Inhibitory Interactions of Aspalathus linearis (Rooibos) Extracts and Compounds, Aspalathin and Z-2-(β-d-Glucopyranosyloxy)-3-phenylpropenoic Acid, on Cytochromes Metabolizing Hypoglycemic and Hypolipidemic Drugs
Abstract
:1. Introduction
2. Results
2.1. PPAG and Flavonoid Content of Extracts
2.2. Qualitative Screening of Extracts and Compounds
2.3. IC50 Determination
2.4. Concentration-Dependent Screening of Compounds and Extracts
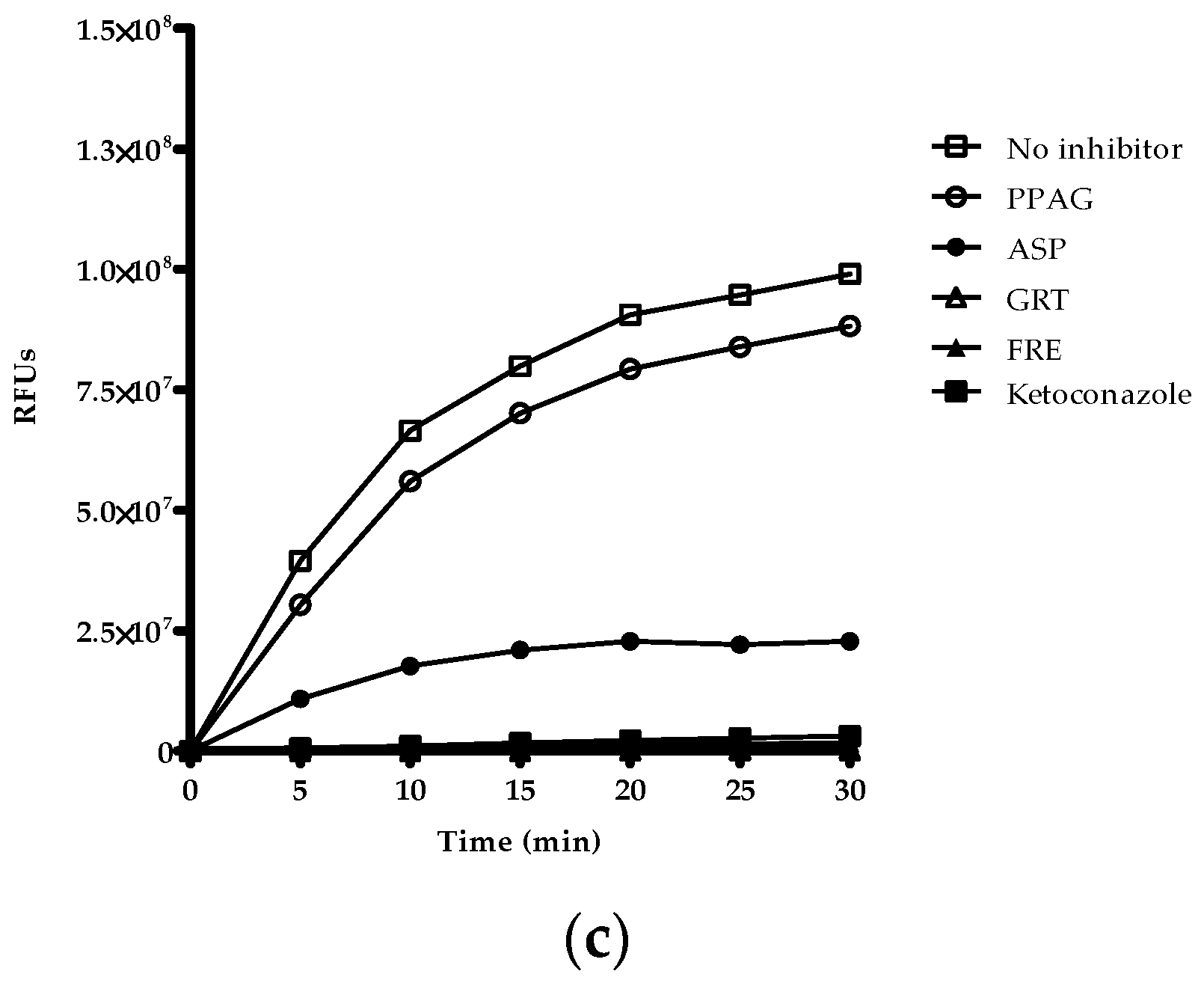
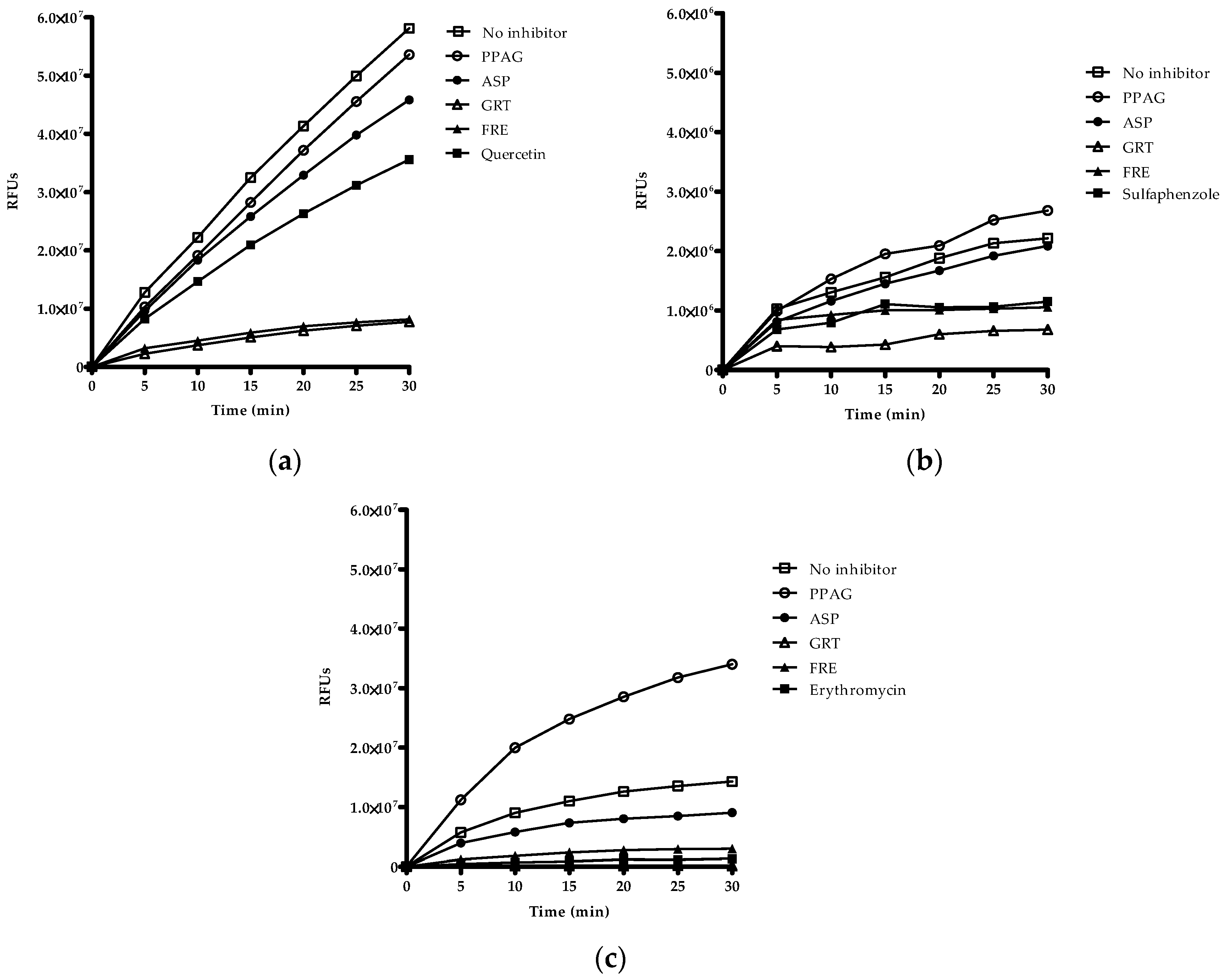
2.5. Time-Dependent Screening of Compounds and Extracts on Enzyme Activity
3. Discussion
4. Materials and Methods
4.1. Plant Extracts
4.2. Pure Compounds
4.3. Chemicals and Reagents
4.4. Solvent Effect on CYPs
4.5. Qualitative Screening of Extracts and Compounds
4.6. Quantitative Screening of Extracts and Compounds and IC50 Determination
4.7. Time-Dependent Screening
4.8. Concentration-Dependent Screening
4.9. Data Analysis
4.9.1. Activity and IC50 Determination
4.9.2. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Joubert, E.; de Beer, D. Rooibos (Aspalathus linearis) beyond the farm gate: From herbal tea to potential phytopharmaceutical. S. Afr. J. Bot. 2011, 77, 869–886. [Google Scholar] [CrossRef]
- Baba, H.; Ohtsuka, Y.; Haruna, H.; Lee, T.; Nagata, S.; Maeda, M.; Yamashiro, Y.; Shimizu, T. Studies of anti-inflammatory effects of Rooibos tea in rats. Pediatr. Int. 2009, 51, 700–704. [Google Scholar] [CrossRef] [PubMed]
- Marnewick, J.L.; Gelderblom, W.C.; Joubert, E. An investigation on the antimutagenic properties of South African herbal teas. Mutat. Res. 2000, 471, 157–166. [Google Scholar] [CrossRef]
- Marnewick, J.; Joubert, E.; Joseph, S.; Swanevelder, S.; Swart, P.; Gelderblom, W. Inhibition of tumour promotion in mouse skin by extracts of rooibos (Aspalathus linearis) and honeybush (Cyclopia intermedia), unique South African herbal teas. Cancer Lett. 2005, 224, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Marnewick, J.L.; van der Westhuizen, F.H.; Joubert, E.; Swanevelder, S.; Swart, P.; Gelderblom, W.C. Chemoprotective properties of rooibos (Aspalathus linearis), honeybush (Cyclopia intermedia) herbal and green and black (Camellia sinensis) teas against cancer promotion induced by fumonisin B1 in rat liver. Food Chem. Toxicol. 2009, 47, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Sissing, L.; Marnewick, J.; de Kock, M.; Swanevelder, S.; Joubert, E.; Gelderblom, W. Modulating effects of rooibos and honeybush herbal teas on the development of esophageal papillomas in rats. Nutr. Cancer 2011, 63, 600–610. [Google Scholar] [CrossRef] [PubMed]
