Effect of Exogenous Abscisic Acid and Methyl Jasmonate on Anthocyanin Composition, Fatty Acids, and Volatile Compounds of Cabernet Sauvignon (Vitis vinifera L.) Grape Berries
Abstract
:1. Introduction
2. Results and Discussion
2.1. Physico-Chemical Properties and TPC and TAC Analysis
2.2. Effect of Exogenous ABA and MeJA on Individual Anthocyanins
2.3. Effect of Exogenous ABA and MeJA on Fatty Acid Composition and LOX Activity
2.3.1. Analysis of Fatty Acid Composition
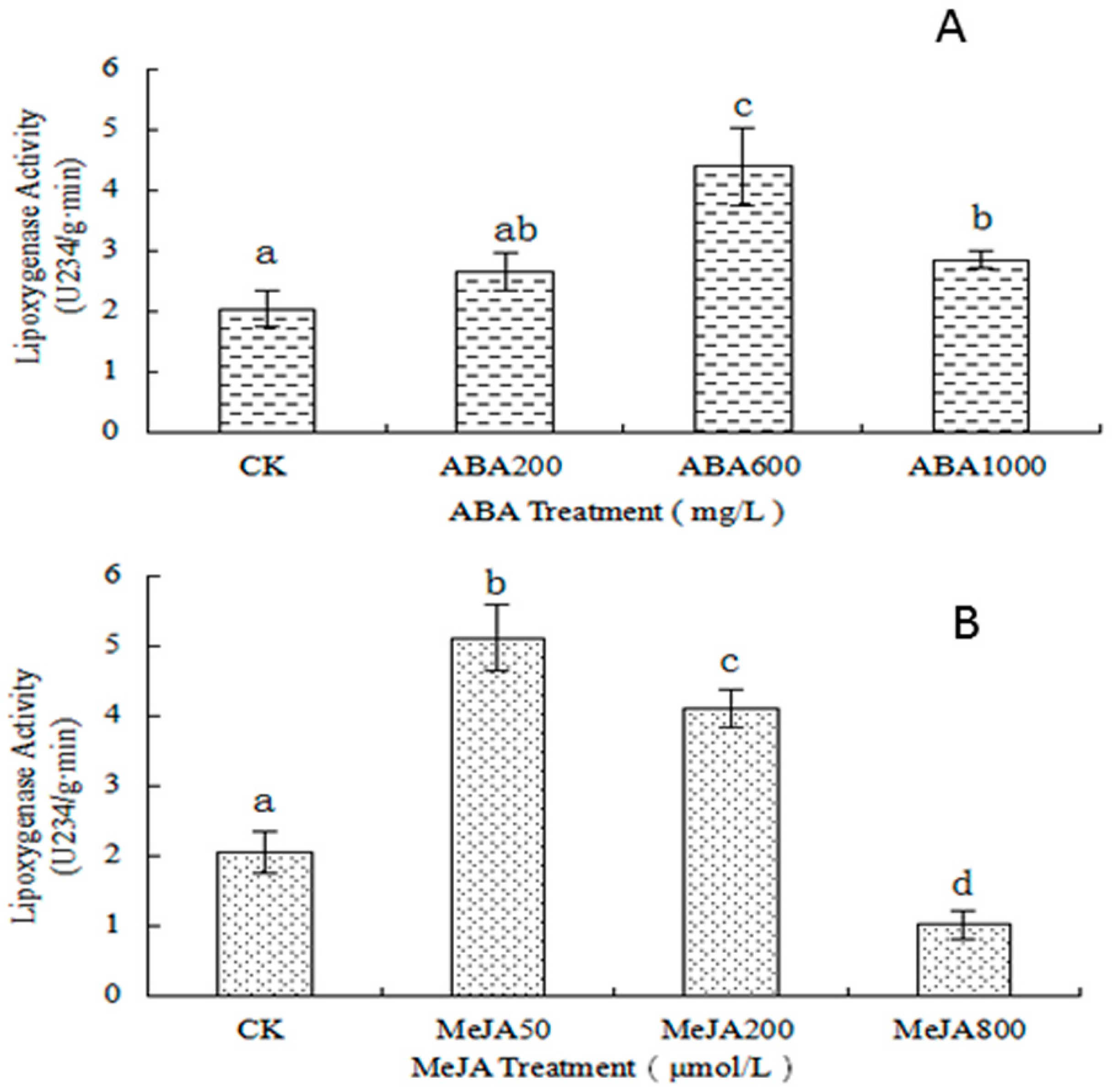
2.3.2. Determination of LOX Activity
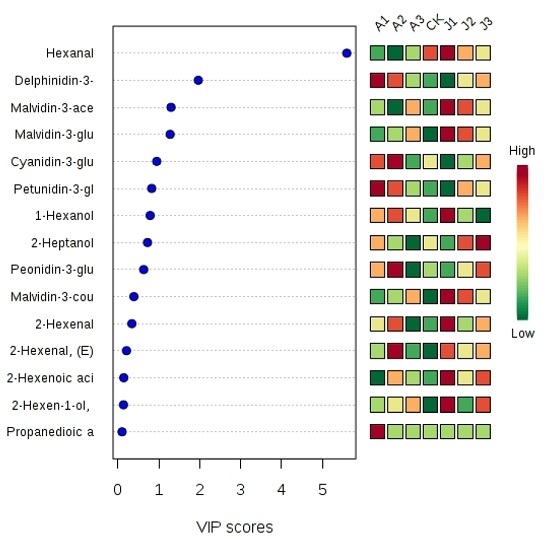
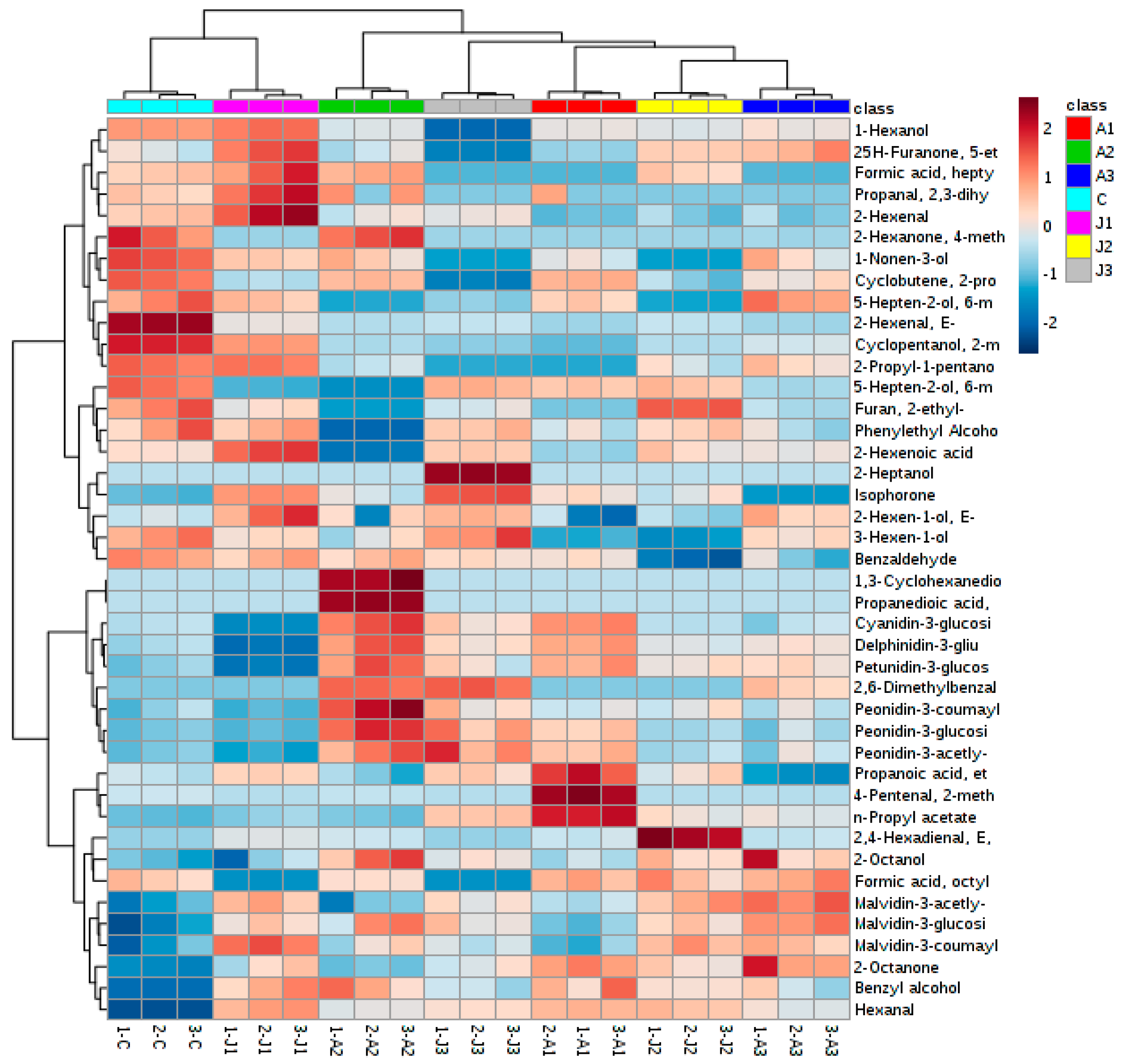
2.4. Effect of Exogenous ABA and MeJA on Volatile Aromas
2.5. Multivariate Statistical Analysis of Anthocyanins and Volatile Aromas
3. Materials and Methods
3.1. Field Conditions and Materials
3.2. Determination of Brix, pH, Total Phenolic (TPC), and Total Anthocyanin Content (TAC)
3.3. Determination of Anthocyanins
3.3.1. Extraction of Anthocyanins
3.3.2. HPLC Analysis of Anthocyanins
3.4. Determination of the LOX Activity
3.4.1. Extraction of the Crude Enzyme
3.4.2. Determination of the LOX Enzyme Activity
3.4.3. Calculation of the LOX Enzyme Activity
3.5. Determination of Fatty Acids
3.5.1. Fatty Acid Extraction
3.5.2. GC-MS Analysis of the Fatty Acid Composition
3.6. Determination of Volatile Compounds
3.6.1. Volatile Compound Extraction
3.6.2. GC-MS Analysis of Volatile Compounds
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Liang, Z.C.; Wu, B.H.; Fan, P.G.; Yang, C.X.; Duan, W.; Zheng, X.B.; Liu, C.Y.; Li, S.H. Anthocyanin composition and content in grape berry skin in Vitis germplasm. Food Chem. 2008, 111, 837–844. [Google Scholar] [CrossRef]
- Langlois, J.; Ballester, J.; Campo, E.; Dacremont, C.; Peyron, D. Combining Olfactory and Gustatory Clues in the Judgment of Aging Potential of Red Wine by Wine Professionals. Am. J. Enol. Vitic. 2010, 61, 15–22. [Google Scholar]
