Bee Venom Inhibits Porphyromonas gingivalis Lipopolysaccharides-Induced Pro-Inflammatory Cytokines through Suppression of NF-κB and AP-1 Signaling Pathways
Abstract
:1. Introduction
2. Results
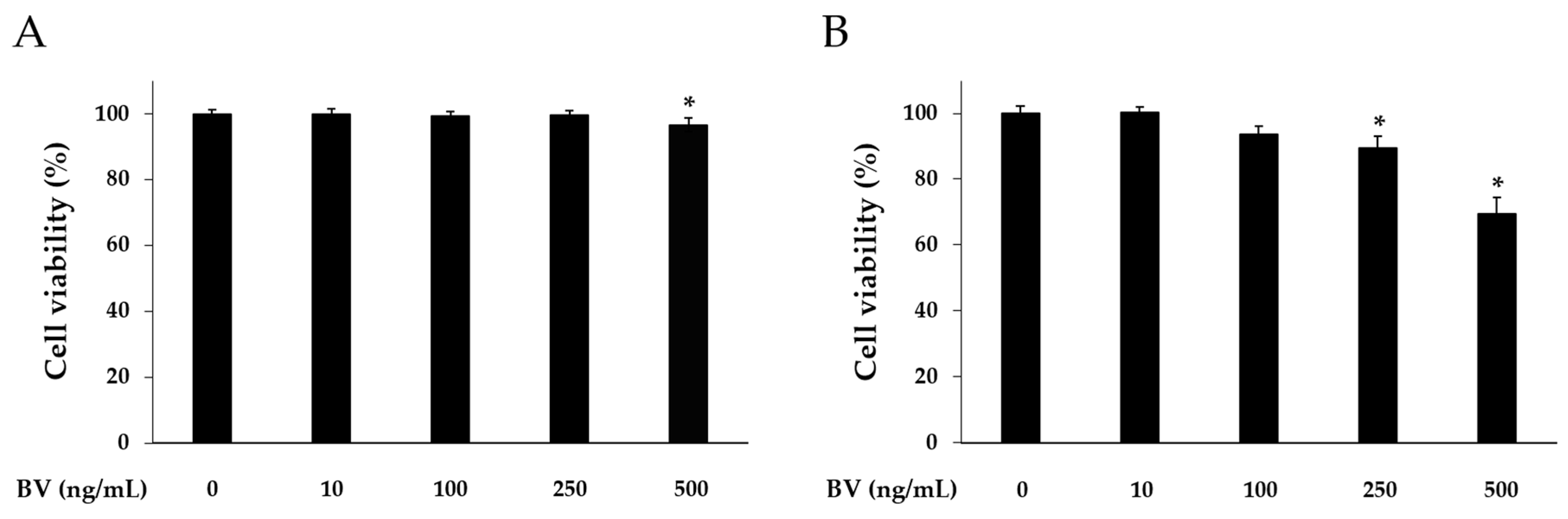
2.1. Effects of BV on Cell Viability
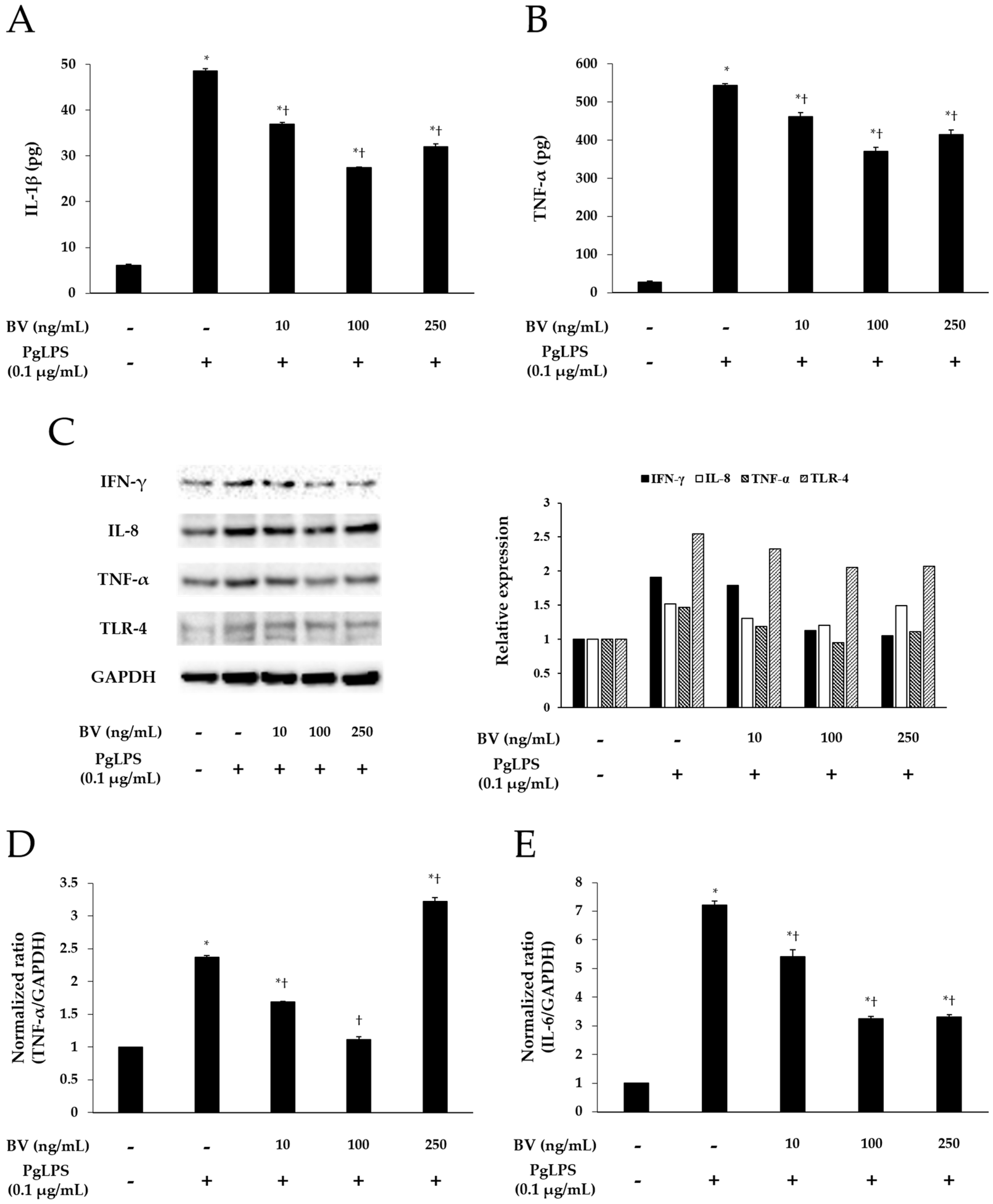
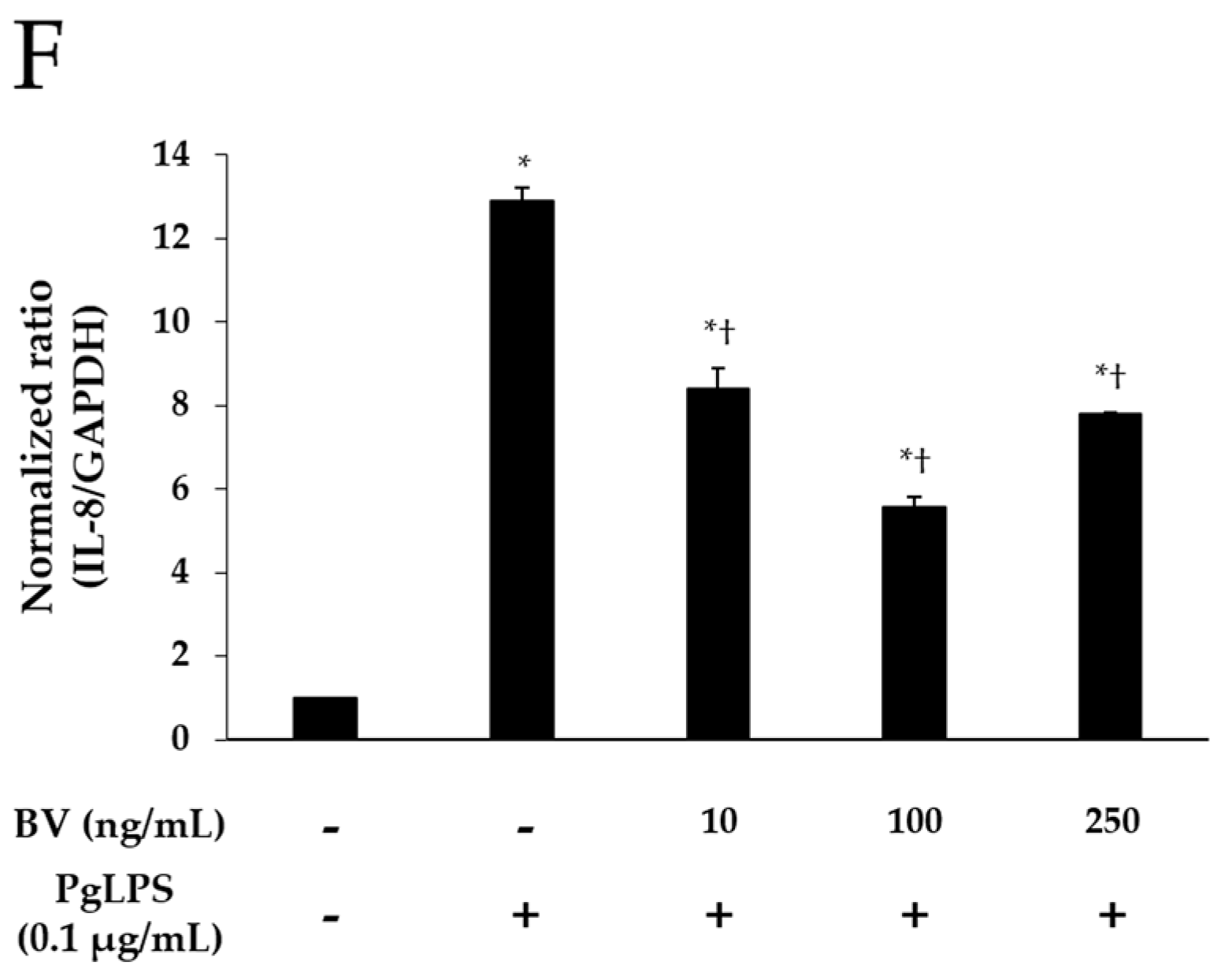
2.2. BV Inhibits PgLPS-Induced the Pro-Inflammatory Cytomokines and TLR-4
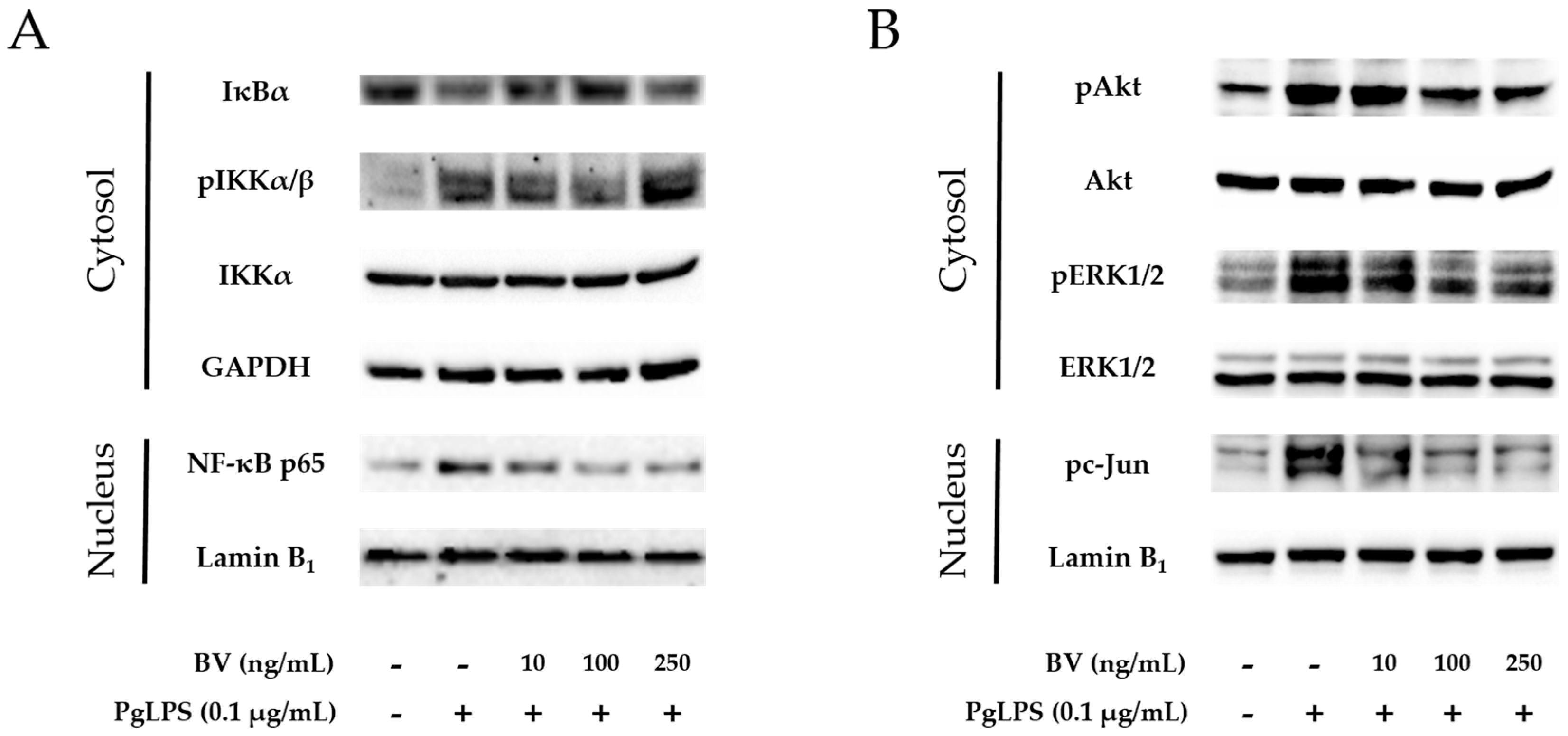
2.3. BV Suppresses Signaling Pathways of Inflammatory Transcription Factors in PgLPS-Treated HaCaT Cells
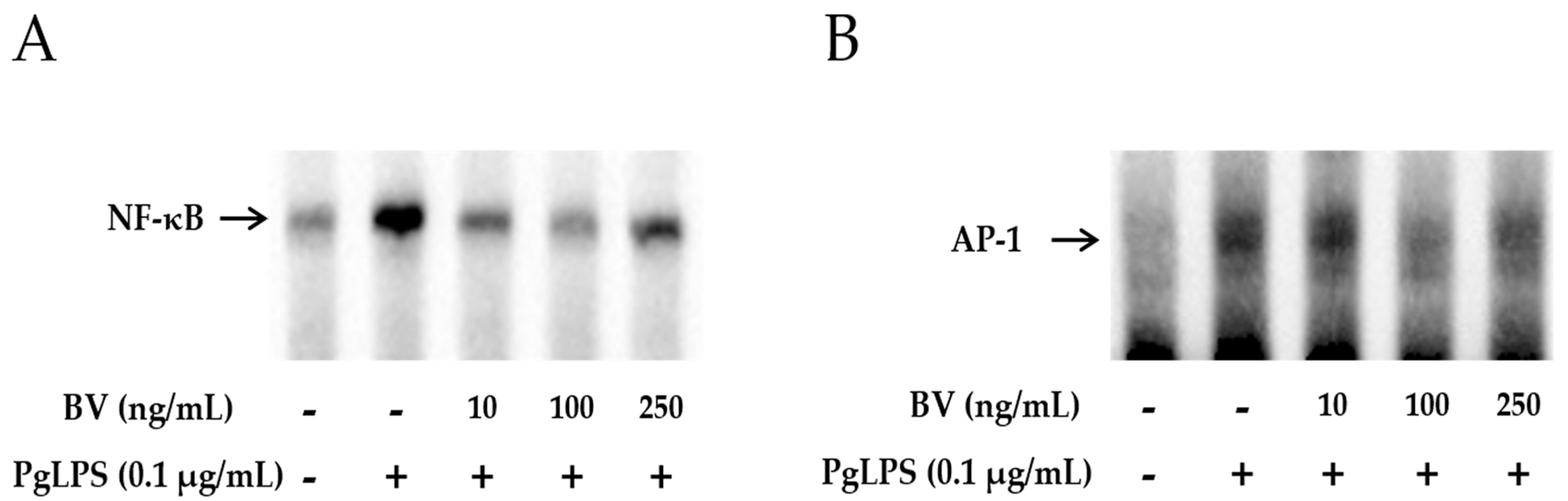
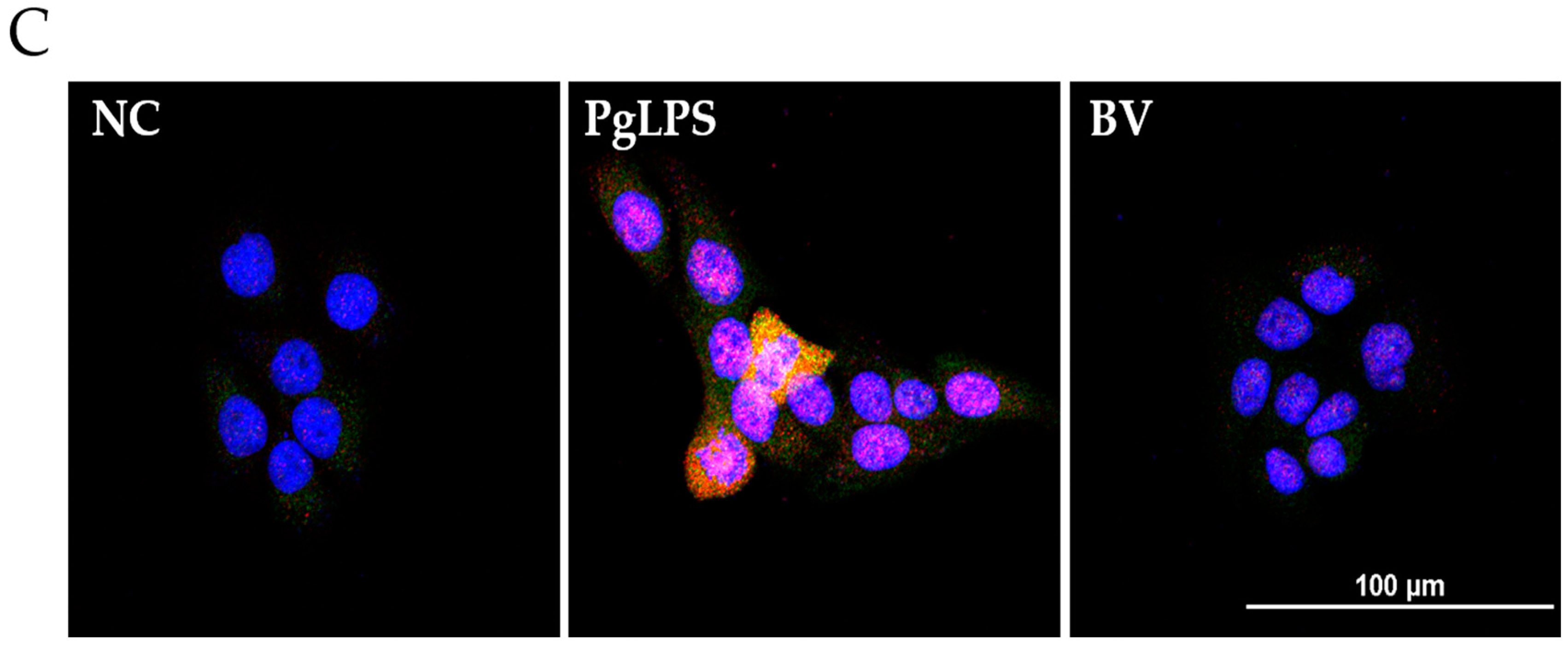
2.4. BV Reduces the DNA-Binding Activity of Inflammatory Transcription Factors in PgLPS-Treated HaCaT Cells
3. Discussion
4. Materials and Methods
4.1. Bee Venom
4.2. Cell Culture and Reagents
4.3. Cell Viability Assay
4.4. Quantitative Real-Time PCR
4.5. ELISA
4.6. Western Blotting
4.7. EMSA
4.8. Immunofluorescence Analysis
4.9. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Liu, J.; Tang, X.; Li, C.; Pan, C.; Li, Q.; Geng, F.; Pan, Y. Porphyromonas gingivalis promotes the cell cycle and inflammatory cytokine production in periodontal ligament fibroblasts. Arch. Oral Biol. 2015, 60, 1153–1161. [Google Scholar] [CrossRef] [PubMed]
- Darveau, R.P. Periodontitis: A polymicrobial disruption of host homeostasis. Nat. Rev. Microbiol. 2010, 8, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Hajishengallis, G. Periodontitis: From microbial immune subversion to systemic inflammation. Nat. Rev. Immunol. 2015, 15, 30–44. [Google Scholar] [CrossRef] [PubMed]
- Lamont, R.J.; Jenkinson, H.F. Subgingival colonization by Porphyromonas gingivalis. Oral Microbiol. Immunol. 2000, 15, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Kuboniwa, M.; Lamont, R.J. Subgingival biofilm formation. Periodontology 2000 2010, 52, 38–52. [Google Scholar] [CrossRef] [PubMed]
- Al Batran, R.; Al-Bayaty, F.H.; Al-Obaidi, M.M. In Vivo effect of andrographolide on alveolar bone resorption induced by Porphyromonas gingivalis and its relation with antioxidant enzymes. BioMed Res. Int. 2013, 2013, 276329. [Google Scholar] [CrossRef] [PubMed]
- Bostanci, N.; Belibasakis, G.N. Porphyromonas gingivalis: An invasive and evasive opportunistic oral pathogen. FEMS Microbiol. Lett. 2012, 333, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Andrian, E.; Grenier, D.; Rouabhia, M. Porphyromonas gingivalis-epithelial cell interactions in periodontitis. J. Dent. Res. 2006, 85, 392–403. [Google Scholar] [CrossRef] [PubMed]
- Amano, A. Disruption of epithelial barrier and impairment of cellular function by Porphyromonas gingivalis. Front. Biosci. J. Virtual Libr. 2007, 12, 3965–3974. [Google Scholar] [CrossRef]