- Beltran-Debon, R.; Rull, A.; Rodriguez-Sanabria, F.; Iswaldi, I.; Herranz-Lopez, M.; Aragones, G.; Camps, J.; Alonso-Villaverde, C.; Menendez, J.A.; Micol, V.; et al. Continuous administration of polyphenols from aqueous rooibos (Aspalathus linearis) extract ameliorates dietary-induced metabolic disturbances in hyperlipidemic mice. Phytomedicine 2011, 18, 414–424. [Google Scholar] [CrossRef] [PubMed]
- Mazibuko, S.E.; Muller, C.J.; Joubert, E.; de Beer, D.; Johnson, R.; Opoku, A.R.; Louw, J. Amelioration of palmitate-induced insulin resistance in C2C12 muscle cells by rooibos (Aspalathus linearis). Phytomedicine 2013, 20, 813–819. [Google Scholar] [CrossRef] [PubMed]
- Muller, C.J.; Joubert, E.; de Beer, D.; Sanderson, M.; Malherbe, C.J.; Fey, S.J.; Louw, J. Acute assessment of an aspalathin-enriched green rooibos (Aspalathus linearis) extract with hypoglycemic potential. Phytomedicine 2012, 20, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Kamakura, R.; Son, M.J.; de Beer, D.; Joubert, E.; Miura, Y.; Yagasaki, K. Antidiabetic effect of green rooibos (Aspalathus linearis) extract in cultured cells and type 2 diabetic model KK-A(y) mice. Cytotechnology 2015, 67, 699–710. [Google Scholar] [CrossRef] [PubMed]
- Sanderson, M.; Mazibuko, S.E.; Joubert, E.; de Beer, D.; Johnson, R.; Pheiffer, C.; Louw, J.; Muller, C.J. Effects of fermented rooibos (Aspalathus linearis) on adipocyte differentiation. Phytomedicine 2014, 21, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Marnewick, J.L.; Rautenbach, F.; Venter, I.; Neethling, H.; Blackhurst, D.M.; Wolmarans, P.; Macharia, M. Effects of rooibos (Aspalathus linearis) on oxidative stress and biochemical parameters in adults at risk for cardiovascular disease. J. Ethnopharmacol. 2011, 133, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Pantsi, W.G.; Marnewick, J.L.; Esterhuyse, A.J.; Rautenbach, F.; van Rooyen, J. Rooibos (Aspalathus linearis) offers cardiac protection against ischaemia/reperfusion in the isolated perfused rat heart. Phytomedicine 2011, 18, 1220–1228. [Google Scholar] [CrossRef] [PubMed]