- Saenz-Navajas, M.P.; Tao, Y.S.; Dizy, M.; Ferreira, V.; Fernandez-Zurbano, P. Relationship between nonvolatile composition and sensory properties of premium Spanish red wines and their correlation to quality perception. J. Agric. Food Chem. 2010, 58, 12407–12416. [Google Scholar] [CrossRef] [PubMed]
- Juhász, C.; Tóbiás, I.; Ádám, A.L.; Kátay, G.; Gullner, G. Pepper 9- and 13-lipoxygenase genes are differentially activated by two tobamoviruses and by hormone treatments. Physiol. Mol. Plant Pathol. 2015, 92, 59–69. [Google Scholar] [CrossRef]
- Han, Y.F.; Sheryl, B. Formation of Volatiles in the Lipoxygenase Pathway as Affected by Fruit Type and Temperature. J. Exp. Food Chem. 2015, 1, 102–109. [Google Scholar] [CrossRef]
- Andreou, A.; Brodhun, F.; Feussner, I. Biosynthesis of oxylipins in non-mammals. Prog. Lipid Res. 2009, 48, 148–170. [Google Scholar] [CrossRef] [PubMed]
- Baysal, T.; Demirdöven, A. Lipoxygenase in fruits and vegetables: A review. Enzyme Microb. Technol. 2007, 40, 491–496. [Google Scholar] [CrossRef]
- Kuhn, H.; Thiele, B.J. The diversity of the lipoxygenase family many sequence data but little information on biological significance. FEBS Lett. 1999, 44, 7–11. [Google Scholar] [CrossRef]
- Baldwin, E.A. Fruit volatile metabolism and consumer perceptions. In Fruit Quality and Its Biological Basis; Sheffield Academic: Boca Raton, FL, USA, 2002; Volume 8, pp. 89–106. [Google Scholar]
- Theerakulkait, C.; Barrett, D.M.; Mcdaniel, M. Sweet corn germ enzymes affect odor formation. J. Food Sci. 1995, 60, 1034–1040. [Google Scholar] [CrossRef]
- Brash, A.R. Lipoxygenases: Occurrence, Functions, Catalysis and Acquisition of substrate. J. Biol. Chem. 1999, 274, 23679–23682. [Google Scholar] [CrossRef] [PubMed]
- Pinto, D.M.; Nerg, A.M.; Holopainen, J.K. The role of ozone-reactive compounds, terpenes, and green leaf volatiles (GLVs), in the orientation of Cotesia plutellae. J. Chem. Ecol. 2007, 33, 2218–2228. [Google Scholar] [CrossRef] [PubMed]
- Olias, J.M.; Perez, A.G.; Rios, J.J. Aroma components and free aroma acids in strawberry variety Chandler during ripening. J. Agric. Food Chem. 1992, 40, 2232–2235. [Google Scholar]