- Colombo, A.V.; da Silva, C.M.; Haffajee, A.; Colombo, A.P. Identification of intracellular oral species within human crevicular epithelial cells from subjects with chronic periodontitis by fluorescence in situ hybridization. J. Periodontal Res. 2007, 42, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Lamont, R.J.; Yilmaz, O. In or out: The invasiveness of oral bacteria. Periodontology 2000 2002, 30, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Kinane, D.F.; Galicia, J.C.; Gorr, S.U.; Stathopoulou, P.G.; Benakanakere, M.P. Gingivalis interactions with epithelial cells. Front. Biosci. J. Virtual Libr. 2008, 13, 966–984. [Google Scholar] [CrossRef]
- Macpherson, A.; Zoheir, N.; Awang, R.A.; Culshaw, S.; Ramage, G.; Lappin, D.F.; Nile, C.J. The alpha 7 nicotinic receptor agonist pha-543613 hydrochloride inhibits Porphyromonas gingivalis-induced expression of interleukin-8 by oral keratinocytes. Inflamm. Res. 2014, 63, 557–568. [Google Scholar] [CrossRef] [PubMed]
- Raingeaud, J.; Pierre, J. Interleukin-4 downregulates tnfalpha-induced il-8 production in keratinocytes. FEBS Lett. 2005, 579, 3953–3959. [Google Scholar] [CrossRef] [PubMed]
- Feliciani, C.; Gupta, A.K.; Sauder, D.N. Keratinocytes and cytokine/growth factors. Crit. Rev. Oral Biol. Med. 1996, 7, 300–318. [Google Scholar] [CrossRef] [PubMed]
- Tribble, G.D.; Kerr, J.E.; Wang, B.Y. Genetic diversity in the oral pathogen Porphyromonas gingivalis: Molecular mechanisms and biological consequences. Future Microbiol. 2013, 8, 607–620. [Google Scholar] [CrossRef] [PubMed]
- Armitage, G.C. Classifying periodontal diseases—A long-standing dilemma. Periodontology 2000 2002, 30, 9–23. [Google Scholar] [CrossRef] [PubMed]
- Colombo, A.P.; Bennet, S.; Cotton, S.L.; Goodson, J.M.; Kent, R.; Haffajee, A.D.; Socransky, S.S.; Hasturk, H.; Van Dyke, T.E.; Dewhirst, F.E.; et al. Impact of periodontal therapy on the subgingival microbiota of severe periodontitis: Comparison between good responders and individuals with refractory periodontitis using the human oral microbe identification microarray. J. Periodontol. 2012, 83, 1279–1287. [Google Scholar] [CrossRef] [PubMed]
- Kornman, K.S.; Holt, S.C.; Robertson, P.B. The microbiology of ligature-induced periodontitis in the cynomolgus monkey. J. Periodontal Res. 1981, 16, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Brecx, M.C.; Nalbandian, J.; Ooya, K.; Kornman, K.S.; Robertson, P.B. Morphological studies on periodontal disease in the cynomolgus monkey. Ii. Light microscopic observations on ligature-induced periodontitis. J. Periodontal Res. 1985, 20, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Holt, S.C.; Ebersole, J.; Felton, J.; Brunsvold, M.; Kornman, K.S. Implantation of bacteroides gingivalis in nonhuman primates initiates progression of periodontitis. Science 1988, 239, 55–57. [Google Scholar] [CrossRef] [PubMed]