- Dludla, P.V.; Muller, C.J.; Louw, J.; Joubert, E.; Salie, R.; Opoku, A.R.; Johnson, R. The cardioprotective effect of an aqueous extract of fermented rooibos (Aspalathus linearis) on cultured cardiomyocytes derived from diabetic rats. Phytomedicine 2014, 21, 595–601. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.B. Globalization of diabetes: The role of diet, lifestyle, and genes. Diabetes Care 2011, 34, 1249–1257. [Google Scholar] [CrossRef] [PubMed]
- Wild, S.; Roglic, G.; Green, A.; Sicree, R.; King, H. Global prevalence of diabetes: Estimates for the year 2000 and projections for 2030. Diabetes Care 2004, 27, 1047–1053. [Google Scholar] [CrossRef] [PubMed]
- Hossain, P.; Kawar, B.; El Nahas, M. Obesity and diabetes in the developing world—A growing challenge. N. Engl. J. Med. 2007, 356, 213–215. [Google Scholar] [CrossRef] [PubMed]
- Kawano, A.; Nakamura, H.; Hata, S.; Minakawa, M.; Miura, Y.; Yagasaki, K. Hypoglycemic effect of aspalathin, a rooibos tea component from Aspalathus linearis, in type 2 diabetic model db/db mice. Phytomedicine 2009, 16, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Son, M.J.; Minakawa, M.; Miura, Y.; Yagasaki, K. Aspalathin improves hyperglycemia and glucose intolerance in obese diabetic ob/ob mice. Eur. J. Nutr. 2013, 52, 1607–1619. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.; Dludla, P.; Joubert, E.; February, F.; Mazibuko, S.; Ghoor, S.; Muller, C.; Louw, J. Aspalathin, a dihydrochalcone C-glucoside, protects H9c2 cardiomyocytes against high glucose-induced shifts in substrate preference and apoptosis. Mol. Nutr. Food. Res. 2016, 60, 922–934. [Google Scholar] [CrossRef] [PubMed]
- Ku, S.K.; Kwak, S.; Kim, Y.; Bae, J.S. Aspalathin and nothofagin from rooibos (Aspalathus linearis) inhibits high glucose-induced inflammation in vitro and in vivo. Inflammation 2015, 38, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Muller, C.J.; Joubert, E.; Pheiffer, C.; Ghoor, S.; Sanderson, M.; Chellan, N.; Fey, S.J.; Louw, J. Z-2-(β-D-glucopyranosyloxy)-3-phenylpropenoic acid, an alpha-hydroxy acid from rooibos (Aspalathus linearis) with hypoglycemic activity. Mol. Nutr. Food Res. 2013, 57, 2216–2222. [Google Scholar] [CrossRef] [PubMed]
- Himpe, E.; Cunha, D.A.; Song, I.; Bugliani, M.; Marchetti, P.; Cnop, M.; Bouwens, L. Phenylpropenoic acid glucoside from rooibos protects pancreatic beta cells against cell death induced by acute injury. PLoS ONE 2016, 11, 11. [Google Scholar] [CrossRef] [PubMed]