- Kim, Y.J.; Jang, M.G.; Sukweenadhi, J. Functional characterization of the pathogenesis-related protein family 10 gene, PgPR10-4, from Panax ginseng in response to environmental stresses. Plant Cell Tissue Organ Cult. 2014, 118, 531–543. [Google Scholar] [CrossRef]
- Agarwal, S.; Sairam, R.K.; Srivastava, G.C. Changes in antioxidant enzymes activity and oxidative stress by abscisic and salicylic acid in wheat genotypes. Biol. Plant 2005, 49, 541–550. [Google Scholar] [CrossRef]
- Matsui, K. Green leaf volatiles: Hydroperoxide lyase pathway of oxylipin metabolism. Curr. Opin. Plant Biol. 2006, 9, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.Q.; Cheng, G.; Duan, L.L.; Jiang, R.; Pan, Q.H.; Duan, C.Q.; Wang, J. Effect of training systems on fatty acids and their derived volatiles in Cabernet Sauvignon grapes and wines of the north foot of Mt. Tianshan. Food Chem. 2015, 181, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-García, Y.; López-Roca, J.M.; Bautista-Ortín, A.B.; Gil-Muñoz, R.; Gómez-Plaza, E. Effect of combined use of benzothiadiazole and methyl jasmonate on volatile compounds of Monastrell wine. Am. J. Enol. Vitic. 2014, 65, 238–243. [Google Scholar] [CrossRef]
- Gómez-Plaza, E.; Mestre-Ortuño, L.; Ruiz-García, Y.; Fernández-Fernández, J.I.; López-Roca, J.M. Effect of benzothiadiazole and methyl jasmonate on the volatile compound composition of monastrell grapes and wines. Am. J. Enol. Vitic. 2012, 63, 394–401. [Google Scholar] [CrossRef]
- Cheng, G.; He, Y.N.; Yue, T.X.; Wang, J.; Zhang, Z.W. Effects of climatic conditions and soil properties on Cabernet Sauvignon berry growth and anthocyanin profiles. Molecules 2014, 19, 13683–13703. [Google Scholar] [CrossRef] [PubMed]
- Traverso, J.A.; Pulido, A.; Rodríguez-García, M.I.; Alché, J.D. Thiol-based redox regulation in sexual plant re-production: New insights and perspectives. Front. Plant Sci. 2013, 4, 465. [Google Scholar] [CrossRef] [PubMed]
- Luan, L.Y. Study on the Regulation of Anthocyanin Synthesis of Grape and the Quality of Wine after Brassinolide and Abscisic Acid Treatment; Northwest A&F University: Yangling, Shaanxi, China, 2014. [Google Scholar]
