Optimization Extraction, Preliminary Characterization and Antioxidant Activities of Polysaccharides from Semen Juglandis
Abstract
:1. Introduction
2. Results and Discussion
2.1. Optimization of the SJP Yield
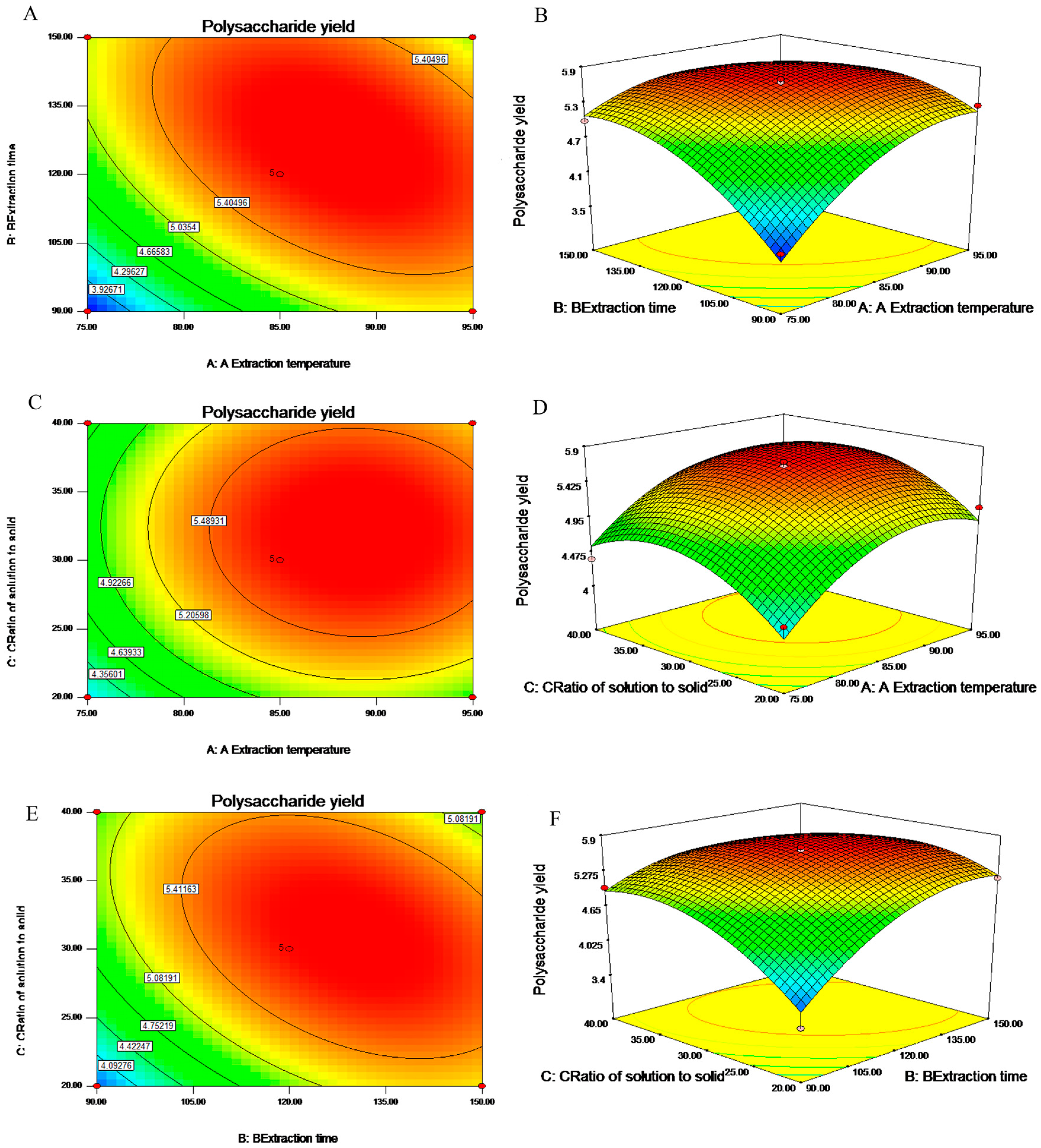
2.2. Analysis of Response Surface
2.3. Optimization of Extracting Parameters and Validation of the Model
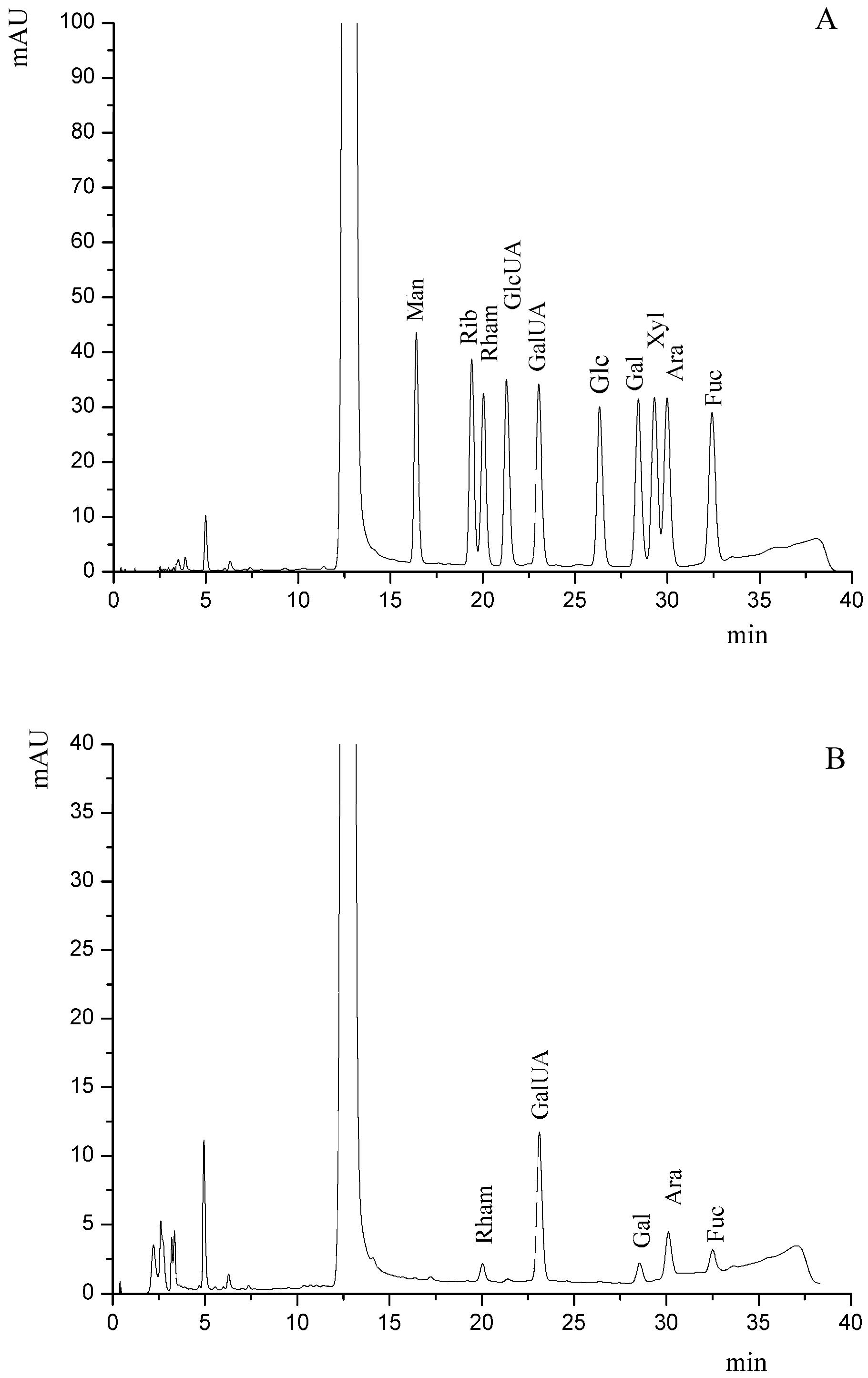
2.4. Preliminary Characterization of the Purified SJP
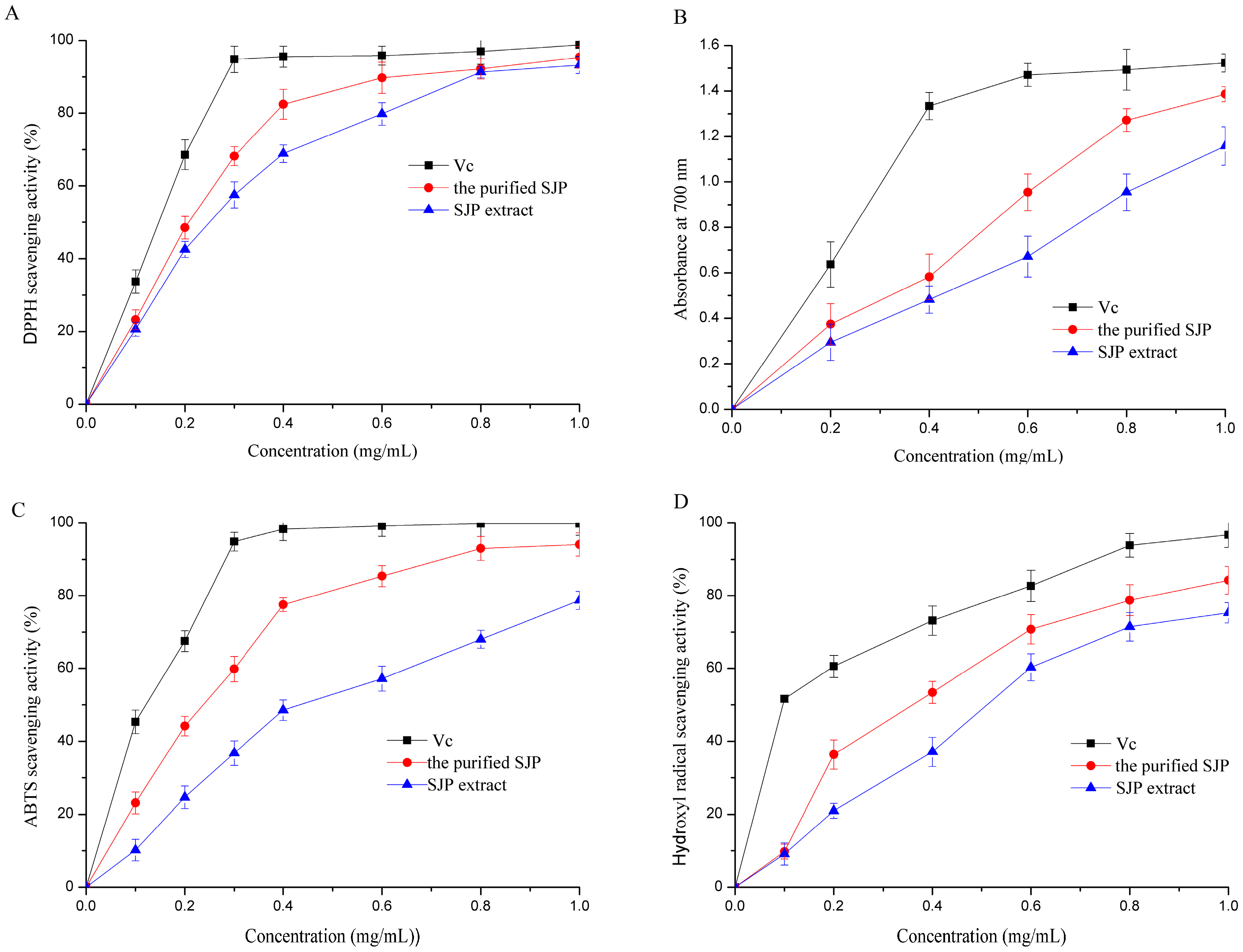
2.5. Antioxidant Activities of SJP
2.5.1. DPPH Scavenging Activity
2.5.2. Reducing Power Activity
2.5.3. ABTS Scavenging Activity
2.5.4. Hydroxyl Radical Scavenging Activity
3. Materials and Methods
3.1. Materials and Chemicals
3.2. Preparation of SJP
3.3. Experimental Design of RSM for Extraction Process
3.4. Fractionation of SJP Extract
3.5. Component Analysis of the Purified SJP
3.6. Determination of Molecular Weight
3.7. In Vitro Antioxidant Activities Assays
3.7.1. DPPH Scavenging Assay
3.7.2. Reducing Power Assay
3.7.3. ABTS Scavenging Assay
3.7.4. Hydroxyl Radical Scavenging Assay
3.8. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| SJP | Polysaccharides from Semen Juglandis |
| RSM | Response surface methodology |
| BBD | Box-Behnken design |
| ANOVA | Analysis of variance |
| SEC-MALLS | Size Exclusion Chromatography with a multi-angle laser light scattering system |
References
- Verma, R.S.; Padalia, R.C.; Chauhan, A.; Thul, S.T. Phytochemical analysis of the leaf volatile oil of walnut tree (Juglans regia L.) from western Himalaya. Ind. Crops Prod. 2013, 42, 195–201. [Google Scholar] [CrossRef]