- Assuma, R.; Oates, T.; Cochran, D.; Amar, S.; Graves, D.T. Il-1 and tnf antagonists inhibit the inflammatory response and bone loss in experimental periodontitis. J. Immunol. 1998, 160, 403–409. [Google Scholar] [PubMed]
- Lee, W.R.; Kim, K.H.; An, H.J.; Kim, J.Y.; Chang, Y.C.; Chung, H.; Park, Y.Y.; Lee, M.L.; Park, K.K. The protective effects of melittin on propionibacterium acnes-induced inflammatory responses in vitro and in vivo. J. Investig. Dermatol. 2014, 134, 1922–1930. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Lee, W.R.; Kim, K.H.; An, H.J.; Chang, Y.C.; Han, S.M.; Park, Y.Y.; Pak, S.C.; Park, K.K. Effects of bee venom against propionibacterium acnes-induced inflammation in human keratinocytes and monocytes. Int. J. Mol. Med. 2015, 35, 1651–1656. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.B.; Lee, J.D.; Lee, H.J.; Han, H.J.; Mar, W.C.; Kang, S.K.; Beitz, A.J.; Lee, J.H. Bee venom injection into an acupuncture point reduces arthritis associated edema and nociceptive responses. Pain 2001, 90, 271–280. [Google Scholar] [CrossRef]
- Park, H.J.; Lee, S.H.; Son, D.J.; Oh, K.W.; Kim, K.H.; Song, H.S.; Kim, G.J.; Oh, G.T.; Yoon, D.Y.; Hong, J.T. Antiarthritic effect of bee venom: Inhibition of inflammation mediator generation by suppression of nf-kappab through interaction with the p50 subunit. Arthritis Rheum. 2004, 50, 3504–3515. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.B.; Lee, H.J.; Han, H.J.; Mar, W.C.; Kang, S.K.; Yoon, O.B.; Beitz, A.J.; Lee, J.H. The water-soluble fraction of bee venom produces antinociceptive and anti-inflammatory effects on rheumatoid arthritis in rats. Life Sci. 2002, 71, 191–204. [Google Scholar] [CrossRef]
- Taylor, J.J. Cytokine regulation of immune responses to Porphyromonas gingivalis. Periodontology 2000 2010, 54, 160–194. [Google Scholar] [CrossRef] [PubMed]
- Pathirana, R.D.; O’Brien-Simpson, N.M.; Reynolds, E.C. Host immune responses to Porphyromonas gingivalis antigens. Periodontology 2000 2010, 52, 218–237. [Google Scholar] [CrossRef] [PubMed]