- Symons, G.M.; Davies, C.; Shavrukov, Y.; Dry, I.B.; Reid, J.B.; Thomas, M.R. Grapes on steroids. Brassinosteroids are involved in grape berry ripening. Plant Physiol. 2006, 140, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Núñez, V.; Monagas, M.; Gomez-Cordovés, M.C.; Bartolomé, B. Vitis vinifera L. cv. Graciano grapes characterized by its anthocyanin profile. Postharvest Biol. Technol. 2004, 31, 69–79. [Google Scholar] [CrossRef]
- Farmer, E.E.; Ryan, C.A. Octadecanoid precursors of jasmonic acid activate the synthesis of wound-inducible proteinase inhibitors. Plant Cell 1992, 4, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Whitehead, I.M.; Muller, B.L.; Dean, C. Industrial use of soybean lipoxygenase for the production of natural green note flavor compounds. Cereal Foods World 1995, 40, 193–197. [Google Scholar]
- Santos, L.P.; Morais, D.R.; Souza, N.E.; Cottica, S.M.; Boroski, M.; Visentainer, J.V. Phenolic compounds and fatty acids in different parts of Vitis labrusca and V. vinifera grapes. Food Res. Int. 2011, 44, 1414–1418. [Google Scholar] [CrossRef]
- Aydin, A. Determination of Fatty Acid Compositions of Some Raisin Cultivars in Turkey. Asian J. Chem. 2011, 23, 1819–1821. [Google Scholar]
- Jia, H.; Zhang, C.; Pervaiz, T.; Zhao, P.; Liu, Z.; Wang, B.; Qian, J. Jasmonic acid involves in grape fruit ripening and resistant against Botrytis cinerea. Funct. Integr. Genom. 2016, 16, 79–94. [Google Scholar] [CrossRef] [PubMed]
- Feussner, I.; Wasternack, C. The lipoxygenase pathway. Annu. Rev. Plant Biol. 2002, 53, 275–297. [Google Scholar] [CrossRef] [PubMed]
- OIV. International Code of Oenological Practices. Available online: http://www.oiv.int/oiv/info/enpratiquesoenologiques (accessed on 1 January 2012).
