Pilot-Scale Production and Thermostability Improvement of the M23 Protease Pseudoalterin from the Deep Sea Bacterium Pseudoalteromonas sp. CF6-2
Abstract
:1. Introduction
2. Results
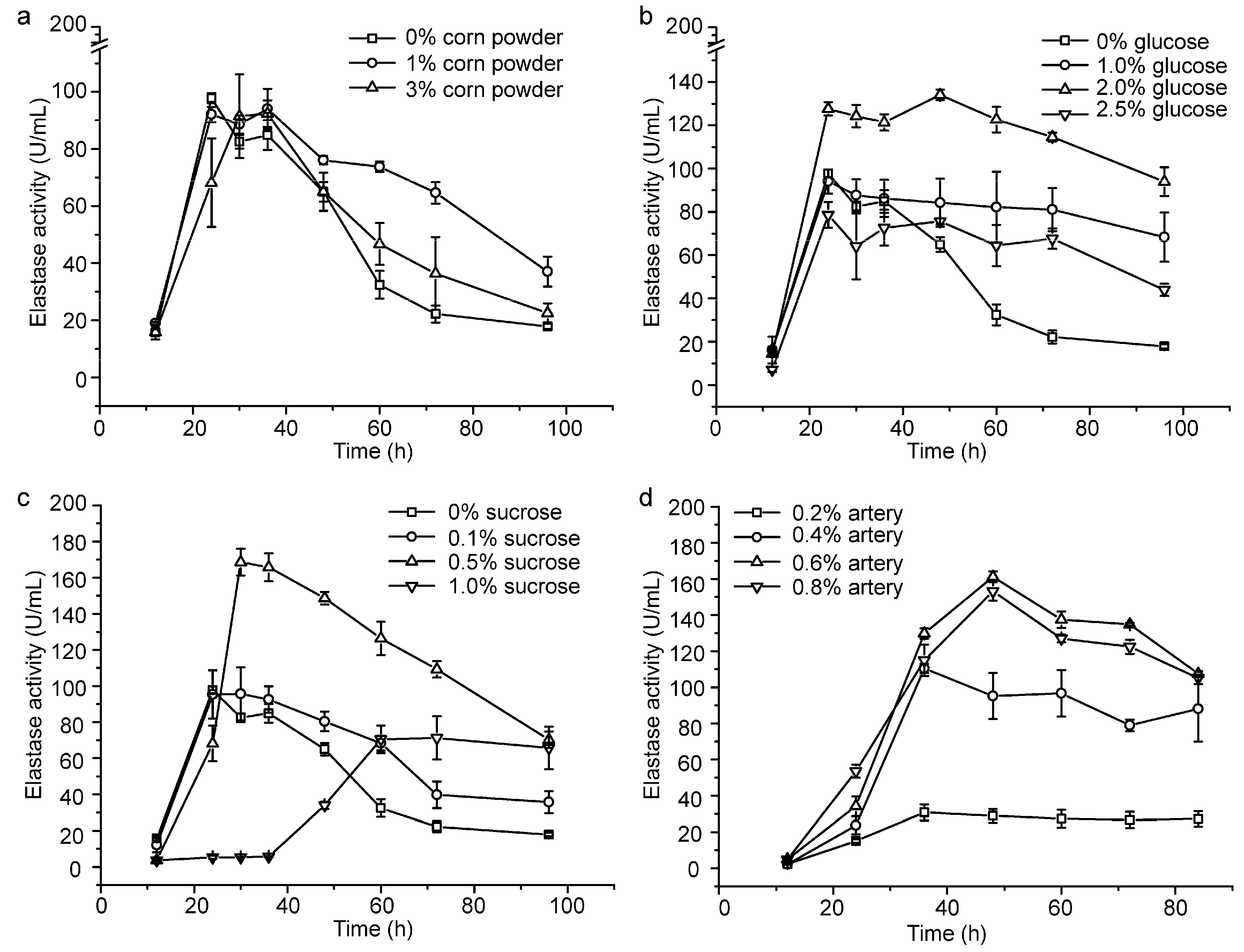
2.1. Optimization of the Fermentation Medium by Increasing the Content of the Carbon Source and Lowering the Usage of Artery Powder
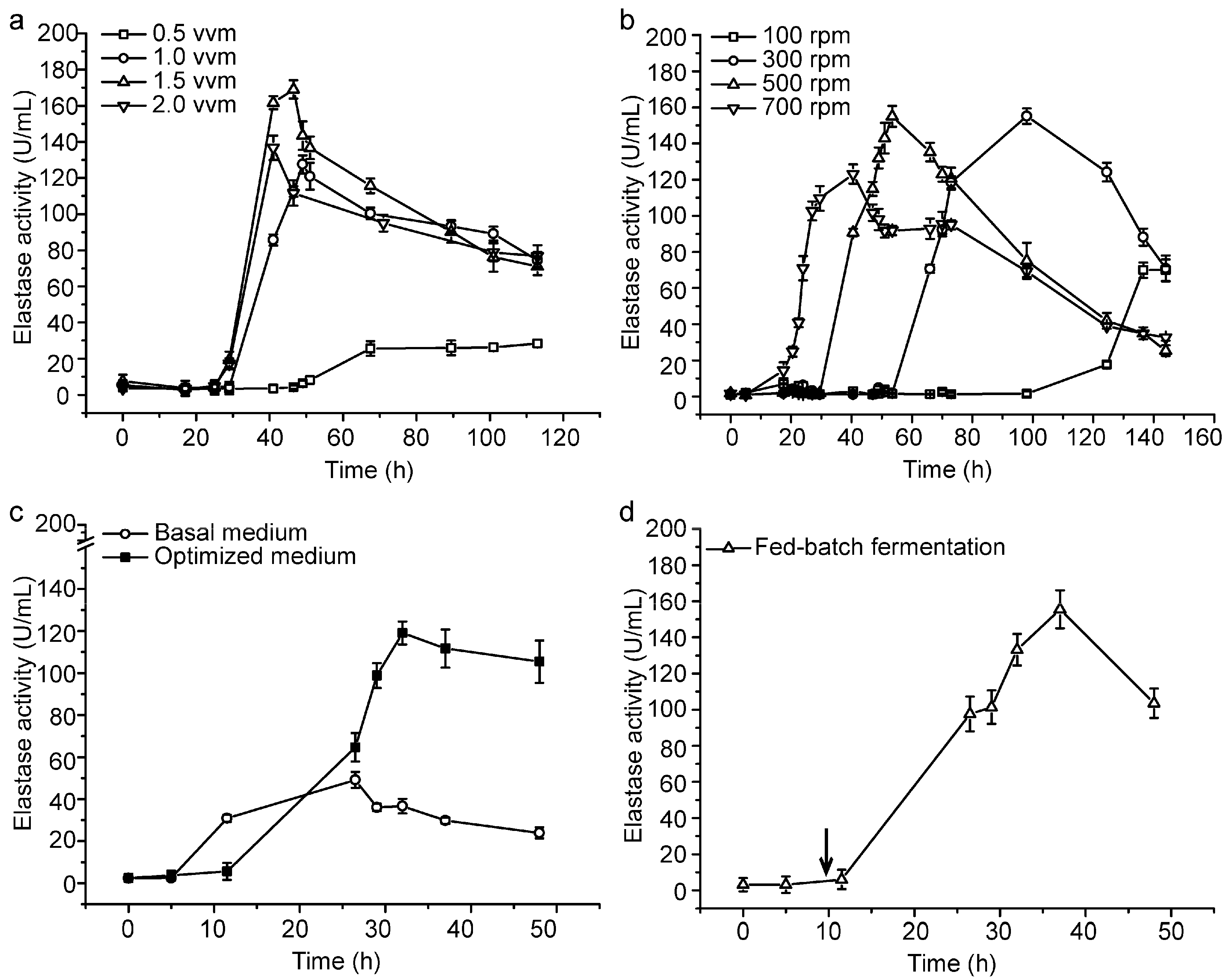
2.2. Small-Scale Fermentation of CF6-2 for Pseudoalterin Production
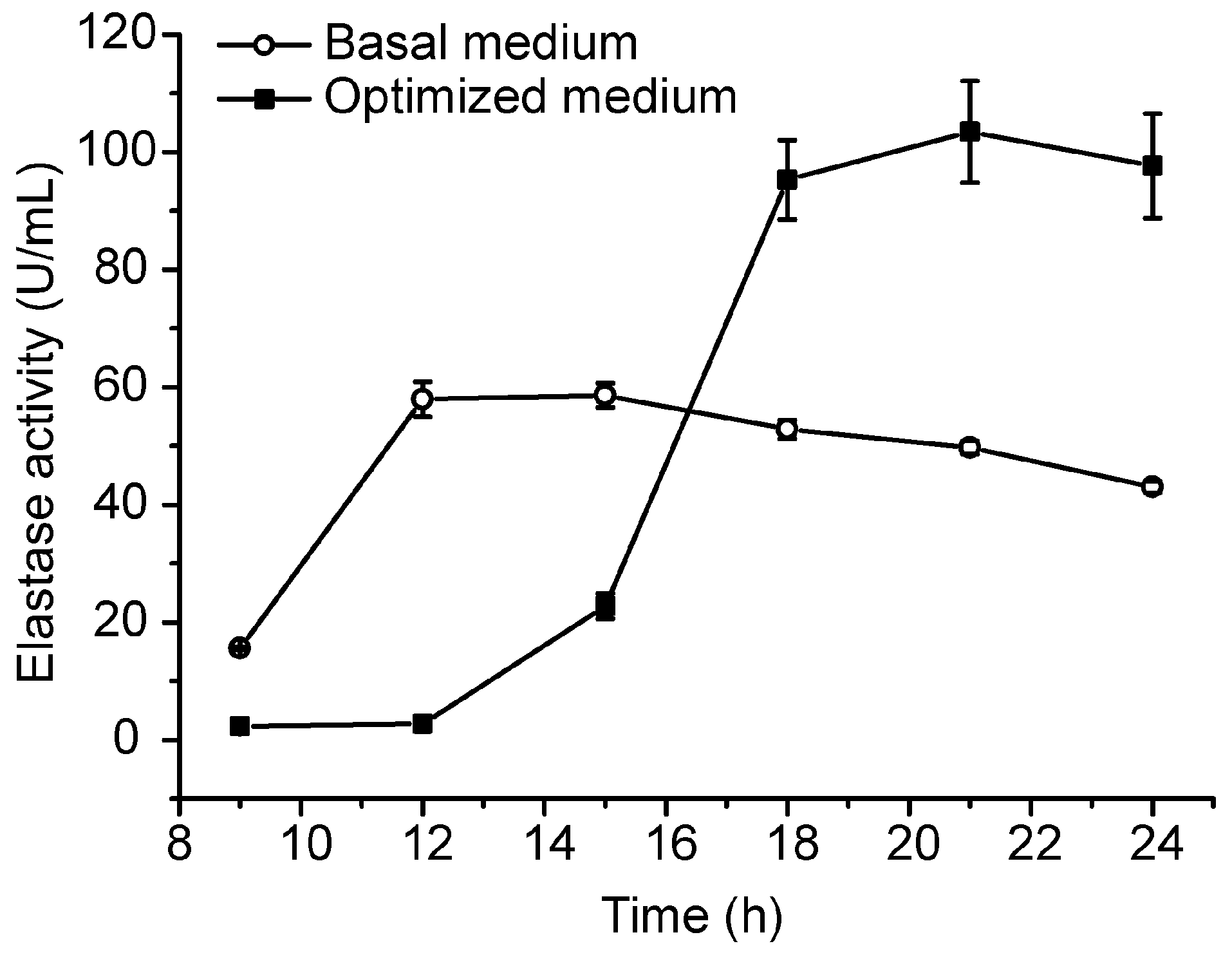
2.3. Pilot-Scale Fermentation of CF6-2 for Pseudoalterin Production
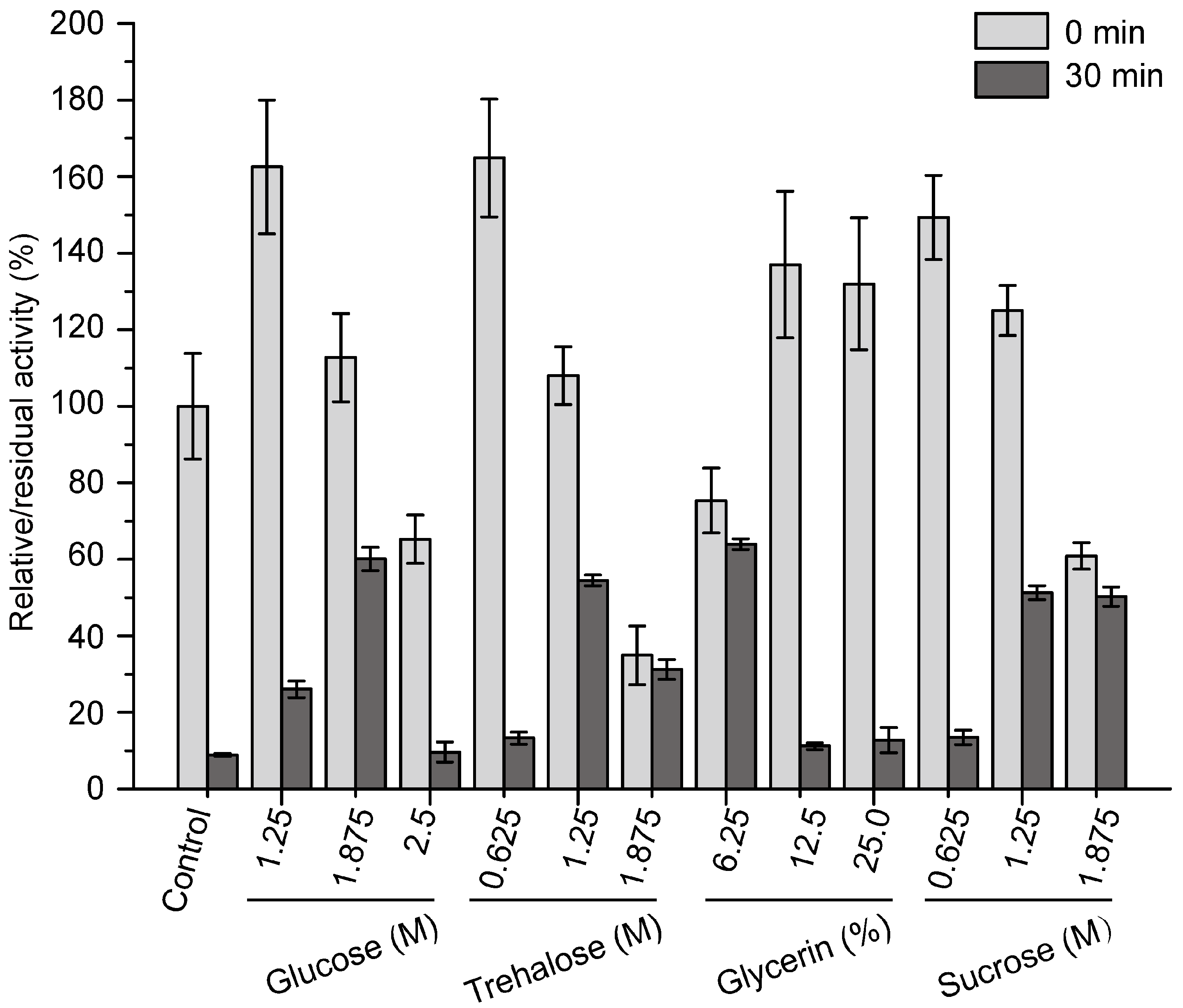
2.4. Effects of Sugars and Polyols on the Thermostability of Pseudoalterin
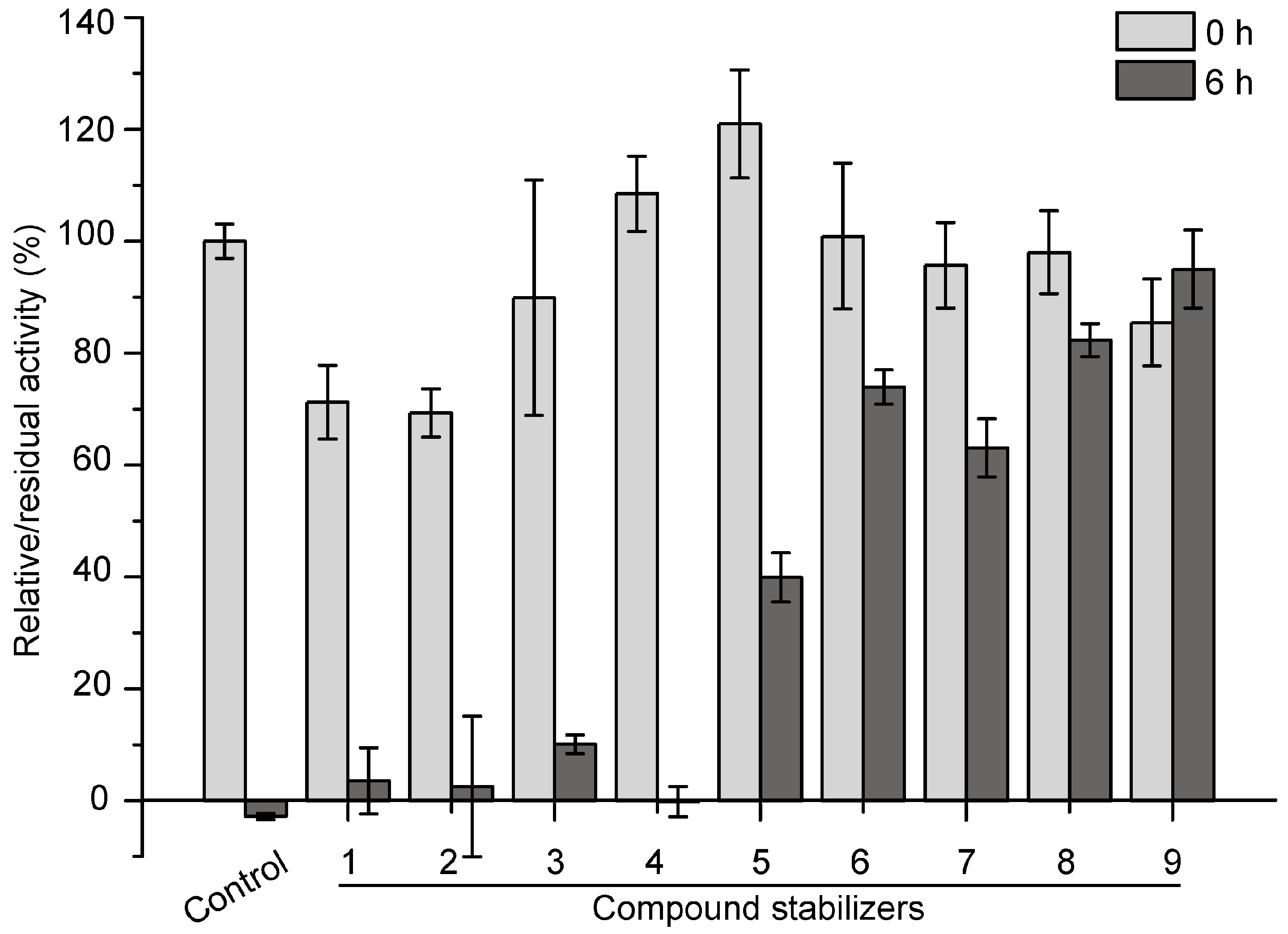
2.5. Development of an Effective Compound Stabilizer for Pseudoalterin by an Orthogonal Test
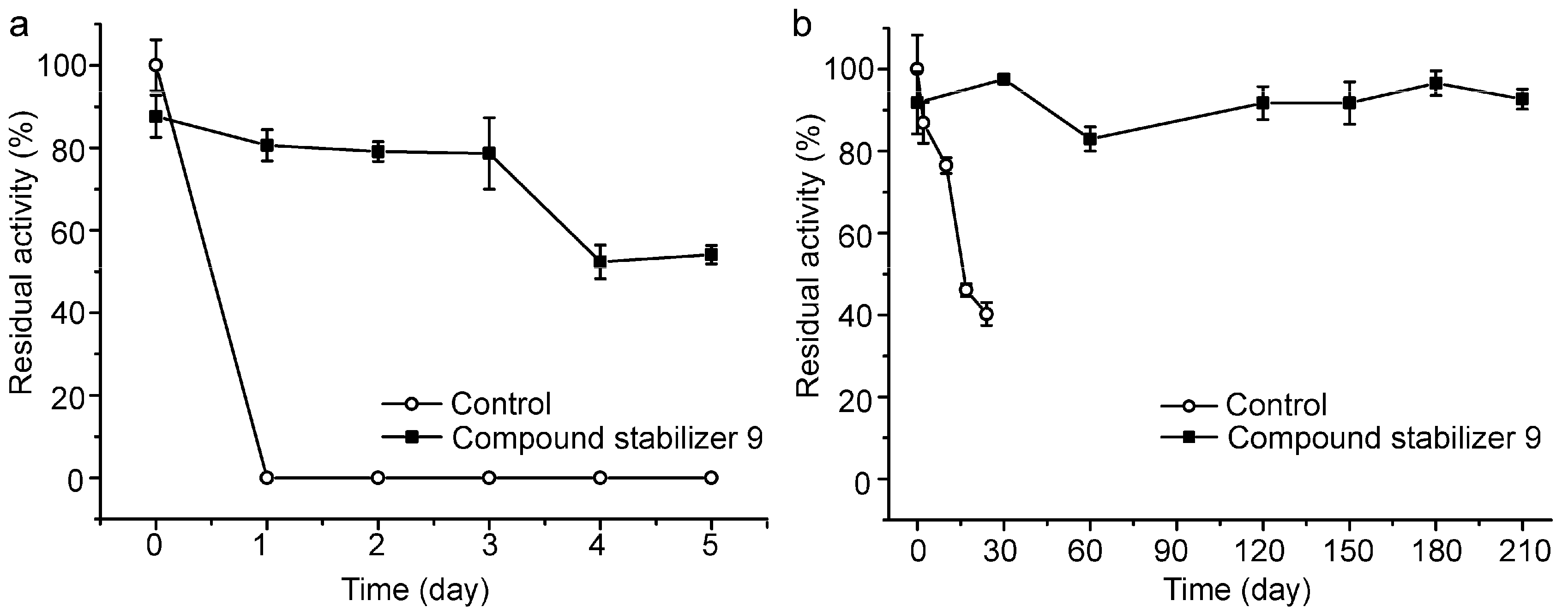
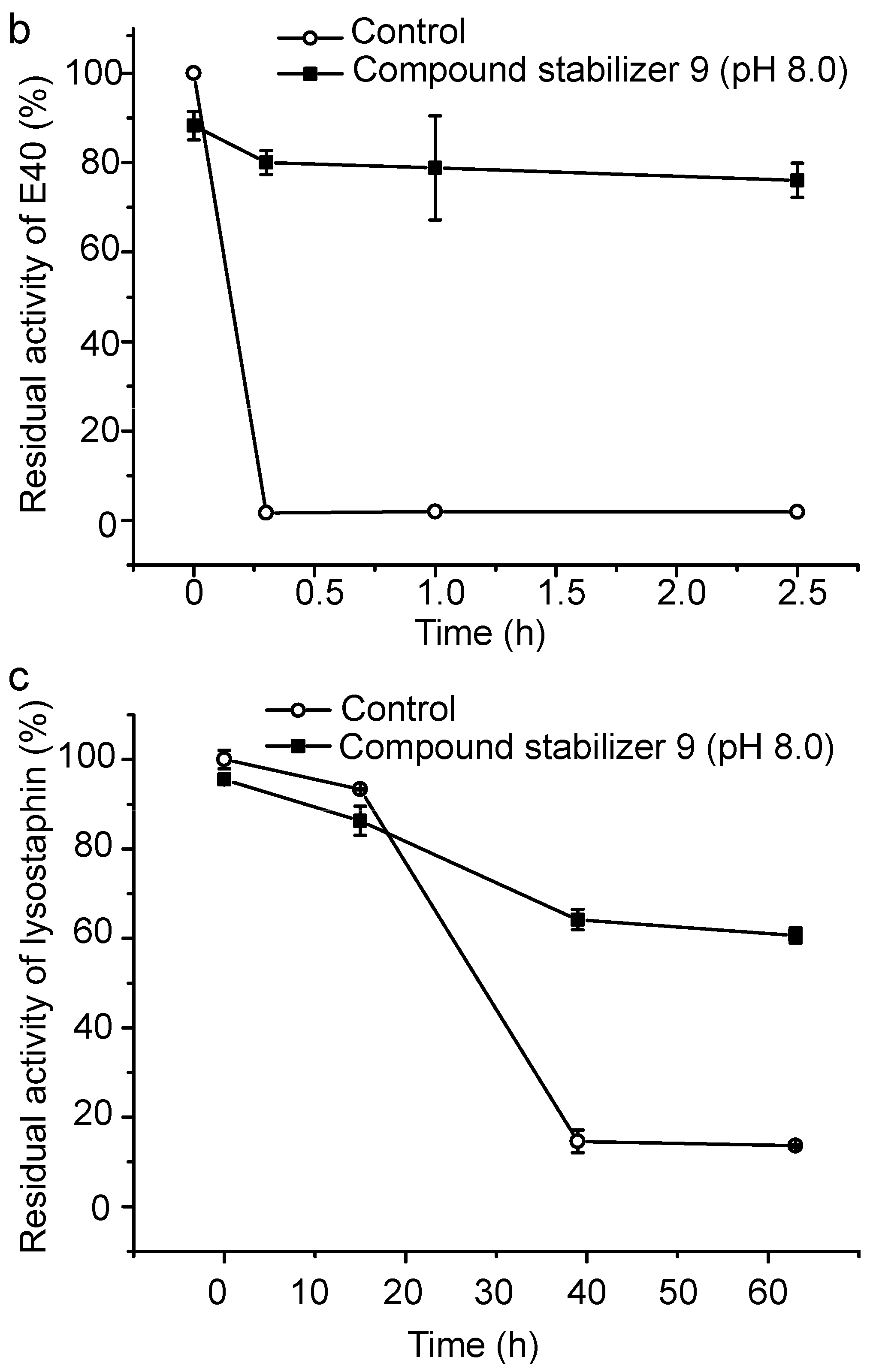
2.6. Effect of Compound Stabilizer 9 on the Thermostability of Pseudoalterin as Well as Other Enzymes
3. Discussion
4. Materials and Methods
4.1. Strains and Media
4.2. Inoculum Preparation and Flask Fermentation
4.3. Enzyme Assay
4.4. Optimization of the Fermentation Medium
4.5. Optimization of Aeration Rate and Stirring Speed in a Mini-in Parallel Fermenter System
4.6. Small-Scale Fermentation
4.7. Pilot-Scale Fermentation
4.8. Assay for the Effects of Sugars and Polyols on Pseudoalterin Thermostability
4.9. Compound Stabilizer Design by Orthogonal Test
