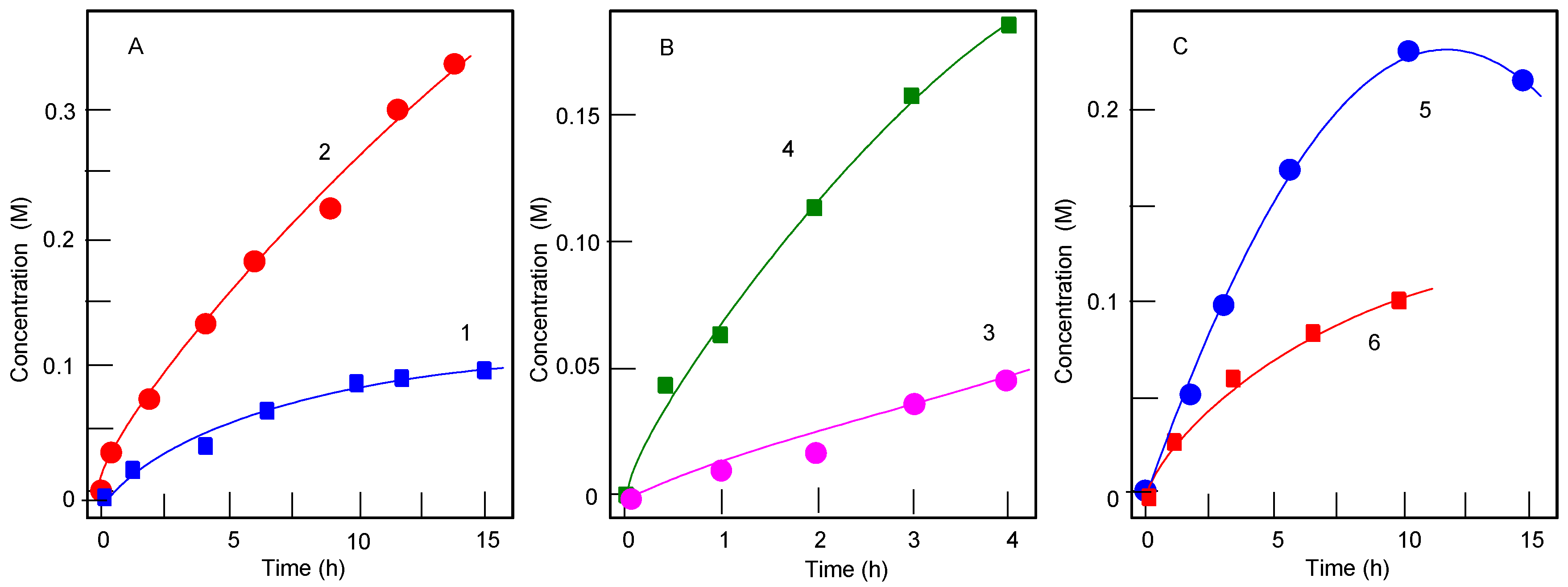

| Entry | Cat | Time (min) | Reduction with PPh3 | Cyclohexanone (mM) | Cyclohexanol (mM) | TON | TOF (h‒1) |
|---|---|---|---|---|---|---|---|
| 1 | 1 | 30 | No | 0.05 | 0.07 | 0.24 | 0.48 |
| 2 | 30 | Yes | 0 | 0.3 | 0.6 | 1.2 | |
| 3 | 120 | No | 0.05 | 0.08 | |||
| 4 | 120 | Yes | 0 | 0.7 | |||
| 5 | 5 2 | 60 | Yes | 0.1 | 0.6 | ||
| 6 | 180 | Yes | 0.5 | 1.0 | |||
| 7 | 7 | 120 | Yes | 0 | 1.4 | ||
| 8 | 7 2 | 60 | Yes | 0.2 | 1.0 | ||
| 9 | 120 | Yes | 1.0 | 2.3 | 6.6 | 3.3 |
| Entry | Cat | Time (min) | HNO3 (mM) | Cyclohexanone (mM) | Cyclohexanol (mM) | Total Yield (%) |
|---|---|---|---|---|---|---|
| 1 | 1 | 120 | 50 | 18 | 5 | 16 |
| 2 | 5 | 120 | 0 | 26 | 6 | 23 |
| 3 | 120 | 50 | 20 | 4.5 | 18 | |
| 4 | 6 | 60 | 50 | 0.7 | 2.6 | 3 |
| 5 | 180 | 50 | 1.9 | 5.3 | 5 |
| Run | Cat | Substrate | [HNO3] (mM) | Time (min) | t-Dimethyl-Alcohol (mM) | c-Dimethyl-Alcohol (mM) | Yield (%) | Ratio trans/cis |
|---|---|---|---|---|---|---|---|---|
| 1 | None | cis-1,2-DMCH | 0 | 30 | 0.13 | 0.17 | 0.2 | 0.76 |
| 2 | None | cis-1,2-DMCH | 0 | 120 | 2.9 | 4.1 | 5 | 0.74 |
| 3 | None | cis-1,2-DMCH | 50 | 30 | 0.07 | 0.5 | 0.4 | 0.14 |
| 4 | 1 | cis-1,2-DMCH | 0 | 30 | 3 | 23 | 19 | 0.13 |
| 5 | cis-1,2-DMCH | 0 | 120 | 4 | 29 | 24 | 0.14 | |
| 6 | cis-1,2-DMCH 2 | 50 | 120 | 2.4 | 52 | 39 | 0.05 | |
| 7 | cis-1,2-DMCH | 50 3 | 30 | 3 | 11 | 10 | 0.30 | |
| 8 | cis-1,2-DMCH | 50 3 | 120 | 5 | 19 | 17 | 0.26 | |
| 9 | cis-1,2-DMCH | 50 4 | 30 | 8 | 12 | 14 | 0.70 | |
| 10 | trans-1,2-DMCH | 0 | 30 | 19 | 8 | 19 | 2.4 | |
| 11 | trans-1,2-DMCH | 0 | 120 | 19 | 8 | 19 | 2.4 | |
| 12 | trans-1,2-DMCH | 50 | 30 | 26 | 1 | 19 | 19 | |
| 13 | trans-1,2-DMCH | 50 | 120 | 36 | 2 | 27 | 21 | |
| 14 | trans-1,2-DMCH | 50 | 30 | 13 | 8 | 15 | 1.6 | |