- Ara, T.; Kurata, K.; Hirai, K.; Uchihashi, T.; Uematsu, T.; Imamura, Y.; Furusawa, K.; Kurihara, S.; Wang, P.L. Human gingival fibroblasts are critical in sustaining inflammation in periodontal disease. J. Periodontal Res. 2009, 44, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Gursoy, U.K.; Kononen, E.; Uitto, V.J. Stimulation of epithelial cell matrix metalloproteinase (mmp-2, -9, -13) and interleukin-8 secretion by fusobacteria. Oral Microbiol. Immunol. 2008, 23, 432–434. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.C.; Lerner, U.H.; Teng, Y.T. Cytokine responses against periodontal infection: Protective and destructive roles. Periodontology 2000 2010, 52, 163–206. [Google Scholar] [CrossRef] [PubMed]
- Mans, J.J.; Hendrickson, E.L.; Hackett, M.; Lamont, R.J. Cellular and bacterial profiles associated with oral epithelium-microbiota interactions. Periodontology 2000 2010, 52, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Roberts, F.A.; Hockett, R.D.; Bucy, R.P.; Michalek, S.M. Quantitative assessment of inflammatory cytokine gene expression in chronic adult periodontitis. Oral Microbiol. Immunol. 1997, 12, 336–344. [Google Scholar] [CrossRef] [PubMed]
- Seymour, G.J.; Gemmell, E.; Kjeldsen, M.; Yamazaki, K.; Nakajima, T.; Hara, K. Cellular immunity and hypersensitivity as components of periodontal destruction. Oral Dis. 1996, 2, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Morandini, A.C.; Chaves Souza, P.P.; Ramos-Junior, E.S.; Brozoski, D.T.; Sipert, C.R.; Souza Costa, C.A.; Santos, C.F. Toll-like receptor 2 knockdown modulates interleukin (il)-6 and il-8 but not stromal derived factor-1 (sdf-1/cxcl12) in human periodontal ligament and gingival fibroblasts. J. Periodontol. 2013, 84, 535–544. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.J.; Chen, Y.L.; Kuo, M.Y.; Li, C.L.; Lu, H.K. Measurement of gp130 cytokines oncostatin m and il-6 in gingival crevicular fluid of patients with chronic periodontitis. Cytokine 2005, 30, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Garlet, G.P.; Avila-Campos, M.J.; Milanezi, C.M.; Ferreira, B.R.; Silva, J.S. Actinobacillus actinomycetemcomitans-induced periodontal disease in mice: Patterns of cytokine, chemokine, and chemokine receptor expression and leukocyte migration. Microbes Infect. 2005, 7, 738–747. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Basal, E. Inhibition of propionibacterium acnes-induced mediators of inflammation by indian herbs. Phytomed. Int. J. Phytother. Phytopharmacol. 2003, 10, 34–38. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Cho, S.; Chung, J.H.; Hammerberg, C.; Fisher, G.J.; Voorhees, J.J. Inflammation and extracellular matrix degradation mediated by activated transcription factors nuclear factor-kappab and activator protein-1 in inflammatory acne lesions in vivo. Am. J. Pathol. 2005, 166, 1691–1699. [Google Scholar] [CrossRef]