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Talbot, V.; Bianchi, M. Bacterial proteolytic activity in sediments of the Subantarctic Indian Ocean sector. Deep Sea Res. II Top. Stud. Oceanogr. 1997, 44, 1069–1084. [Google Scholar] [CrossRef]
- Zhou, M.Y.; Zhang, Y.Z. Diversity of both the cultivable protease-producing bacteria and their extracellular proteases in the sediments of the South China sea. Microb. Ecol. 2009, 58, 582–590. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.Y.; Wang, G.L.; Li, D.; Zhao, D.L.; Qin, Q.L.; Chen, X.L.; Chen, B.; Zhou, B.C.; Zhang, X.Y.; Zhang, Y.Z. Diversity of Both the Cultivable Protease-Producing Bacteria and Bacterial Extracellular Proteases in the Coastal Sediments of King George Island, Antarctica. PLoS ONE 2013, 8, e79668. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.L.; Xie, B.B.; Lu, J.T.; He, H.L.; Zhang, Y. A novel type of subtilase from the psychrotolerant bacterium Pseudoalteromonas sp. SM9913: Catalytic and structural properties of deseasin MCP-01. Microbiology 2007, 153, 2116–2125. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.Y.; Chen, X.L.; Zhao, H.L.; Xie, B.B.; Zhou, B.C.; Zhang, Y.Z. Hydrolysis of insoluble collagen by deseasin MCP-01 from deep-sea Pseudoalteromonas sp. SM9913: Collagenolytic characters, collagen-binding ability of C-terminal polycystic kidney disease domain, and implication for its novel role in deep-sea sedimentary particulate organic nitrogen degradation. J. Biol. Chem. 2008, 283, 36100–36107. [Google Scholar] [PubMed]