| 15 | trans-1,2-DMCH | 50 4 | 120 | 14 | 9 | 16 | 1.5 | |
| 16 | cis-1,4-DMCH | 50 | 30 | 3 | 48 | 37 | 0.07 | |
| 17 | cis-1,4-DMCH | 50 | 120 | 14 | 9 | 43 | 0.11 | |
| 18 | trans-1,4-DMCH | 50 | 30 | 30 | 3 | 23 | 12 | |
| 19 | trans-1,4-DMCH | 50 | 120 | 30 | 2 | 23 | 18 | |
| 20 | 2 | cis-1,2-DMCH | 0 | 120 | 10 | 32 | 30 | 0.28 |
| 21 | cis-1,2-DMCH | 50 | 30 | 3.5 | 44 | 34 | 0.08 | |
| 22 | cis-1,2-DMCH | 50 | 120 | 4 | 69 | 53 | 0.06 | |
| 23 | 3 | cis-1,2-DMCH | 50 | 30 | 3.5 | 50 | 35 | 0.07 |
| 24 | cis-1,2-DMCH | 50 | 120 | 4 | 61 | 46 | 0.07 | |
| 25 | 4 | cis-1,2-DMCH | 50 | 30 | 3 | 37 | 26 | 0.08 |
| 26 | cis-1,2-DMCH | 50 | 120 | 3 | 44 | 32 | 0.07 | |
| 27 | trans-1,4-DMCH | 50 | 30 | 24 | 1 | 17 | 27 | |
| 28 | trans-1,4-DMCH | 50 | 120 | 35 | 1 | 26 | 34 | |
| 29 | 5 | cis-1,2-DMCH | 50 | 90 | 9 | 14 | 16 | 0.64 |
| 30 | cis-1,2-DMCH | 50 | 180 | 4 | 10 | 10 | 0.4 | |
| 31 | 6 | cis-1,2-DMCH | 50 | 30 | 3 | 39 | 30 | 0.08 |
| 32 | cis-1,2-DMCH | 50 | 120 | 3 | 40 | 30 | 0.07 | |
| 33 | 7 | cis-1,2-DMCH | 50 | 300 | 3 | 6 | 6 | 0.5 |
| 34 | 8 | cis-1,2-DMCH | 50 | 30 | 17 | 54 | 50 | 0.3 |
| 35 | cis-1,2-DMCH | 50 | 120 | 17 | 57 | 53 | 0.3 | |
| 36 | 9 | cis-1,2-DMCH | 50 | 30 | 4.5 | 6 | 7 | 0.82 |
| 37 | cis-1,2-DMCH | 50 | 120 | 4.5 | 6 | 7 | 0.82 | |
| 38 | 10 | cis-1,2-DMCH | 50 | 30 | 3 | 4 | 5 | 0.84 |
| 39 | cis-1,2-DMCH | 50 | 120 | 3 | 4 | 5 | 0.84 |
| Compound | 7 | 9 | 10 |
|---|---|---|---|
| Empirical formula | C22H23CoF6IN2P | C15H13CoF6IN2P | C20H23CoF6IN2P |
| Formula weight | 646.22 | 552.07 | 622.20 |
| Crystal system | Triclinic | Orthorhombic | Monoclinic |
| Space group | P-1 | Pna21 | P21/n |
| a (Å) | 8.2684(9) | 16.7546(14) | 9.0966(18) |
| b (Å) | 11.4505(13) | 8.4636(7) | 26.448(5) |
| c (Å) | 12.4520(14) | 24.681(2) | 9.2018(17) |
| α (°) | 87.800(2) | - | - |
