Eluent Tolerance and Enantioseparation Recovery of Chiral Packing Materials Based on Chitosan Bis(Phenylcarbamate)-(n-Octyl Urea)s for High Performance Liquid Chromatography †
Abstract
:1. Introduction
2. Results and Discussion
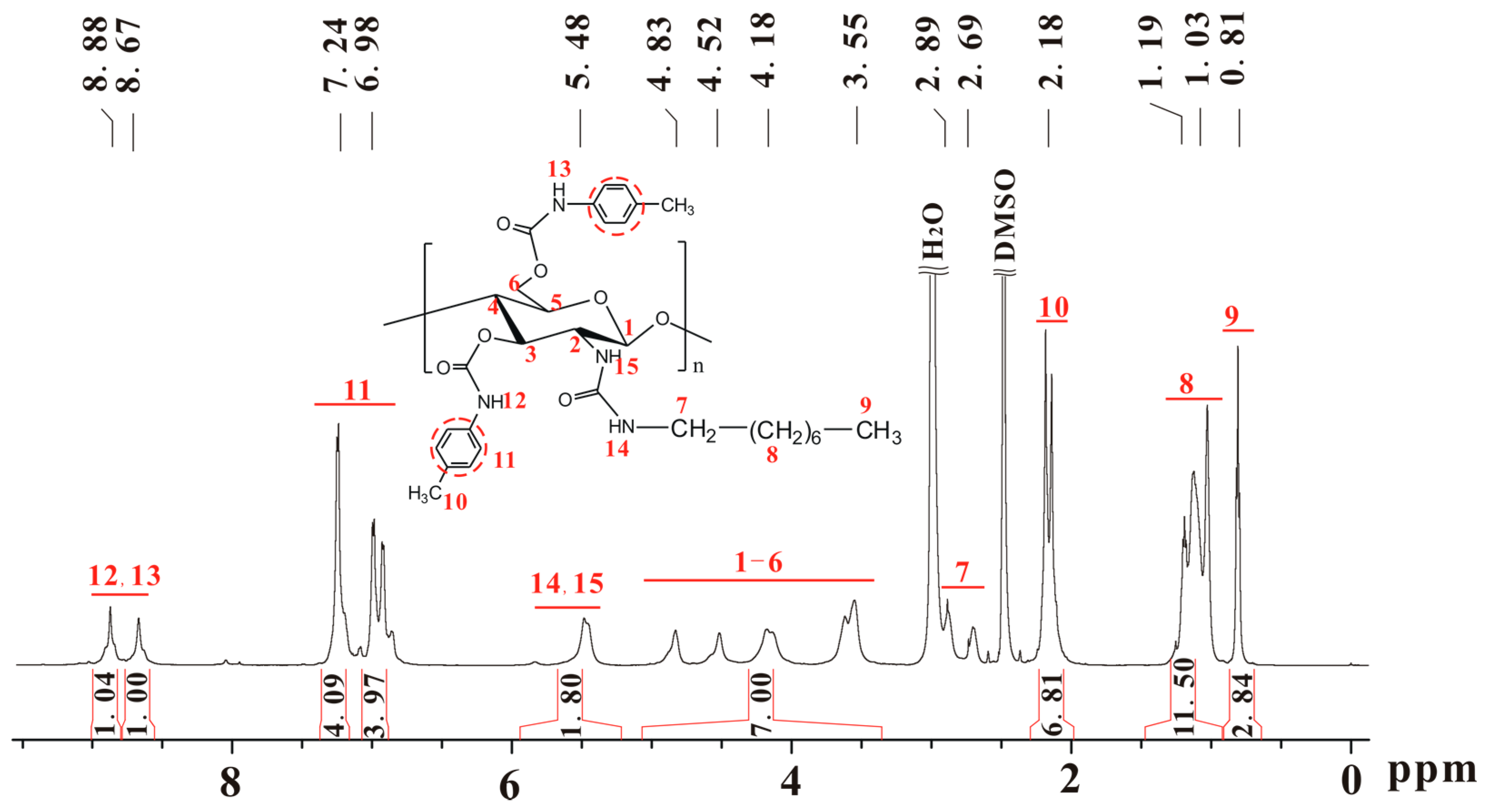
2.1. Characterization of Chitosan Derivatives
2.2. Swelling Capacity of the Chiral Selectors
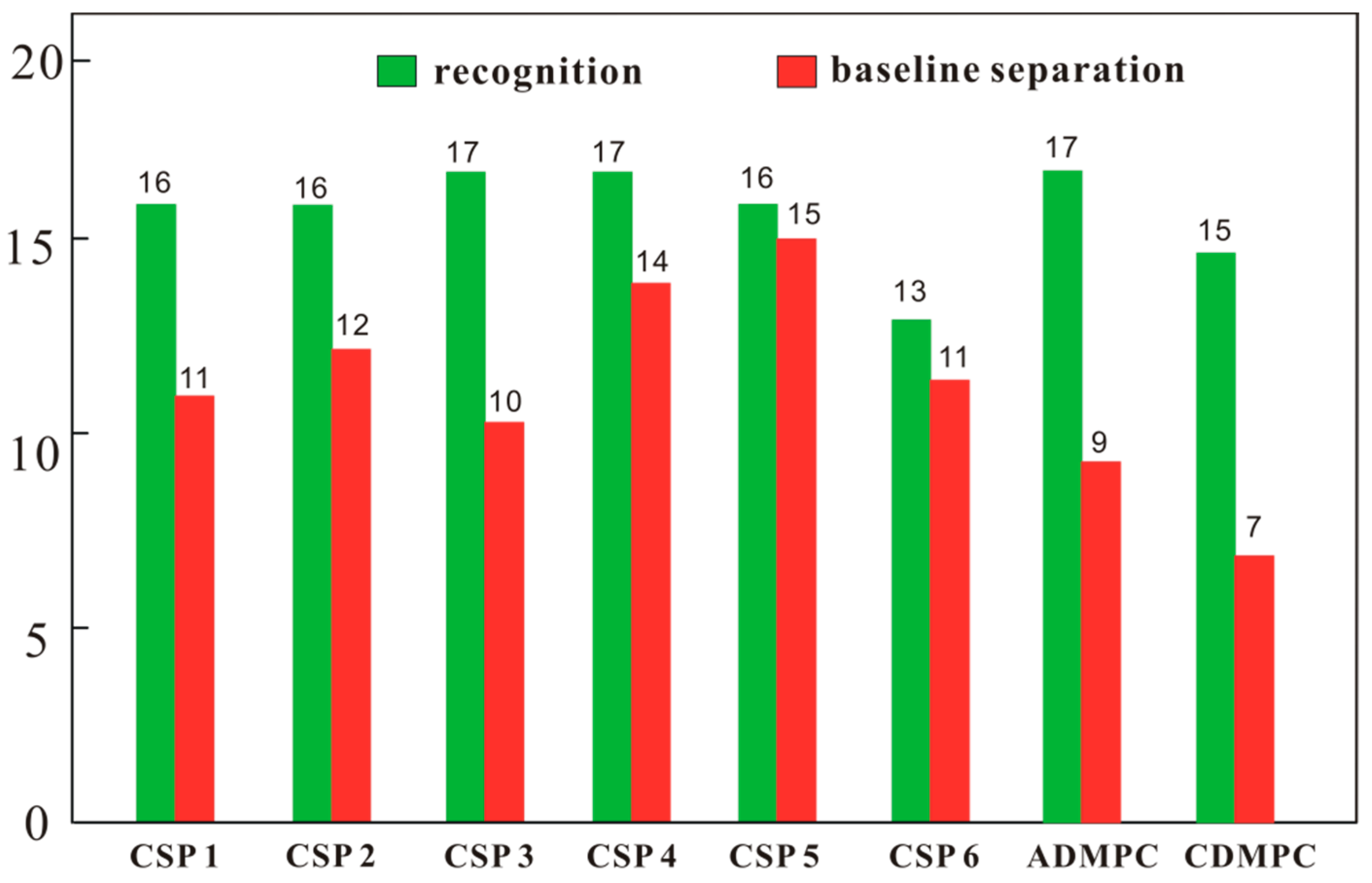
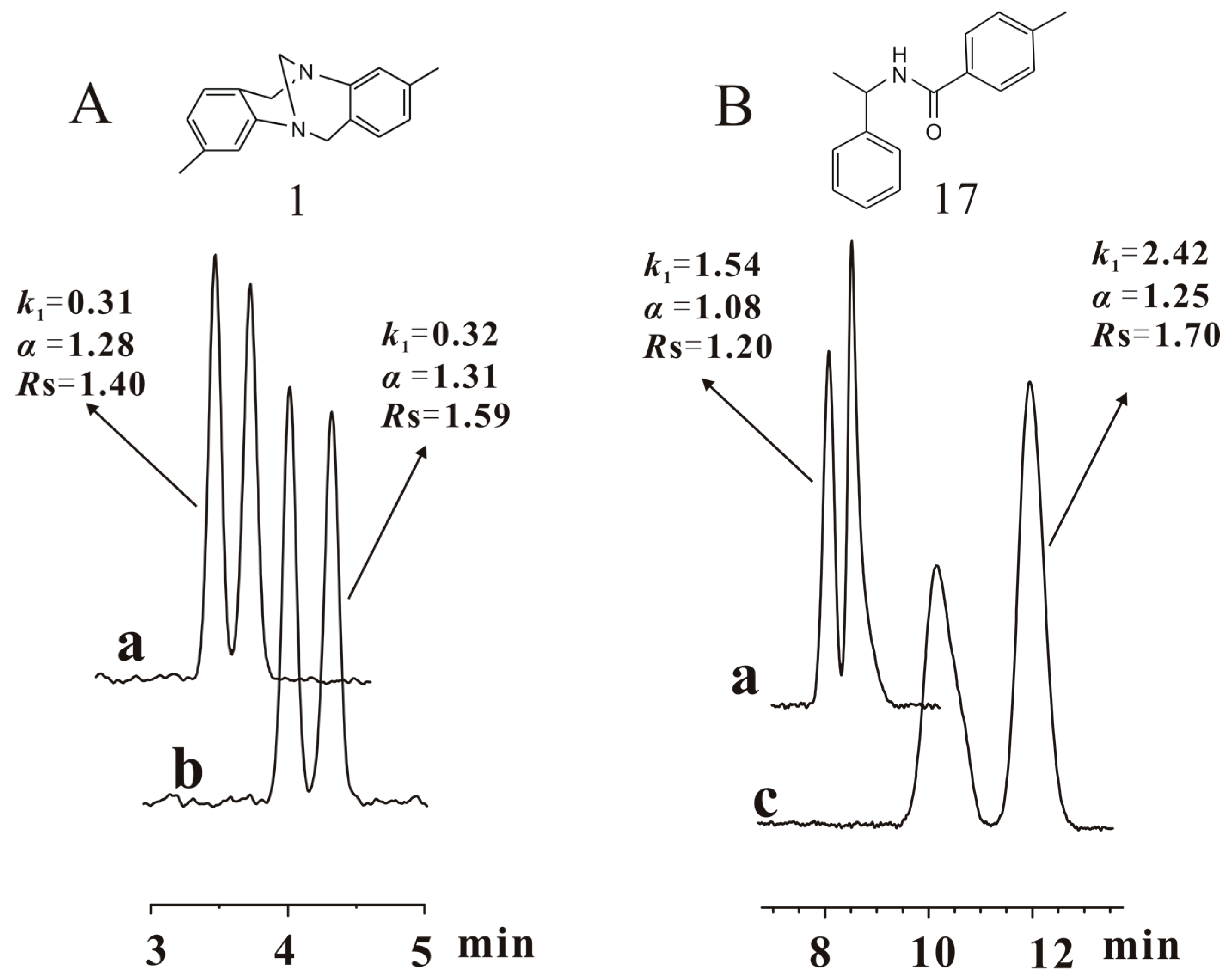
2.3. Enantioseparation Capability of CSPs 1–6
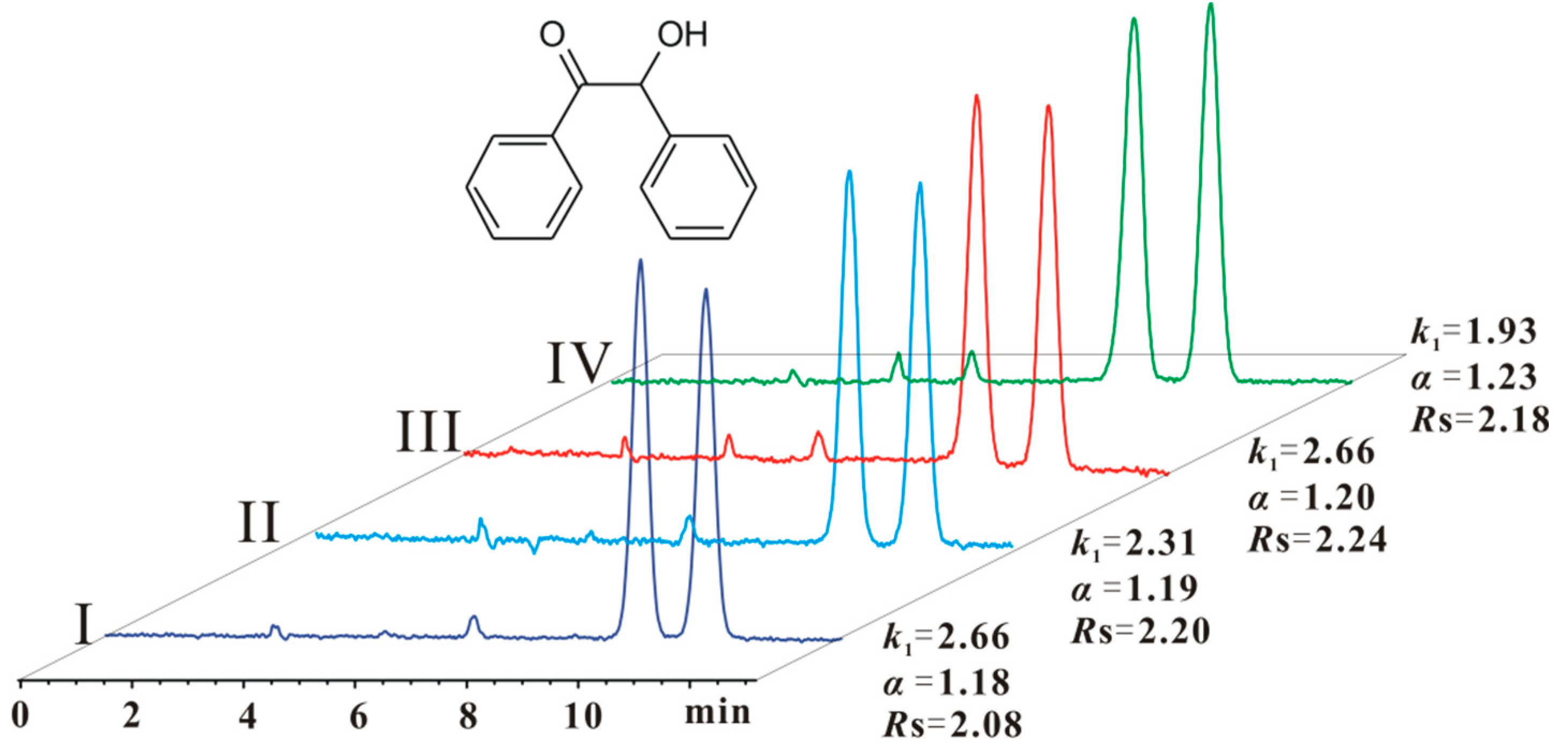
2.4. Eluents Tolerance and Enantioseparation Recovery of CS 5
3. Experimental Section
3.1. Materials and Instruments
3.2. Preparation and Characterization of Chitosan Derivatives
3.3. Swelling Capacity Evaluation of Chitosan Derivatives
3.4. Preparation of Chiral Stationary Phases and Columns Packing
3.5. Enantioseparation Evaluation of Chiral Stationary Phases
4. Conclusions
Supplementary Materials
Acknowledgements
Author Contributions
Conflicts of Interest
References
- Chai, T.; Yang, W.; Qiu, J.; Hou, S. Direct Enantioseparation of nitrogen-heterocyclic pesticides on cellulose-based chiral column by high-performance liquid chromatography. Chirality 2015, 27, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Geryk, R.; Kalíkova, K.; Schmid, M.G.; Tesarova, E. Enantioselective separation of biologically active basic compounds in ultra-performance supercritical fluid chromatography. Anal. Chim. Acta 2016, 932, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Batra, S.; Bhushan, R. Resolution of enantiomers of bupropion and its metabolites by liquid chromatography. Biomed. Chromatogr. 2016, 30, 670–682. [Google Scholar] [CrossRef] [PubMed]