- Ran, L.Y.; Su, H.N.; Zhou, M.Y.; Wang, L.; Chen, X.L.; Xie, B.B.; Song, X.Y.; Shi, M.; Qin, Q.L.; Pang, X.; et al. Characterization of a novel subtilisin-like protease myroicolsin from deep sea bacterium Myroides profundi D25 and molecular insight into its collagenolytic mechanism. J. Biol. Chem. 2014, 289, 6041–6053. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.L.; Xie, B.B.; Bian, F.; Zhao, G.Y.; Zhao, H.L.; He, H.L.; Zhou, B.C.; Zhang, Y.Z. Ecological function of myroilysin, a novel bacterial M12 metalloprotease with elastinolytic activity and a synergistic role in collagen hydrolysis, in biodegradation of deep-sea high-molecular-weight organic nitrogen. Appl. Environ. Microbiol. 2009, 75, 1838–1844. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.Y.; Wang, P.; Li, C.Y.; Dong, S.; Song, X.Y.; Zhang, X.Y.; Xie, B.B.; Zhou, B.C.; Zhang, Y.Z.; Chen, X.L. Characterization of a New M13 Metallopeptidase from Deep-Sea Shewanella sp. E525-6 and Mechanistic Insight into Its Catalysis. Front. Microbiol. 2015, 6, 1498. [Google Scholar] [CrossRef] [PubMed]
- Esin Hameş-Kocabaş, E.; Uzel, A. Alkaline protease production by an actinomycete MA1-1 isolated from marine sediments. Ann. Microbiol. 2007, 5, 71–75. [Google Scholar] [CrossRef]