| β (°) | 84.628(2) | - | 90.574(4) |
| γ (°) | 77.714(2) | - | - |
| V (Å3) | 1146.7(2) | 3499.9(5) | 2213.7(7) |
| Dcalc (g·cm−3) | 1.872 | 2.095 | 1.867 |
| Linear absorption, μ (cm−3) | 22.27 | 28.98 | 23.02 |
| F(000) | 636 | 2128 | 1224 |
| 2θmax, ° | 56 | 52 | 54 |
| Reflections measured | 20,367 | 31,839 | 28,418 |
| Independent reflections | 5529 (Rint = 0.0416) | 6888 (Rint = 0.0642) | 4819 (Rint = 0.1797) |
| Observed reflections (I > 2σ(I)) | 4730 | 6398 | 4320 |
| Parameters | 303 | 484 | 307 |
| R1 | 0.0301 | 0.0620 | 0.0739 |
| wR2 | 0.0800 | 0.1721 | 0.2145 |
| Goodness-of-fit | 1.017 | 1.271 | 1.477 |
| Largest diff. peak and hole (e Å−3) | 1.44 and −0.72 | 2.49 and −1.51 | 2.34 and −1.92 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shul’pin, G.B.; Loginov, D.A.; Shul’pina, L.S.; Ikonnikov, N.S.; Idrisov, V.O.; Vinogradov, M.M.; Osipov, S.N.; Nelyubina, Y.V.; Tyubaeva, P.M. Stereoselective Alkane Oxidation with meta-Chloroperoxybenzoic Acid (MCPBA) Catalyzed by Organometallic Cobalt Complexes. Molecules 2016, 21, 1593. https://doi.org/10.3390/molecules21111593
Shul’pin GB, Loginov DA, Shul’pina LS, Ikonnikov NS, Idrisov VO, Vinogradov MM, Osipov SN, Nelyubina YV, Tyubaeva PM. Stereoselective Alkane Oxidation with meta-Chloroperoxybenzoic Acid (MCPBA) Catalyzed by Organometallic Cobalt Complexes. Molecules. 2016; 21(11):1593. https://doi.org/10.3390/molecules21111593
Chicago/Turabian StyleShul’pin, Georgiy B., Dmitriy A. Loginov, Lidia S. Shul’pina, Nikolay S. Ikonnikov, Vladislav O. Idrisov, Mikhail M. Vinogradov, Sergey N. Osipov, Yulia V. Nelyubina, and Polina M. Tyubaeva. 2016. "Stereoselective Alkane Oxidation with meta-Chloroperoxybenzoic Acid (MCPBA) Catalyzed by Organometallic Cobalt Complexes" Molecules 21, no. 11: 1593. https://doi.org/10.3390/molecules21111593