- Mathijs, I.; da Cunha, D.A.; Himpe, E.; Ladriere, L.; Chellan, N.; Roux, C.R.; Joubert, E.; Muller, C.; Cnop, M.; Louw, J.; et al. Phenylpropenoic acid glucoside augments pancreatic beta cell mass in high-fat diet-fed mice and protects beta cells from ER stress-induced apoptosis. Mol. Nutr. Food Res. 2014, 58, 1980–1990. [Google Scholar] [CrossRef] [PubMed]
- Dludla, P.V.; Muller, C.J.; Joubert, E.; Louw, J.; Gabuza, K.B.; Huisamen, B.; Essop, M.F.; Johnson, R. Phenylpyruvic acid-2-O-β-d-glucoside attenuates high glucose-induced apoptosis in H9c2 cardiomyocytes. Planta Med. 2016. [Google Scholar] [CrossRef] [PubMed]
- Van Andel, T.; Carvalheiro, L.G. Why urban citizens in developing countries use traditional medicines: The case of suriname. J. Evid. Based Complement. Altern. Med. 2013, 2013, 687197. [Google Scholar] [CrossRef] [PubMed]
- Bodeker, G.; Kronenberg, F. A public health agenda for traditional, complementary, and alternative medicine. Am. J. Public Health 2002, 92, 1582–1591. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.M.; Chik, Z.; Ramachandra, M.; Subramaniam, U.; Aziddin, R.E.; Mohamed, Z. Evaluation of the effects of Mitragyna speciosa alkaloid extract on cytochrome P450 enzymes using a high throughput assay. Molecules 2011, 16, 7344–7356. [Google Scholar] [CrossRef] [PubMed]
- Cupp, M.J.; Tracy, T.S. Cytochrome P450: New nomenclature and clinical implications. Am. Fam. Phys. 1998, 57, 107–116. [Google Scholar]
- Ogu, C.C.; Maxa, J.L. Drug interactions due to cytochrome P450. Proceedings (Bayl. Univ. Med. Cent.) 2000, 13, 421–423. [Google Scholar] [PubMed]
- Basheer, L.; Kerem, Z. Interactions between CYP3A4 and dietary polyphenols. Oxid. Med. Cell. Longev. 2015, 2015, 854015. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.Z.; Zhang, Y.L.; Zeng, M.Z.; He, F.Z.; Luo, Z.Y.; Luo, J.Q.; Wen, J.G.; Chen, X.P.; Zhou, H.H.; Zhang, W. Pharmacogenomics and herb-drug interactions: Merge of future and tradition. J. Evid. Based Complement. Altern. Med. 2015, 2015, 321091. [Google Scholar] [CrossRef] [PubMed]
- Hermann, R.; von Richter, O. Clinical evidence of herbal drugs as perpetrators of pharmacokinetic drug interactions. Planta Med. 2012, 78, 1458–1477. [Google Scholar] [CrossRef] [PubMed]
- Bushra, R.; Aslam, N.; Khan, A.Y. Food-drug interactions. Oman Med. J. 2011, 26, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.S. Patient counseling about herbal-drug interactions. Afr. J. Tradit. Complement. Altern. Med. 2011, 8, 152–163. [Google Scholar] [CrossRef] [PubMed]