- Yang, J.; Li, J.; Mai, Z.; Tian, X.; Zhang, S. Purification, characterization, and gene cloning of a cold-adapted thermolysin-like protease from Halobacillus sp. SCSIO 20089. J. Biosci. Bioeng. 2013, 115, 628–632. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.L.; Chen, X.L.; Xie, B.B.; Zhou, M.Y.; Gao, X.; Zhang, X.Y.; Zhou, B.C.; Weiss, A.S.; Zhang, Y.Z. Elastolytic mechanism of a novel M23 metalloprotease pseudoalterin from deep-sea Pseudoalteromonas sp. CF6-2: Cleaving not only glycyl bonds in the hydrophobic regions but also peptide bonds in the hydrophilic regions involved in cross-linking. J. Biol. Chem. 2012, 287, 39710–39720. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, K.; Chohnan, S.; Ohashi, H.; Hirata, T.; Masaki, T.; Sakiyama, F. Purification, bacteriolytic activity, and specificity of beta-lytic protease from Lysobacter sp. IB-9374. J. Biosci. Bioeng. 2003, 95, 27–34. [Google Scholar] [CrossRef]
- Kessler, E.; Safrin, M.; Olson, J.C.; Ohman, D.E. Secreted LasA of Pseudomonas aeruginosa is a staphylolytic protease. J. Biol. Chem. 1993, 268, 7503–7508. [Google Scholar] [PubMed]
- Park, P.W.; Senior, R.M.; Griffin, G.L.; Broekelmann, T.J.; Mudd, M.S.; Mecham, R.P. Binding and degradation of elastin by the staphylolytic enzyme lysostaphin. Int. J. Biochem. Cell Biol. 1995, 27, 139–146. [Google Scholar] [CrossRef]