- Skogsberg, U.; Allenmark, S. Some conformationally restricted chiral stationary phase selector units related to N,N′-diallyl-l-tartardiamide. Chromatographia 2001, 51, 691–695. [Google Scholar] [CrossRef]
- Thunberg, L.; Allenmark, S. Resolution studies on two regioisomeric chiral stationary phases: Effects from reversed orientation of an amide group. J. Chromatogr. A 2004, 1026, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Okamoto, Y. Efficient Separation of enantiomers using stereoregular chiral polymers. Chem. Rev. 2016, 116, 1094–1138. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.-R.; Yip, S.H.; Li, P.; Sun, D.; Mathur, A. From analytical methods to large scale chiral SFC using chlorinated chiral stationary phases. J. Chromatogr. A 2016, 1432, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Chen, S.; Luo, P.; Sun, C.; Meng, L.; Du, Q.; Sun, F. Chiral separations in normal-phase liquid chromatography: updating a screening strategy with a chlorine-containing polysaccharide-based selector. J. Chin. Chem. Soc. 2015, 62, 1059–1067. [Google Scholar] [CrossRef]
- Sharp, V.S.; Gokey, M.A.; Wolfe, C.N.; Rener, G.A.; Cooper, M.R. High performance liquid chromatographic enantioseparation development and analytical method characterization of the carboxylate ester of evacetrapib using an immobilized chiral stationary phase with a non-conventional eluent system. J. Chromatogr. A 2015, 1416, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, L.; Dong, S.; Zhang, X.; Wu, Q.; Zhao, L.; Shi, Y. Nanocellulose 3,5-dimethylphenylcarbamate derivative coated chiral stationary phase: Preparation and enantioseparation performance. Chirality 2016, 28, 376–381. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Wei, P.; Ouyang, P.; Zou, Q. Chiral separation and thermodynamic investigation of ezetimibe optical isomers on a chiralpak IC column. J. Chromatogr. Sci. 2016. [Google Scholar] [CrossRef] [PubMed]