- Xu, F.; Luan, L.Y.; Zhang, Z.W.; Huo, S.S.; Gao, X.; Fang, Y.L.; Xi, Z.M. Phenolic profiles and antioxidant properties of young wines made from Yan73 (Vitis vinifera L.) and Cabernet Sauvignon (Vitis vinifera L.) grapes treated by 24-epibrassinolide. Molecules 2014, 19, 10189–10207. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.; Fang, Y.L.; Gao, J.S.; Qiao, L.L.; Zhang, A.; Guo, M.Y. Phenolics composition andantioxidant activity of wine produced from spine grape (Vitis Davidii Foex) and cherokee rose (Rosa laevigata Michx.) fruits from South China. J. Food Sci. 2012, 77, C8–C14. [Google Scholar] [CrossRef] [PubMed]
- Zoratti, L.; Jaakola, L.; Häggman, H. Anthocyanin Profile in Berries of Wild and Cultivated Vaccinium spp. along Altitudinal Gradients in the Alps. J. Agric. Food Chem. 2015, 63, 8641–8650. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.Q.; Han, F.L.; Wang, Y. Determination of anthocyanin in Granoir grape and wine with HPLC. J. Agric. Univ. Hebei 2008, 31, 59–61. [Google Scholar]
- Veberic, R.; Slatnar, A.; Bizjak, J.; Stampar, F.; Mikulic-Petkovsek, M. Anthocyanin composition of different wild and cultivated berry species. LWT-Food Sci. Technol. 2015, 60, 509–517. [Google Scholar] [CrossRef]
- Ju, Y.L.; Fang, Y.L.; Zhang, L.; Zeng, J.; Liu, J.B. Determination of Lipoxygenase Activity and the Change of the Fatty Acid Composition of Cabernet Sauvignon Grape Berries. Food Sci. 2015, 36, 116–119. [Google Scholar]
- Curtis, J.M.; Berrigan, N.; Dauphinee, P. The determination of n-3 fatty acid in food products containing microencapsulated fish oil using the one step extraction method. Part 1: Measurement in the raw ingredient and dry powdered foods. J. Am. Oil Chem. Soc. 2008, 85, 297–305. [Google Scholar] [CrossRef]
- Ju, Y.L.; Chen, T.; Gao, J.S.; Fang, Y.L.; Wang, K.; Zhang, Z.W. Lipoxygenase Activity and Fatty Acid Content of Cabernet Sauvignon Grape during Berry Development and External Treatment. Acta Bot. Boreali Occident. Sin. 2014, 34, 2283–2287. [Google Scholar]
- Chen, S.; Zhang, R.; Hao, L.; Chen, W.; Cheng, S. Profiling of volatile compounds and associated gene expression and enzyme activity during fruit development in two cucumber cultivars. PLoS ONE 2015, 10, e0119444. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Not Available.