- Elston, C.; Wallach, J.; Saulnier, J. New continuous and specific fluorometric assays for Pseudomonas aeruginosa elastase and LasA protease. Anal. Biochem. 2007, 368, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Donovan, D.M. Bacteriophage and peptidoglycan degrading enzymes with antimicrobial applications. Recent Pat. Biotechnol. 2007, 1, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Dajcs, J.J.; Thibodeaux, B.A.; Hume, E.B.; Zheng, X.; Sloop, G.D.; O’callaghan, R.J. Lysostaphin is effective in treating methicillin-resistant Staphylococcus aureus endophthalmitis in the rabbit. Curr. Eye Res. 2001, 22, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Dajcs, J.J.; Thibodeaux, B.A.; Girgis, D.O.; Shaffer, M.D.; Delvisco, S.M.; O’callaghan, R.J. Immunity to lysostaphin and its therapeutic value for ocular MRSA infections in the rabbit. Investig. Ophthalmol. Vis. Sci. 2002, 43, 3712–3716. [Google Scholar]
- Barequet, I.S.; Ben Simon, G.J.; Safrin, M.; Ohman, D.E.; Kessler, E. Pseudomonas aeruginosa LasA protease in treatment of experimental staphylococcal keratitis. Antimicrob. Agents Chemother. 2004, 48, 1681–1687. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.H.; Babbitt, P.C.; Vasquez, J.R.; West, B.L.; Kenyon, G.L. Cloning and expression of functional rabbit muscle creatine kinase in Escherichia coli. Addressing the problem of microheterogeneity. J. Biol. Chem. 1991, 266, 12053–12057. [Google Scholar] [PubMed]
- Jiang, X.Y.; Wang, C.F.; Zhang, P.J.; He, Z.Y. Cloning and expression of Mycobacterium bovis secreted protein MPB83 in Escherichia coli. J. Biochem. Mol. Biol. 2006, 39, 22–25. [Google Scholar] [PubMed]