- Shibata, M.; Katsuyama, M.; Onodera, T.; Ehama, R.; Hosoi, J.; Tagami, H. Glucocorticoids enhance toll-like receptor 2 expression in human keratinocytes stimulated with propionibacterium acnes or proinflammatory cytokines. J. Investig. Dermatol. 2009, 129, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Webster, G.F. Acne vulgaris. Br. Med. J. 2002, 325, 475–479. [Google Scholar] [CrossRef]
- Heymann, W.R. Toll-like receptors in acne vulgaris. J. Am. Acad. Dermatol. 2006, 55, 691–692. [Google Scholar] [CrossRef] [PubMed]
- Jugeau, S.; Tenaud, I.; Knol, A.C.; Jarrousse, V.; Quereux, G.; Khammari, A.; Dreno, B. Induction of toll-like receptors by propionibacterium acnes. Br. J. Dermatol. 2005, 153, 1105–1113. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Ochoa, M.T.; Krutzik, S.R.; Takeuchi, O.; Uematsu, S.; Legaspi, A.J.; Brightbill, H.D.; Holland, D.; Cunliffe, W.J.; Akira, S.; et al. Activation of toll-like receptor 2 in acne triggers inflammatory cytokine responses. J. Immunol. 2002, 169, 1535–1541. [Google Scholar] [CrossRef] [PubMed]
- Kim, J. Review of the innate immune response in acne vulgaris: Activation of toll-like receptor 2 in acne triggers inflammatory cytokine responses. Dermatology 2005, 211, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Hari, A.; Flach, T.L.; Shi, Y.; Mydlarski, P.R. Toll-like receptors: Role in dermatological disease. Mediat. Inflamm. 2010, 2010, 437246. [Google Scholar] [CrossRef] [PubMed]
- Pivarcsi, A.; Bodai, L.; Rethi, B.; Kenderessy-Szabo, A.; Koreck, A.; Szell, M.; Beer, Z.; Bata-Csorgoo, Z.; Magocsi, M.; Rajnavolgyi, E.; et al. Expression and function of toll-like receptors 2 and 4 in human keratinocytes. Int. Immunol. 2003, 15, 721–730. [Google Scholar] [CrossRef] [PubMed]
- Kollisch, G.; Kalali, B.N.; Voelcker, V.; Wallich, R.; Behrendt, H.; Ring, J.; Bauer, S.; Jakob, T.; Mempel, M.; Ollert, M. Various members of the toll-like receptor family contribute to the innate immune response of human epidermal keratinocytes. Immunology 2005, 114, 531–541. [Google Scholar] [CrossRef] [PubMed]
- Grange, P.A.; Raingeaud, J.; Calvez, V.; Dupin, N. Nicotinamide inhibits propionibacterium acnes-induced il-8 production in keratinocytes through the nf-kappab and mapk pathways. J. Dermatol. Sci. 2009, 56, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Park, D.J.; Kim, Y.H.; Kim, Y.; Choi, Y.W.; Lee, S.J. Schisandra chinensis alpha-iso-cubebenol induces heme oxygenase-1 expression through pi3k/akt and nrf2 signaling and has anti-inflammatory activity in Porphyromonas gingivalis lipopolysaccharide-stimulated macrophages. Int. Immunopharmacol. 2011, 11, 1907–1915. [Google Scholar] [CrossRef] [PubMed]