- Rahou, I.; Sekkoum, K.; Belboukhari, N.; Cheriti, A.; Aboul-Enein, H.Y. Liquid chromatographic separation of novel 4-amino-flavanes series diastereomers on a polysaccharide-type chiral stationary phase. J. Chromatogr. Sci. 2016. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Fan, J.; Lin, C.; Tu, H.; Zheng, S.; Zhang, W. Synthesis and enantioseparation behaviors of novel immobilized 3,5-dimethylphenylcarbamoylated polysaccharide chiral stationary phases. J. Sep. Sci. 2014, 37, 488–494. [Google Scholar] [CrossRef] [PubMed]
- Thurmann, S.; Lotter, C.; Heiland, J.J.; Chankvetadze, B.; Belder, D. Chip-based high-performance liquid chromatography for high-speed enantioseparations. Anal. Chem. 2015, 87, 5568–5576. [Google Scholar] [CrossRef] [PubMed]
- Khater, S.; West, C. Insights into chiral recognition mechanisms in supercritical fluid chromatography V. Effect of the nature and proportion of alcohol mobile phase modifier with amylose and cellulose tris-(3,5-dimethylphenylcarbamate) stationary phases. J. Chromatogr. A 2014, 1373, 197–210. [Google Scholar] [CrossRef] [PubMed]
- Ates, H.; Younes, A.A.; Mangelings, D.; Heyden, Y.V. Enantioselectivity of polysaccharide-based chiral selectors in polar organic solvents chromatography: Implementation of chlorinated selectors in a separation strategy. J. Pharmaceut. Biomed. Anal. 2013, 74, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Rao, R.N.; Kumar, K.N.; Naidu, C.G. Liquid chromatographic separation of darunavir enantiomers on coated and immobilized amylose tris(3,5-dimethylphenylcarbamate) chiral stationary phases. Chirality 2012, 24, 652–660. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, Y.; Ikai, T.; Shen, J. Controlled immobilization of polysaccharide derivatives for efficient chiral separation. Isr. J. Chem. 2011, 51, 1096–1106. [Google Scholar] [CrossRef]
- Ikai, T.; Yamamoto, C.; Kamigaito, M.; Okamoto, Y. Immobilized-type chiral packing materials for HPLC based on polysaccharide derivatives. J. Chromatogr. B 2008, 875, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Francotte, E.; Zhang, T. Preparation and evaluation of immobilized 4-methylbenzoylcellulose stationary phases for enantioselective separations. J. Chromatogr. A 2016, 1467, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, C.; Hayashi, T.; Okamoto, Y. High-performance liquid chromatographic enantioseparation using chitin carbamate derivatives as chiral stationary phases. J. Chromatogr. A 2003, 1021, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Shen, J.; Zuo, W.; Okamoto, Y. Synthesis of chitosan 3,6-diphenylcarbamate-2-urea derivatives andtheir applications as chiral stationary phases for high-performanceliquid chromatography. J. Chromatogr. A 2014, 1365, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, C.; Fujisawa, M.; Kamigaito, M.; Okamoto, Y. Enantioseparation using urea- and imide- bearing chitosan phenylcarbamate derivatives as chiral stationary phases for high-performance liquid chromatography. Chirality 2008, 20, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Bin, Q.; Chen, W.; Bai, Z.-W.; Huang, S.-H. Chiral stationary phases based on chitosanbis(methylphenylcarbamate)-(isobutyrylamide) forhigh-performance liquid chromatography. J. Chromatogr. A 2016, 1440, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, X.