- Cavicchioli, R.; Siddiqui, K.S.; Andrews, D.; Sowers, K.R. Low-temperature extremophiles and their applications. Curr. Opin. Biotechnol. 2002, 13, 253–261. [Google Scholar] [CrossRef]
- Georlette, D.; Blaise, V.; Collins, T.; D’amico, S.; Gratia, E.; Hoyoux, A.; Marx, J.C.; Sonan, G.; Feller, G.; Gerday, C. Some like it cold: Biocatalysis at low temperatures. FEMS Microbiol. Rev. 2004, 28, 25–42. [Google Scholar] [CrossRef] [PubMed]
- Rawlings, N.D.; Barrett, A.J.; Bateman, A. MEROPS: The peptidase database. Nucleic Acids Res. 2010, 38, D227–D233. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.L.; Yang, J.; Chen, X.L.; Su, H.N.; Zhang, X.Y.; Huang, F.; Zhou, B.C.; Xie, B.B. Optimization of fermentation conditions for the production of the M23 protease Pseudoalterin by deep-sea Pseudoalteromonas sp. CF6–2 with artery powder as an inducer. Molecules 2014, 19, 4779–4790. [Google Scholar] [CrossRef] [PubMed]
- Li, P.Y.; Chen, X.L.; Ji, P.; Li, C.Y.; Wang, P.; Zhang, Y.; Xie, B.B.; Qin, Q.L.; Su, H.N.; Zhou, B.C.; et al. Interdomain hydrophobic interactions modulate the thermostability of microbial esterases from the hormone-sensitive lipase family. J. Biol. Chem. 2015, 290, 11188–11198. [Google Scholar] [CrossRef] [PubMed]
- Recsei, P.A.; Gruss, A.D.; Novick, R.P. Cloning, sequence, and expression of the lysostaphin gene from Staphylococcus simulans. Proc. Natl. Acad. Sci. USA 1987, 84, 1127–1131. [Google Scholar] [CrossRef] [PubMed]