- Tachjian, A.; Maria, V.; Jahangir, A. Use of herbal products and potential interactions in patients with cardiovascular diseases. J. Am. Coll. Cardiol. 2010, 55, 515–525. [Google Scholar] [CrossRef] [PubMed]
- Fasinu, P.S.; Bouic, P.J.; Rosenkranz, B. An overview of the evidence and mechanisms of herb-drug interactions. Front. Pharmacol. 2012, 3, 69. [Google Scholar] [CrossRef] [PubMed]
- Fasinu, P.S.; Bouic, P.J.; Rosenkranz, B. The inhibitory activity of the extracts of popular medicinal herbs on CYP1A2, 2C9, 2C19 and 3A4 and the implications for herb-drug interaction. Afr. J. Tradit. Complement. Altern. Med. 2014, 11, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Hansen, T.S.; Nilsen, O.G. In vitro CYP3A4 metabolism: Inhibition by Echinacea purpurea and choice of substrate for the evaluation of herbal inhibition. Basic Clin. Pharmacol. Toxicol. 2008, 103, 445–449. [Google Scholar] [CrossRef] [PubMed]
- Hutzler, J.M.; Cook, J.; Fleishaker, J.C. Drug-drug interactions: Designing development programs and appropriate product labeling. Pharmacokinet. Drug Dev. 2011, 21–56. [Google Scholar]
- Bell, L.; Bickford, S.; Nguyen, P.H.; Wang, J.; He, T.; Zhang, B.; Friche, Y.; Zimmerlin, A.; Urban, L.; Bojanic, D. Evaluation of fluorescence- and mass spectrometry-based CYP inhibition assays for use in drug discovery. J. Biomol. Screen. 2008, 13, 343–353. [Google Scholar] [CrossRef] [PubMed]
- Kimura, Y.; Ito, H.; Ohnishi, R.; Hatano, T. Inhibitory effects of polyphenols on human cytochrome P450 3A4 and 2C9 activity. Food Chem. Toxicol. 2010, 48, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Yamaori, S.; Ebisawa, J.; Okushima, Y.; Yamamoto, I.; Watanabe, K. Potent inhibition of human cytochrome P450 3A isoforms by cannabidiol: Role of phenolic hydroxyl groups in the resorcinol moiety. Life Sci. 2011, 88, 730–736. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Tiong, K.H.; Abd-Rashid, B.A.; Ismail, Z.; Ismail, R.; Mak, J.W.; Ong, C.E. Inhibitory effects of cytochrome P450 enzymes CYP2C8, CYP2C9, CYP2C19 and CYP3A4 by Labisia pumila extracts. J. Ethnopharmacol. 2012, 143, 586–591. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, K.; Nishimura, Y.; Kurata, N.; Iwase, M.; Yasuhara, H. Effects of continuous ingestion of herbal teas on intestinal CYP3A in the rat. J. Pharmacol. Sci. 2007, 103, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Quintieri, L.; Palatini, P.; Nassi, A.; Ruzza, P.; Floreani, M. Flavonoids diosmetin and luteolin inhibit midazolam metabolism by human liver microsomes and recombinant CYP 3A4 and CYP3A5 enzymes. Biochem. Pharmacol. 2008, 75, 1426–1437. [Google Scholar] [CrossRef] [PubMed]