-C.; Chen, W.; Bai, Z.-W. Synthesis of substituted phenylcarbamates of N-cyclobutylformylated chitosan and their application as chiral selectors in enantioseparation. Analyst 2016, 141, 4470–4480. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.-W.; Huang, S.-H.; Chen, W.; Bai, Z.-W. Selective and full derivatization of amino group in chitosan with alkyl chloroformate of low stereo-hindrance. Macromol. Res. 2016, 24, 650–653. [Google Scholar] [CrossRef]
- Feng, Z.-W.; Chen, W.; Bai, Z.-W. Chiral stationary phases based on chitosan bis(4-methylphenylcarbamate)-(alkoxyformamide). J. Sep. Sci. 2016, 39, 3728–3735. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Jiang, J.-Z.; Chen, W.; Bai, Z.-W. Synthesis and characterization of chitosan alkyl urea. Carbohydr. Polym. 2016, 145, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xi, J.-B.; Chen, W.; Huang, S.-H.; Bai, Z.-W. High performance chiral separation materials based on chitosan bis(3,5-dimethylphenylcarbamate)-(alkyl urea)s. Carbohydr. Polym. 2017, 156, 481–489. [Google Scholar] [CrossRef]
- Shen, J.; Li, G.; Yang, Z.; Okamoto, Y. Synthesis and chiral recognition of amylose derivatives bearing regioselective phenylcarbamate substituents at 2,6- and 3-positions for high-performance liquid chromatography. J. Chromatogr. A 2016, 1467, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Shen, S.; Lee, H.; Eriksson, M.; Zeng, X.; Xu, J.; Fandrick, K.; Yee, N.; Senanayake, C.; Grinberg, N. Mechanistic studies on the chiral recognition of polysaccharide-based chiral stationary phases using liquid chromatography and vibrational circular dichroism. J. Chromatogr. A 2009, 1216, 3784–3793. [Google Scholar] [CrossRef] [PubMed]