- Arpigny, J.L.; Feller, G.; Gerday, C. Cloning, sequence and structural features of a lipase from the antarctic facultative psychrophile Psychrobacter immobilis B10. Biochim. Biophys. Acta 1993, 1171, 331–333. [Google Scholar] [CrossRef]
- Davail, S.; Feller, G.; Narinx, E.; Gerday, C. Cold adaptation of proteins. Purification, characterization, and sequence of the heat-labile subtilisin from the antarctic psychrophile Bacillus TA41. J. Biol. Chem. 1994, 269, 17448–17453. [Google Scholar] [PubMed]
- Lee, J.C.; Timasheff, S.N. The stabilization of proteins by sucrose. J. Biol. Chem. 1981, 256, 7193–7201. [Google Scholar] [PubMed]
- Samborska, K.; Guiavarc’h, Y.; Loey, A.V.; Hendrickx, M. The thermal stability of Aspergillus oryzae alpha-amylase in presence of sugars and polyols. J. Food Process Eng. 2006, 19, 287–303. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds pseudoalterin and compound stabilizers are available from the authors.

| Level | Factor | |||
|---|---|---|---|---|
| A | B | C | D | |
| Glucose (M) | Trehalose (M) | Glycerin (%) | Sucrose (M) | |
| 1 | 0.25 | 0.25 | 1.25 | 0.25 |
| 2 | 0.9375 | 0.625 | 2.5 | 0.125 |
| 3 | 1.875 | 1.25 | 6.25 | 0.0625 |
| Test No. | Glucose | Trehalose | Glycerin | Sucrose |
|---|---|---|---|---|
| 1 | 1 | 1 | 1 | 1 |
| 2 | 1 | 2 | 2 | 2 |
| 3 | 1 | 3 | 3 | 3 |
| 4 | 2 | 1 | 2 | 3 |
| 5 | 2 | 2 | 3 | 1 |
| 6 | 2 | 3 | 1 | 2 |
| 7 | 3 | 1 | 3 | 2 |