- Xu, F.; Deng, G.; Cheng, S.Y.; Zhang, W.W.; Huang, X.H.; Li, L.L.; Cheng, H.; Rong, X.F.; Li, J.B. Molecular cloning, characterization and expression of the phenylalanine ammonia-lyase gene from Juglans regia. Molecules 2012, 17, 7810–7823. [Google Scholar] [CrossRef] [PubMed]
- Anderson, K.J.; Teuber, S.S.; Gobeille, A.; Cremin, P.; Waterhouse, A.L.; Steinberg, F.M. Walnut polyphenolics inhibit in vitro human plasma and LDL oxidation. J. Nutr. 2001, 131, 2837–2842. [Google Scholar] [PubMed]
- Samaranayaka, A.G.; John, J.A.; Shahidi, F. Antioxidant activity of English walnut (Juglans regia L.). J. Food Lipids 2008, 15, 384–397. [Google Scholar] [CrossRef]
- Sharma, N.; Ghosh, P.; Sharma, U.K.; Sinha, A.K. Antioxidant potential of Juglans regia bark: Quantification of seven phenolic compounds by RP-HPLC. Chem. Nat. Compd. 2012, 5, 881–882. [Google Scholar] [CrossRef]
- Arranz, S.; Pérez-Jiménez, J.; Saura-Calixto, F. Antioxidant capacity of walnut (Juglans regia L.): Contribution of oil and defatted matter. Eur. Food Res. Technol. 2008, 227, 425–431. [Google Scholar] [CrossRef]
- Fenoradosoa, T.A.; Delattre, C.; Laroche, C.; Wadouachi, A.; Dulong, V.; Picton, L.; Andriamadio, P.; Michaud, P. Highly sulphated galactan from Halymenia durvillei (Halymeniales, Rhodophyta), a red seaweed of Madagascar marine coasts. Int. J. Biol. Macromol. 2009, 45, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Delattre, C.; Fenoradosoa, A.; Michaud, P. Galactans: An overview of their most important sourcing and applications as natural polysaccharides. Braz. Arch. Biol. Technol. 2011, 54, 1075–1092. [Google Scholar]