- Bodet, C.; La, V.D.; Gafner, S.; Bergeron, C.; Grenier, D. A licorice extract reduces lipopolysaccharide-induced proinflammatory cytokine secretion by macrophages and whole blood. J. Periodontol. 2008, 79, 1752–1761. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; McFadden, G. Modulation of nf-kappab signalling by microbial pathogens. Nat. Rev. Microbiol. 2011, 9, 291–306. [Google Scholar] [CrossRef] [PubMed]
- Bozinovski, S.; Jones, J.E.; Vlahos, R.; Hamilton, J.A.; Anderson, G.P. Granulocyte/macrophage-colony-stimulating factor (gm-csf) regulates lung innate immunity to lipopolysaccharide through akt/erk activation of nfkappa b and ap-1 in vivo. J. Biol. Chem. 2002, 277, 42808–42814. [Google Scholar] [CrossRef] [PubMed]
- Palm, N.W.; Medzhitov, R. Role of the inflammasome in defense against venoms. Proc. Natl. Acad. Sci. USA 2013, 110, 1809–1814. [Google Scholar] [CrossRef] [PubMed]
- Dombrowski, Y.; Peric, M.; Koglin, S.; Kaymakanov, N.; Schmezer, V.; Reinholz, M.; Ruzicka, T.; Schauber, J. Honey bee (apis mellifera) venom induces aim2 inflammasome activation in human keratinocytes. Allergy 2012, 67, 1400–1407. [Google Scholar] [CrossRef] [PubMed]
- Tada, H.; Matsuyama, T.; Nishioka, T.; Hagiwara, M.; Kiyoura, Y.; Shimauchi, H.; Matsushita, K. Porphyromonas gingivalis gingipain-dependently enhances il-33 production in human gingival epithelial cells. PLoS ONE 2016, 11, e0152794. [Google Scholar] [CrossRef] [PubMed]
- de Camargo Pereira, G.; Guimaraes, G.N.; Planello, A.C.; Santamaria, M.P.; de Souza, A.P.; Line, S.R.; Marques, M.R. Porphyromonas gingivalis lps stimulation downregulates dnmt1, dnmt3a, and jmjd3 gene expression levels in human hacat keratinocytes. Clin. Oral Investig. 2013, 17, 1279–1285. [Google Scholar] [CrossRef] [PubMed]
- Kato, H.; Taguchi, Y.; Tominaga, K.; Umeda, M.; Tanaka, A. Porphyromonas gingivalis lps inhibits osteoblastic differentiation and promotes pro-inflammatory cytokine production in human periodontal ligament stem cells. Arch. Oral Biol. 2014, 59, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Roberts, F.A.; McCaffery, K.A.; Michalek, S.M. Profile of cytokine mrna expression in chronic adult periodontitis. J. Dent. Res. 1997, 76, 1833–1839. [Google Scholar] [CrossRef] [PubMed]
- Perlman, H.; Bradley, K.; Liu, H.; Cole, S.; Shamiyeh, E.; Smith, R.C.; Walsh, K.; Fiore, S.; Koch, A.E.; Firestein, G.S.; et al. Il-6 and matrix metalloproteinase-1 are regulated by the cyclin-dependent kinase inhibitor p21 in synovial fibroblasts. J. Immunol. 2003, 170, 838–845. [Google Scholar] [CrossRef] [PubMed]
- Yanti; Lee, M.; Kim, D.; Hwang, J.K. Inhibitory effect of panduratin a on c-jun n-terminal kinase and activator protein-1 signaling involved in Porphyromonas gingivalis supernatant-stimulated matrix metalloproteinase-9 expression in human oral epidermoid cells. Biol. Pharm. Bull. 2009, 32, 1770–1775. [Google Scholar] [CrossRef] [PubMed]