| 8 | 3 | 2 | 1 | 3 |
| 9 | 3 | 3 | 2 | 1 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.; Yu, Y.; Tang, B.-L.; Zhong, S.; Shi, M.; Xie, B.-B.; Zhang, X.-Y.; Zhou, B.-C.; Zhang, Y.-Z.; Chen, X.-L. Pilot-Scale Production and Thermostability Improvement of the M23 Protease Pseudoalterin from the Deep Sea Bacterium Pseudoalteromonas sp. CF6-2. Molecules 2016, 21, 1567. https://doi.org/10.3390/molecules21111567
Yang J, Yu Y, Tang B-L, Zhong S, Shi M, Xie B-B, Zhang X-Y, Zhou B-C, Zhang Y-Z, Chen X-L. Pilot-Scale Production and Thermostability Improvement of the M23 Protease Pseudoalterin from the Deep Sea Bacterium Pseudoalteromonas sp. CF6-2. Molecules. 2016; 21(11):1567. https://doi.org/10.3390/molecules21111567
Chicago/Turabian StyleYang, Jie, Yang Yu, Bai-Lu Tang, Shuai Zhong, Mei Shi, Bin-Bin Xie, Xi-Ying Zhang, Bai-Cheng Zhou, Yu-Zhong Zhang, and Xiu-Lan Chen. 2016. "Pilot-Scale Production and Thermostability Improvement of the M23 Protease Pseudoalterin from the Deep Sea Bacterium Pseudoalteromonas sp. CF6-2" Molecules 21, no. 11: 1567. https://doi.org/10.3390/molecules21111567
APA StyleYang, J., Yu, Y., Tang, B.-L., Zhong, S., Shi, M., Xie, B.-B., Zhang, X.-Y., Zhou, B.-C., Zhang, Y.-Z., & Chen, X.-L. (2016). Pilot-Scale Production and Thermostability Improvement of the M23 Protease Pseudoalterin from the Deep Sea Bacterium Pseudoalteromonas sp. CF6-2. Molecules, 21(11), 1567. https://doi.org/10.3390/molecules21111567