- Mima, S.; Miya, M.; Iwamoto, R.; Yoshikawa, S. Highly deacetylated chitosan and its properties. J. Appl. Polym. Sci. 1983, 28, 1909–1917. [Google Scholar] [CrossRef]
- Tolaimate, A.; Desbrieres, J.; Rhazi, M.; Alagui, A. Contribution to the preparation of chitins and chitosans with controlled physico-chemical properties. Polymer 2003, 44, 7939–7952. [Google Scholar] [CrossRef]
- Wei, W.-J.; Deng, H.-W.; Chen, W.; Bai, Z.-W.; Li, S.-R. Preparation and enantioseparation of a mixed selector chiral stationary phase derived from benzoylated tartaric acid and 1,2-diphenylethylenediamine. Chirality 2010, 22, 604–611. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.-H.; Bai, Z.-W.; Feng, J.-W. Chiral self-discrimination of the enantiomers of α-phenylethylamine derivatives in proton NMR. Magn. Reson. Chem. 2009, 47, 423–427. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.-Q.; Liu, J.-D.; Chen, W.; Bai, Z.-W. Enantioseparation characteristics of biselector chiral stationary phases based on derivatives of cellulose and amylase. J. Chromatogr. A 2014, 1346, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-C.; Zhang, J.; Xu, X.-Q.; Chen, W.; Yang, Y.-G.; Bai, Z.-W. Enantioseparation characteristics of chiral stationary phases based on the derivatives of cellulose and chitin. Anal. Methods 2015, 7, 2786–2793. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds CSs 1–6 are available from the authors.



| Solvent | Swelling Capacity | |||||
|---|---|---|---|---|---|---|
| CS 1 | CS 2 | CS 3 | CS 4 | CS 5 | CS 6 | |
| Ethyl acetate | 2.5 | 2.4 | 2.5 | 3.2 | 3.3 | 2.7 |