| Variable | ABA | MeJA | C | ||||
|---|---|---|---|---|---|---|---|
| A1 | A2 | A3 | J1 | J2 | J3 | ||
| Brix | 21.83 ± 0.12 a | 21.97 ± 0.12 a | 22.17 ± 0.06 a | 21.03 ± 0.40 a | 22.03 ± 0.06 b | 21.23 ± 0.08 a | 21.13 ± 0.29 a |
| pH | 3.95 ± 0.03 a | 4.01 ± 0.01 a | 3.92 ± 0.01 a | 3.82 ± 0.02 b | 3.94 ± 0.01 a | 3.91 ± 0.03 a | 3.97 ± 0.01 a |
| Total Acid (g L−1) | 3.65 ± 0.03 a | 3.57 ± 0.06 a | 3.47 ± 0.04 b | 3.66 ± 0.05 a | 3.67 ± 0.30 a | 4.07 ± 0.06 c | 3.49 ± 0.01 b |
| TPC (mg·g−1) | 21.18 ± 0.28 b | 20.29 ± 0.24 c | 19.18 ± 0.06 d | 22.19 ± 0.05 b | 21.93 ± 0.10 c | 19.18 ± 0.06 c | 18.19 ± 0.28 a |
| TAC (mg·g−1) | 12.28 ± 0.34 b | 10.53 ± 0.08 c | 9.81 ± 0.32 d | 9.82 ± 0.10 b | 9.56 ± 0.03 b | 9.60 ± 0.15 b | 8.85 ± 0.09 a |
| Treatments | Delphinidin-3-O-glucoside | Cyanidin-3-O-glucoside | Petunidin-3-O-glucoside | Peonidin-3-O-glucoside | Malvidin-3-O-glucoside | Non-Acylated (%) | Peonidin-3-O-(6-O-acetyl)-glucoside | Malvidin-3-O-(6-O-acetyl)-glucoside | Acetyl Derivatives (%) | Peonidin-3-O-(6-O-coumaryl)-glucoside | Malvidin-3-O-(6-O-coumaryl)-glucoside | Coumaryl Derivatives (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C | 171.68 ± 3.91 a | 40.32 ± 0.78 a | 100.47 ± 2.35 a | 107.88 ± 2.45 a | 565.77 ± 12.98 a | 72.29 | 30.90 ± 0.59 a | 266.09 ± 6.75 ab | 21.77 | 13.29 ± 0.67 a | 67.70 ± 3.34 a | 5.94 |
| J1 | 130.17 ± 3.19 b | 23.70 ± 0.50 b | 88.63 ± 2.54 b | 110.32 ± 3.35 a | 678.66 ± 19.14 b | 68.50 | 30.44 ± 1.05 a | 334.56 ± 12.19 d | 24.24 | 13.32 ± 0.33 a | 96.03 ± 3.25 d | 7.26 |
| J2 | 177.82 ± 5.55 a | 38.06 ± 0.66 a | 107.52 ± 3.85 ac | 109.53 ± 3.39 a | 617.42 ± 26.11 a | 71.16 | 30.71 ± 1.16 a | 298.41 ± 14.08 c | 22.30 | 14.22 ± 0.83 a | 82.27 ± 4.98 c | 6.54 |
| J3 | 189.55 ± 9.34 a | 49.12 ± 5.89 c | 112.94 ± 5.61 cd | 146.90 ± 18.30 b | 617.04 ± 26.96 a | 72.64 | 40.89 ± 5.17 c | 288.86 ± 10.60 bc | 21.47 | 15.80 ± 1.60 a | 74.56 ± 2.76 abc | 5.88 |
| A1 | 217.68 ± 3.20 c | 61.94 ± 0.96 d | 123.55 ± 2.24 d | 138.56 ± 2.46 b | 589.32 ± 12.32 a | 73.50 | 37.94 ± 0.64 c | 284.51 ± 6.05 bc | 20.95 | 14.54 ± 0.24 a | 70.88 ± 1.64 ab | 5.55 |
| A2 | 216.52 ± 7.66 c | 62.60 ± 1.85 d | 123.03 ± 3.86 d | 158.81 ± 4.43 b | 597.93 ± 15.09 a | 75.48 | 37.77 ± 0.97 b | 248.15 ± 6.14 a | 18.62 | 18.74 ± 0.48 b | 71.82 ± 1.45 ab | 5.90 |
| A3 | 176.41 ± 14.54 a | 33.93 ± 7.04 a | 107.05 ± 8.31 ac | 105.55 ± 19.10 a | 608.18 ± 37.50 a | 71.16 | 30.22 ± 5.16 a | 296.49 ± 14.58 c | 22.55 | 13.44 ± 1.78 a | 77.76 ± 3.91 bc | 6.29 |