- Delattre, C.; Pierre, G.; Gardarin, C.; Traikia, M.; Elboutachfaiti, R.; Isogai, A.; Michaud, P. Antioxidant activities of a polyglucuronic acid sodium salt obtainedfrom TEMPO-mediated oxidation of xanthan. Carbohydr. Polym. 2015, 116, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Redouan, E.; Emmanuel, P.; Michelle, P.; Bernard, C.; Josiane, C.; Cédric, D. Evaluation of antioxidant capacity of ulvan-like polymer obtained by regioselective oxidation of gellan exopolysaccharide. Food Chem. 2011, 127, 976–983. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.L.; Yang, W.; Tang, Y.Y.; Xu, Q.; Huang, S.L.; Yao, J.; Zhang, J.; Lei, Z.Q. Regioselective sulfation of Artemisia sphaerocephala polysaccharide: Solution conformation and antioxidant activities in vitro. Carbohydr. Polym. 2016, 136, 527–536. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Y.; Li, S.; Wang, X.H.; Zhang, L.N.; Cheung, P.C.K. Advances in lentinan: Isolation, structure, chain conformation and bioactivities. Food Hydrocoll. 2011, 25, 196–206. [Google Scholar] [CrossRef]
- Miyajima, T.; Ogawa, H.; Koike, I. Water-extractable polysaccharides in marine sediments: Abundance, molecular size distribution, and monosaccharide composition. Geochim. Cosmochim. Acta 2001, 65, 1455–1466. [Google Scholar] [CrossRef]
- Dong, C.H.; Xie, X.Q.; Wang, X.L.; Zhan, Y.; Yao, Y.J. Application of Box-Behnken design in optimisation for polysaccharides extraction from cultured mycelium of Cordyceps sinensis. Food Bioprod. Process. 2009, 87, 139–144. [Google Scholar] [CrossRef]