| Acetone | 1.7 | 1.2 | 1.9 | 1.8 | 1.6 | 2.1 |
| THF | 9.7 | 4.6 | 4.0 | 11.6 | 20.2 | 5.3 |
| S.N. | I | II | III | IV | V | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| k1 | α | RS | k1 | α | RS | k1 | α | RS | k1 | α | RS | k1 | α | RS | |
| 1 | 0.34 | 1.37 | 1.54 | 0.34 | 1.32 | 1.61 | 0.34 | 1.31 | 1.55 | 0.30 | 1.26 | 1.16 | 0.31 | 1.28 | 1.40 |
| 2 | 0.62 | 1.00 | 0.00 | 0.65 | 1.00 | 0.00 | 0.63 | 1.00 | 0.00 | 0.55 | 1.00 | 0.00 | 0.57 | 1.00 | 0.00 |
| 3 | 2.26 | 1.18 | 2.08 | 2.31 | 1.19 | 2.20 | 2.26 | 1.20 | 2.24 | 1.93 | 1.23 | 2.18 | 2.06 | 1.22 | 2.25 |
| 4 | 1.26 | 1.27 | 3.01 | 1.29 | 1.29 | 2.98 | 1.25 | 1.29 | 2.91 | 1.05 | 1.27 | 2.54 | 1.16 | 1.29 | 2.99 |
| 5 | 3.57 | 1.16 | 2.25 | 3.70 | 1.18 | 2.53 | 3.69 | 1.17 | 2.34 | 3.19 | 1.16 | 1.92 | 3.29 | 1.17 | 2.25 |
| 6 | 2.19 | 1.00 | 0.00 | 2.10 | 1.00 | 0.00 | 2.10 | 1.00 | 0.00 | 2.01 | 1.00 | 0.00 | 2.13 | 1.00 | 0.00 |
| 7 | 0.47 | 1.00 | 0.00 | 0.41 | 1.00 | 0.00 | 0.43 | 1.00 | 0.00 | 0.41 | 1.00 | 0.00 | 0.42 | 1.00 | 0.00 |
| 8 | 2.57 | 1.00 | 0.00 | 2.27 | 1.00 | 0.00 | 2.34 | 1.00 | 0.00 | 2.19 | 1.00 | 0.00 | 2.44 | 1.00 | 0.00 |
| 9 | 0.91 | 1.00 | 0.00 | 0.91 | 1.00 | 0.00 | 1.08 | 1.00 | 0.00 | 0.96 | 1.00 | 0.00 | 0.95 | 1.00 | 0.00 |
| 10 | 1.38 | 1.24 | 2.20 | 1.46 | 1.24 | 2.40 | 1.44 | 1.22 | 2.18 | 1.28 | 1.18 | 1.73 | 1.37 | 1.20 | 2.14 |
| 11 | 2.63 | 1.14 | 1.53 | 2.59 | 1.12 | 1.29 | 2.49 | 1.13 | 1.35 | 2.12 | 1.16 | 1.57 | 2.30 | 1.16 | 1.60 |
| 12 | 1.92 | 2.16 | 8.46 | 2.04 | 2.28 | 9.14 | 1.84 | 2.27 | 8.85 | 1.59 | 2.07 | 7.41 | 1.79 | 2.20 | 8.71 |
| 13 | 0.93 | 1.98 | 9.80 | 0.98 | 2.01 | 10.54 | 0.95 | 2.02 | 10.06 | 0.83 | 1.97 | 7.47 | 0.87 | 2.03 | 8.52 |