| Fatty acid | C | A1 | A2 | A3 | J1 | J2 | J3 |
|---|---|---|---|---|---|---|---|
| linoleic acid | 138.23 ± 7.92 a | 116.75 ± 8.40 b | 178.08 ± 5.30 c | 147.3 ± 9.23 d | 127.32 ± 8.35 e | 136.72 ± 3.26 a | 120.49 ± 5.24 b |
| elaidic acid | 72.22 ± 7.22 a | 90.02 ± 5.37 b | 102.75 ± 3.42 c | 124.70 ± 6.29 d | 109.12 ± 7.39 cd | 104.33 ± 0.28 c | 104.44 ± 6.27 c |
| 9-hexadecenoic acid | 0.61 ± 0.052 a | 1.16 ± 0.058 b | 1.15 ± 0.082 b | 0.89 ± 0.032 c | 1.09 ± 0.069 d | 0.89 ± 0.072 c | 1.05 ± 0.127 d |
| hexadecanoic acid | 126.67 ± 9.03 a | 146.72 ± 7.93 b | 187.36 ± 8.19 c | 164.58 ± 5.09 d | 159.26 ± 5.83 e | 147.43 ± 6.83 b | 193.17 ± 5.23 ce |
| stearic acid | 15.34 ± 1.14 a | 20.02 ± 1.62 b | 21.00 ± 1.78 bc | 21.88 ± 1.29 bc | 21.02 ± 1.42 bc | 15.84 ± 1.52 a | 28.21 ± 1.37 d |
| docosanoic acid | 7.17 ± 0.51 a | 11.99 ± 0.38 b | 10.99 ± 0.69 bc | 10.66 ± 0.59 bc | 13.27 ± 0.61 d | 7.53 ± 0.26 a | 15.89 ± 0.39 d |
| eicosanoic acid | 3.40 ± 0.21 a | 5.07 ± 0.26 b | 4.55 ± 0.19 c | 5.42 ± 0.32 de | 5.29 ± 0.33 e | 4.21 ± 0.31 c | 3.18 ± 0.19 a |
| PUFA | 211.06 ± 8.92 a | 207.93 ± 7.98 a | 281.98 ± 10.61 b | 272.89 ± 9.45 c | 237.53 ± 6.98 d | 241.94 ± 7.46 d | 225.98 ± 7.34 e |
| MUFA | 152.58 ± 7.23 a | 183.8 ± 8.79 b | 223.9 ± 7.84 c | 202.54 ± 10.54 bc | 198.84 ± 9.57 c | 175.01 ± 7.98 d | 240.45 ± 7.21 e |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ju, Y.-L.; Liu, M.; Zhao, H.; Meng, J.-F.; Fang, Y.-L. Effect of Exogenous Abscisic Acid and Methyl Jasmonate on Anthocyanin Composition, Fatty Acids, and Volatile Compounds of Cabernet Sauvignon (Vitis vinifera L.) Grape Berries. Molecules 2016, 21, 1354. https://doi.org/10.3390/molecules21101354
Ju Y-L, Liu M, Zhao H, Meng J-F, Fang Y-L. Effect of Exogenous Abscisic Acid and Methyl Jasmonate on Anthocyanin Composition, Fatty Acids, and Volatile Compounds of Cabernet Sauvignon (Vitis vinifera L.) Grape Berries. Molecules. 2016; 21(10):1354. https://doi.org/10.3390/molecules21101354
Chicago/Turabian StyleJu, Yan-Lun, Min Liu, Hui Zhao, Jiang-Fei Meng, and Yu-Lin Fang. 2016. "Effect of Exogenous Abscisic Acid and Methyl Jasmonate on Anthocyanin Composition, Fatty Acids, and Volatile Compounds of Cabernet Sauvignon (Vitis vinifera L.) Grape Berries" Molecules 21, no. 10: 1354. https://doi.org/10.3390/molecules21101354