- Li, P.Q.; Lu, S.Q.; Shan, T.J.; Mou, Y.; Li, Y.; Sun, W.B.; Zhou, L.G. Extraction optimization of water-extracted mycelial polysaccharide from endophytic fungus Fusarium oxysporum Dzf17 by Response Surface Methodology. Int. J. Mol. Sci. 2012, 13, 5441–5453. [Google Scholar] [CrossRef] [PubMed]
- Vitali, R. Response Surface Methods for High Dimensional Structural Design Problems. Ph.D. Thesis, University of Florida, Gainesville, FL, USA, 2000. [Google Scholar]
- Liang, R.J. Optimization of extraction process of Glycyrrhiza glabra polysaccharides by response surface methodology. Carbohydr. Polym. 2008, 74, 858–861. [Google Scholar]
- Qiao, D.L.; Hu, B.; Gan, D.; Sun, Y.; Ye, H.; Zeng, X.X. Fraction optimized by using response surface methodology, purification and preliminary characterization of polysaccharides from Hyriopsis cuminagii. Carbohydr. Polym. 2009, 76, 422–429. [Google Scholar] [CrossRef]
- Qu, Y.; Li, C.X.; Zhang, C.; Zeng, R.; Fu, C.M. Optimization of infrared-assisted extraction of Bletilla striata polysaccharides based on response surface methodology and their antioxidant activities. Carbohydr. Polym. 2016, 148, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.X.; Liu, J.C.; Kennedy, J.F. Extraction optimization of antioxidant polysaccharides from the fruiting bodies of Chroogomphis rutilus (Schaeff.: Fr.) O.K. Miller by Box-Behnken statistical design. Carbohydr. Polym. 2010, 82, 209–214. [Google Scholar] [CrossRef]