| 14 | 2.42 | 1.28 | 1.23 | 2.69 | 1.25 | 1.64 | 2.68 | 1.25 | 1.72 | 2.16 | 1.23 | 0.75 | 2.38 | 1.24 | 2.07 |
| 15 | 1.87 | 2.48 | 10.65 | 1.94 | 2.62 | 11.74 | 1.96 | 2.04 | 11.85 | 1.69 | 2.42 | 11.03 | 1.81 | 2.49 | 9.06 |
| 16 | 1.44 | 2.08 | 6.33 | 1.58 | 2.16 | 6.71 | 1.51 | 2.09 | 6.52 | 1.32 | 1.96 | 5.21 | 1.39 | 2.06 | 6.75 |
| 17 | 1.65 | 1.14 | 2.09 | 1.77 | 1.13 | 2.00 | 1.74 | 1.11 | 1.69 | 1.54 | 1.08 | 1.20 | 1.66 | 1.09 | 1.16 |
| 18 | 11.07 | 1.10 | 1.32 | 11.21 | 1.10 | 1.34 | 10.74 | 1.10 | 1.15 | 8.66 | 1.10 | 1.05 | 9.56 | 1.11 | 1.28 |
| 19 | 10.90 | 1.48 | 3.87 | 11.36 | 1.61 | 5.61 | 10.61 | 1.58 | 5.37 | 9.01 | 1.45 | 4.07 | 10.19 | 1.45 | 3.72 |
| 20 | 3.85 | 5.83 | 19.50 | 3.99 | 5.85 | 19.96 | 3.81 | 5.77 | 19.23 | 3.23 | 5.43 | 17.35 | 3.54 | 5.76 | 19.07 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Huang, S.-H.; Chen, W.; Bai, Z.-W. Eluent Tolerance and Enantioseparation Recovery of Chiral Packing Materials Based on Chitosan Bis(Phenylcarbamate)-(n-Octyl Urea)s for High Performance Liquid Chromatography. Molecules 2016, 21, 1528. https://doi.org/10.3390/molecules21111528
Wang J, Huang S-H, Chen W, Bai Z-W. Eluent Tolerance and Enantioseparation Recovery of Chiral Packing Materials Based on Chitosan Bis(Phenylcarbamate)-(n-Octyl Urea)s for High Performance Liquid Chromatography. Molecules. 2016; 21(11):1528. https://doi.org/10.3390/molecules21111528
Chicago/Turabian StyleWang, Jing, Shao-Hua Huang, Wei Chen, and Zheng-Wu Bai. 2016. "Eluent Tolerance and Enantioseparation Recovery of Chiral Packing Materials Based on Chitosan Bis(Phenylcarbamate)-(n-Octyl Urea)s for High Performance Liquid Chromatography" Molecules 21, no. 11: 1528. https://doi.org/10.3390/molecules21111528