- Patel, J.; Buddha, B.; Dey, S.; Pal, D.; Mitra, A.K. In vitro interaction of the HIV protease inhibitor ritonavir with herbal constituents: Changes in P-gp and CYP3A4 activity. Am. J. Ther. 2004, 11, 262–277. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.P.; Wu, P.P.; Hou, Y.C.; Lin, S.P.; Tsai, S.Y.; Chen, C.T.; Chao, P.D. Quercetin and rutin reduced the bioavailability of cyclosporine from Neoral, an immunosuppressant, through activating P-glycoprotein and CYP 3A4. J. Agric. Food Chem. 2011, 59, 4644–4648. [Google Scholar] [CrossRef] [PubMed]
- Raucy, J.L. Regulation of CYP3A4 expression in human hepatocytes by pharmaceuticals and natural products. Drug Metab. Dispos. 2003, 31, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Vijayakumar, T.M.; Kumar, R.M.; Agrawal, A.; Dubey, G.P.; Ilango, K. Comparative inhibitory potential of selected dietary bioactive polyphenols, phytosterols on CYP3A4 and CYP2D6 with fluorometric high-throughput screening. J. Food Sci. Technol. 2015, 52, 4537–4543. [Google Scholar] [CrossRef] [PubMed]
- Belpaire, F.M.; Bogaert, M.G. Cytochrome P450: Genetic polymorphism and drug interactions. Acta Clin. Belg. 1996, 51, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Ali, Z.K.; Kim, R.J.; Ysla, F.M. CYP2C9 polymorphisms: Considerations in NSAID therapy. Curr. Opin. Drug Discov. Dev. 2009, 12, 108–114. [Google Scholar]
- Lee, C.R.; Goldstein, J.A.; Pieper, J.A. Cytochrome P450 2C9 polymorphisms: A comprehensive review of the in vitro and human data. Pharmacogenetics 2002, 12, 251–263. [Google Scholar] [CrossRef] [PubMed]
- Wanwimolruk, S.; Phopin, K.; Prachayasittikul, V. Cytochrome P450 enzyme mediated herbal drug interactions (Part 2). EXCLI J. 2014, 13, 869–896. [Google Scholar] [PubMed]
- Van Booven, D.; Marsh, S.; McLeod, H.; Carrillo, M.W.; Sangkuhl, K.; Klein, T.E.; Altman, R.B. Cytochrome P450 2C9-CYP2C9. Pharmacogenet. Genom. 2010, 20, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Pirmohamed, M.; Park, B.K. Cytochrome P450 enzyme polymorphisms and adverse drug reactions. Toxicology 2003, 192, 23–32. [Google Scholar] [CrossRef]