- Yin, G.H.; Dan, Y.L. Optimization of extraction technology of the Lycium barbarum polysaccharides by Box-Behnken statistical design. Carbohydr. Polym. 2008, 74, 603–610. [Google Scholar] [CrossRef]
- Guo, Q.B.; Cui, S.; Kang, J.; Ding, H.H.; Wang, Q.; Wang, C. Non-starch polysaccharides from American ginseng: Physicochemical investigation and structural characterization. Food Hydrocoll. 2015, 44, 320–327. [Google Scholar] [CrossRef]
- Shakhmatova, S.G.; Toukachb, P.V.; Michailowac, E.; Makarovaa, E. Structural studies of arabinan-rich pectic polysaccharides from Abies sibirica L. Biological activity of pectins of A. sibirica. Carbohydr. Polym. 2014, 113, 515–524. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.T.; Xu, X.J.; Zheng, H.; Li, J.L.; Chao, D.; Xu, Z.H.; Chen, J.H. Structural characterization, chain conformation, and morphology of a β-(1→3)-d-Glucan isolated from the fruiting body of Dictyophora indusiata. J. Agri. Food Chem. 2009, 57, 5918–5924. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, I.; Sousa, A.; Ferreira, I.C.F.R.; Bento, A.; Estevinho, L.; Pereira, J.A. Total phenols, antioxidant potential and antimicrobial activity of walnut (Juglans regia L.) green husks. Food Chem. Toxicol. 2008, 46, 2326–2331. [Google Scholar] [CrossRef] [PubMed]
- Luo, A.X.; He, X.J.; Zhou, S.D.; Fan, Y.J.; Luo, A.S.; Chun, Z. Purification, composition analysis and antioxidant activity of the polysaccharides from Dendrobium nobile Lindl. Carbohydr Polym. 2010, 79, 1014–1019. [Google Scholar] [CrossRef]
- Yu, H.H.; Liu, X.G.; Xing, R.E.; Liu, S.; Guo, Z.Y.; Wang, P.B.; Li, C.P.; Li, P.C. In vitro determination of antioxidant activity of proteins from jellyfish Rhopilema esculentum. Food Chem. 2006, 95, 123–130. [Google Scholar] [CrossRef]