- Kawa, K.; Tsutsui, H.; Uchiyama, R.; Kato, J.; Matsui, K.; Iwakura, Y.; Matsumoto, T.; Nakanishi, K. Ifn-gamma is a master regulator of endotoxin shock syndrome in mice primed with heat-killed propionibacterium acnes. Int. Immunol. 2010, 22, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, N.; Gans, E.H. Tretinoin: A review of its anti-inflammatory properties in the treatment of acne. J. Clin. Aesthet. Dermatol. 2011, 4, 22–29. [Google Scholar] [PubMed]
- Vowels, B.R.; Yang, S.; Leyden, J.J. Induction of proinflammatory cytokines by a soluble factor of propionibacterium acnes: Implications for chronic inflammatory acne. Infect. Immun. 1995, 63, 3158–3165. [Google Scholar] [PubMed]
- Basal, E.; Jain, A.; Kaushal, G.P. Antibody response to crude cell lysate of propionibacterium acnes and induction of pro-inflammatory cytokines in patients with acne and normal healthy subjects. J. Microbiol. 2004, 42, 117–125. [Google Scholar] [PubMed]
- Ryan, K.A.; Smith, M.F., Jr.; Sanders, M.K.; Ernst, P.B. Reactive oxygen and nitrogen species differentially regulate toll-like receptor 4-mediated activation of nf-kappa b and interleukin-8 expression. Infect. Immun. 2004, 72, 2123–2130. [Google Scholar] [CrossRef] [PubMed]
- Netea, M.G.; van der Graaf, C.; Van der Meer, J.W.; Kullberg, B.J. Toll-like receptors and the host defense against microbial pathogens: Bringing specificity to the innate-immune system. J. Leukoc. Biol. 2004, 75, 749–755. [Google Scholar] [CrossRef] [PubMed]
- Teng, Y.T. Protective and destructive immunity in the periodontium: Part 1—Innate and humoral immunity and the periodontium. J. Dent. Res. 2006, 85, 198–208. [Google Scholar] [CrossRef] [PubMed]
- Aderem, A.; Ulevitch, R.J. Toll-like receptors in the induction of the innate immune response. Nature 2000, 406, 782–787. [Google Scholar] [CrossRef] [PubMed]
- Miggin, S.M.; O’Neill, L.A. New insights into the regulation of tlr signaling. J. Leukoc. Biol. 2006, 80, 220–226. [Google Scholar] [CrossRef] [PubMed]
- Yaron, A.; Hatzubai, A.; Davis, M.; Lavon, I.; Amit, S.; Manning, A.M.; Andersen, J.S.; Mann, M.; Mercurio, F.; Ben-Neriah, Y. Identification of the receptor component of the ikappabalpha-ubiquitin ligase. Nature 1998, 396, 590–594. [Google Scholar] [PubMed]
- Spencer, E.; Jiang, J.; Chen, Z.J. Signal-induced ubiquitination of ikappabalpha by the f-box protein slimb/beta-trcp. Genes Dev. 1999, 13, 284–294. [Google Scholar] [CrossRef] [PubMed]