- Huang, S.M.; Temple, R.; Throckmorton, D.; Lesko, L. Drug interaction studies: Study design, data analysis, and implications for dosing and labeling. Clin. Pharmacol. Ther. 2007, 81, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Tirkkonen, T.; Heikkila, P.; Huupponen, R.; Laine, K. Potential CYP2C9-mediated drug-drug interactions in hospitalized type 2 diabetes mellitus patients treated with the sulphonylureas glibenclamide, glimepiride or glipizide. J. Intern. Med. 2010, 268, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Tornio, A.; Niemi, M.; Neuvonen, P.J.; Backman, J.T. Drug interactions with oral antidiabetic agents: Pharmacokinetic mechanisms and clinical implications. Trends Pharmacol. Sci. 2012, 33, 312–322. [Google Scholar] [CrossRef] [PubMed]
- Ho, P.C.; Saville, D.J.; Wanwimolruk, S. Inhibition of human CYP3A4 activity by grapefruit flavonoids, furanocoumarins and related compounds. J. Pharm. Pharm. Sci. 2001, 4, 217–227. [Google Scholar] [PubMed]
- Beelders, T.; Sigge, G.O.; Joubert, E.; de Beer, D.; de Villiers, A. Kinetic optimisation of the reversed phase liquid chromatographic separation of rooibos tea (Aspalathus linearis) phenolics on conventional high performance liquid chromatographic instrumentation. J. Chromatogr. A 2012, 1219, 128–139. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Aspalathin is available from commercial sources.





© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patel, O.; Muller, C.; Joubert, E.; Louw, J.; Rosenkranz, B.; Awortwe, C. Inhibitory Interactions of Aspalathus linearis (Rooibos) Extracts and Compounds, Aspalathin and Z-2-(β-d-Glucopyranosyloxy)-3-phenylpropenoic Acid, on Cytochromes Metabolizing Hypoglycemic and Hypolipidemic Drugs. Molecules 2016, 21, 1515. https://doi.org/10.3390/molecules21111515
Patel O, Muller C, Joubert E, Louw J, Rosenkranz B, Awortwe C. Inhibitory Interactions of Aspalathus linearis (Rooibos) Extracts and Compounds, Aspalathin and Z-2-(β-d-Glucopyranosyloxy)-3-phenylpropenoic Acid, on Cytochromes Metabolizing Hypoglycemic and Hypolipidemic Drugs. Molecules. 2016; 21(11):1515. https://doi.org/10.3390/molecules21111515
Chicago/Turabian StylePatel, Oelfah, Christo Muller, Elizabeth Joubert, Johan Louw, Bernd Rosenkranz, and Charles Awortwe. 2016. "Inhibitory Interactions of Aspalathus linearis (Rooibos) Extracts and Compounds, Aspalathin and Z-2-(β-d-Glucopyranosyloxy)-3-phenylpropenoic Acid, on Cytochromes Metabolizing Hypoglycemic and Hypolipidemic Drugs" Molecules 21, no. 11: 1515. https://doi.org/10.3390/molecules21111515