- Yang, X.M.; Yu, W.; Ou, Z.P.; Ma, H.L.; Liu, W.M.; Ji, X.L. Antioxidant and immunity activity of water extract and crude polysaccharide from Ficus carica L. fruit. Plant Food. Hum. Nutr. 2009, 64, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Ren, X.Y.; He, L.; Cheng, J.W.; Chang, J.M. Optimization of the solid-state fermentation and properties of a polysaccharide from Paecilomyces cicadae (Miquel) Samson and its antioxidant activities in vitro. PLoS ONE 2014, 9, e87578. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Ji, P.F.; Gong, X.G.; Li, W.Q.; Cheng, J.W.; Qian, H.; Song, X.L. Physico-chemical characterization, antioxidant and anticancer activities in vitro of a novel polysaccharide Melia toosendan Sieb. Et Zucc fruit. Int. J. Biol. Macromol. 2016, 49, 422–427. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Yang, X.B.; Zhao, Y.; Ruan, Y.; Yang, Y.; Wang, Z.Z. Separation and quantification of component monosaccharides of the tea polysaccharides from Gynostemma pentaphyllum by HPLC with indirect UV detection. Food Chem. 2009, 112, 742–746. [Google Scholar] [CrossRef]
- Mao, G.; Zou, Y.; Feng, W.; Wang, W.; Zhao, T.; Ye, C.; Zhu, Y.; Wu, X.S.; Yang, L.Q.; Wu, X.Y. Extraction, preliminary characterization and antioxidant activity of Se-enriched Maitake polysaccharide. Carbohydr. Polym. 2014, 101, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds SJP (Polysaccharides from Semen Juglandis) are available from the authors.