- Duesbery, N.S.; Webb, C.P.; Leppla, S.H.; Gordon, V.M.; Klimpel, K.R.; Copeland, T.D.; Ahn, N.G.; Oskarsson, M.K.; Fukasawa, K.; Paull, K.D.; et al. Proteolytic inactivation of map-kinase-kinase by anthrax lethal factor. Science 1998, 280, 734–737. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Kim, D.H.; Baek, S.H.; Lee, H.J.; Kim, M.R.; Kwon, H.J.; Lee, C.H. Rengyolone inhibits inducible nitric oxide synthase expression and nitric oxide production by down-regulation of nf-kappab and p38 map kinase activity in lps-stimulated raw 264.7 cells. Biochem. Pharmacol. 2006, 71, 1198–1205. [Google Scholar] [CrossRef] [PubMed]
- Moon, D.O.; Park, S.Y.; Lee, K.J.; Heo, M.S.; Kim, K.C.; Kim, M.O.; Lee, J.D.; Choi, Y.H.; Kim, G.Y. Bee venom and melittin reduce proinflammatory mediators in lipopolysaccharide-stimulated bv2 microglia. Int. Immunopharmacol. 2007, 7, 1092–1101. [Google Scholar] [CrossRef] [PubMed]
- Han, S.M.; Kim, J.M.; Hong, I.P.; Woo, S.O.; Kim, S.G.; Jang, H.R.; Pak, S.C. Antibacterial activity and antibiotic-enhancing effects of honeybee venom against methicillin-resistant staphylococcus aureus. Molecules 2016, 21, 79. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Not Available.
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, W.-H.; An, H.-J.; Kim, J.-Y.; Gwon, M.-G.; Gu, H.; Park, J.-B.; Sung, W.J.; Kwon, Y.-C.; Park, K.-D.; Han, S.M.; et al. Bee Venom Inhibits Porphyromonas gingivalis Lipopolysaccharides-Induced Pro-Inflammatory Cytokines through Suppression of NF-κB and AP-1 Signaling Pathways. Molecules 2016, 21, 1508. https://doi.org/10.3390/molecules21111508
Kim W-H, An H-J, Kim J-Y, Gwon M-G, Gu H, Park J-B, Sung WJ, Kwon Y-C, Park K-D, Han SM, et al. Bee Venom Inhibits Porphyromonas gingivalis Lipopolysaccharides-Induced Pro-Inflammatory Cytokines through Suppression of NF-κB and AP-1 Signaling Pathways. Molecules. 2016; 21(11):1508. https://doi.org/10.3390/molecules21111508
Chicago/Turabian StyleKim, Woon-Hae, Hyun-Jin An, Jung-Yeon Kim, Mi-Gyeong Gwon, Hyemin Gu, Jae-Bok Park, Woo Jung Sung, Yong-Chul Kwon, Kyung-Duck Park, Sang Mi Han, and et al. 2016. "Bee Venom Inhibits Porphyromonas gingivalis Lipopolysaccharides-Induced Pro-Inflammatory Cytokines through Suppression of NF-κB and AP-1 Signaling Pathways" Molecules 21, no. 11: 1508. https://doi.org/10.3390/molecules21111508
APA StyleKim, W.-H., An, H.-J., Kim, J.-Y., Gwon, M.-G., Gu, H., Park, J.-B., Sung, W. J., Kwon, Y.-C., Park, K.-D., Han, S. M., & Park, K.-K. (2016). Bee Venom Inhibits Porphyromonas gingivalis Lipopolysaccharides-Induced Pro-Inflammatory Cytokines through Suppression of NF-κB and AP-1 Signaling Pathways. Molecules, 21(11), 1508. https://doi.org/10.3390/molecules21111508