| Run Order | Coded Levels | Yield of SJP (%) | |||
|---|---|---|---|---|---|
| X1 | X2 | X3 | Observed | Predicted | |
| 1 | −1 | −1 | 0 | 3.69 | 3.56 |
| 2 | 1 | −1 | 0 | 5.25 | 5.16 |
| 3 | −1 | 1 | 0 | 4.99 | 5.09 |
| 4 | 1 | 1 | 0 | 4.92 | 5.05 |
| 5 | −1 | 0 | −1 | 4.22 | 4.07 |
| 6 | 1 | 0 | −1 | 5.09 | 4.91 |
| 7 | −1 | 0 | 1 | 4.38 | 4.56 |
| 8 | 1 | 0 | 1 | 5.13 | 5.28 |
| 9 | 0 | −1 | −1 | 3.49 | 3.76 |
| 10 | 0 | 1 | −1 | 5.16 | 5.22 |
| 11 | 0 | −1 | 1 | 4.98 | 4.93 |
| 12 | 0 | 1 | 1 | 5.17 | 4.90 |
| 13 | 0 | 0 | 0 | 5.69 | 5.67 |
| 14 | 0 | 0 | 0 | 5.69 | 5.67 |
| 15 | 0 | 0 | 0 | 5.68 | 5.67 |
| 16 | 0 | 0 | 0 | 5.65 | 5.67 |
| 17 | 0 | 0 | 0 | 5.64 | 5.67 |
| Source | Sum of Squares | Degree of Freedom | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|
| Model | 7.11 | 9 | 0.79 | 17.46 | <0.0005 |
| X1 | 1.22 | 1 | 1.22 | 26.85 | 0.0013 |
| X2 | 1.01 | 1 | 1.01 | 22.25 | 0.0022 |
| X3 | 0.36 | 1 | 0.36 | 7.95 | 0.0258 |
| X1X2 | 0.67 | 1 | 0.67 | 14.88 | 0.0062 |
| X1X3 | 0.00352 | 1 | 0.00352 | 0.078 | 0.7886 |
| X2X3 | 0.55 | 1 | 0.55 | 12.19 | 0.0101 |
| X12 | 0.97 | 1 | 0.97 | 21.33 | 0.0024 |
| X22 | 0.98 | 1 | 0.98 | 21.59 | 0.0024 |
| X32 | 1.01 | 1 | 1.01 | 22.30 | 0.0022 |
| Residual | 0.32 | 7 | 0.045 | 26.85 | 0.0013 |
| Lack of fit | 0.31 | 3 | 0.10 | 137.46 | 0.0002 |
| Pure error | 0.003044 | 4 | 0.000761 | ||
| Cor. Total | 7.43 | 16 | |||
| R2 = 0.9573, R2adj = 0.9025, C.V.% = 4.26% | |||||
| Independent Variables | Variable Levels | ||
|---|---|---|---|
| −1 | 0 | 1 | |
| Extraction temperature (°C) | 75 | 85 | 95 |
| Extraction time (min) | 90 | 120 | 150 |
| Ratio of solution to solid (mL/g) | 20 | 30 | 40 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, X.; He, L.; Wang, Y.; Cheng, J. Optimization Extraction, Preliminary Characterization and Antioxidant Activities of Polysaccharides from Semen Juglandis. Molecules 2016, 21, 1335. https://doi.org/10.3390/molecules21101335
Ren X, He L, Wang Y, Cheng J. Optimization Extraction, Preliminary Characterization and Antioxidant Activities of Polysaccharides from Semen Juglandis. Molecules. 2016; 21(10):1335. https://doi.org/10.3390/molecules21101335
Chicago/Turabian StyleRen, Xueyong, Liang He, Yanbin Wang, and Junwen Cheng. 2016. "Optimization Extraction, Preliminary Characterization and Antioxidant Activities of Polysaccharides from Semen Juglandis" Molecules 21, no. 10: 1335. https://doi.org/10.3390/molecules21101335
APA StyleRen, X., He, L., Wang, Y., & Cheng, J. (2016). Optimization Extraction, Preliminary Characterization and Antioxidant Activities of Polysaccharides from Semen Juglandis. Molecules, 21(10), 1335. https://doi.org/10.3390/molecules21101335






