2.1. Optimization of the Chromatographic Conditions
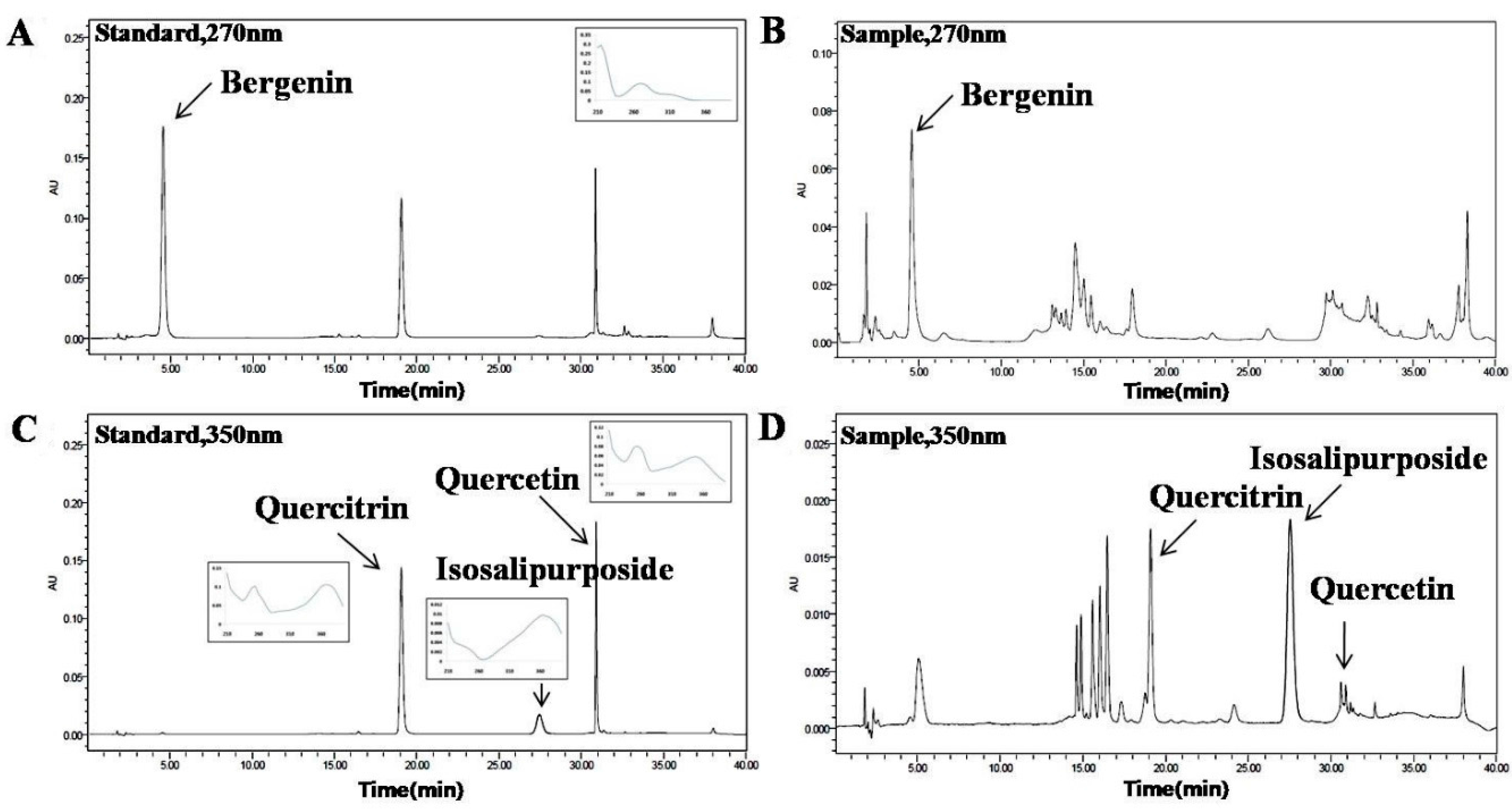
The ratio of methanol and water containing phosphoric acid as the mobile phase, column temperature, wavelength and flow rate were investigated for good separation (data not shown). A gradient program was used to separate the four active markers in a single run within a reasonable period of time. In the present study, two different detection wavelengths were set according to the UV absorption maxima of the compounds. Bergenin was detected at 270 nm, quercetin, quercitrin and isosalipurposide were detected at 350 nm. Under the proposed analytical conditions, baseline resolution was obtained for all the analytes. Chromatograms of the standards and sample solutions are shown in
Figure 1.
Figure 1.
Identification of active compounds in C. coreana Uyeki flos by HPLC. (A) standard mixture at 270 nm; (B) sample extract at 270 nm; (C) standard mixture at 350 nm; (D) sample extract at 350 nm, A mobile phase consisting of mixture of solvent A (acetonitrile) and B (water containing 0.2% phosphoric acid) and employing a gradient elution (from 10:90 to 100:0, v/v) at a flow rate of 0.8 mL/min. The detection wavelength was set at 270 nm for bergenin, at 350 nm for quercetin, quercitrin and isosalipurposide.
Figure 1.
Identification of active compounds in C. coreana Uyeki flos by HPLC. (A) standard mixture at 270 nm; (B) sample extract at 270 nm; (C) standard mixture at 350 nm; (D) sample extract at 350 nm, A mobile phase consisting of mixture of solvent A (acetonitrile) and B (water containing 0.2% phosphoric acid) and employing a gradient elution (from 10:90 to 100:0, v/v) at a flow rate of 0.8 mL/min. The detection wavelength was set at 270 nm for bergenin, at 350 nm for quercetin, quercitrin and isosalipurposide.
2.3 Antimicrobial Activity of C. coreana Uyeki Flos Extracts
Samples were extracted with six different solvent compositions in order to select the best extraction solvent conditions: water, 20% ethanol, 40% ethanol, 60% ethanol, 80% ethanol and 100% ethanol (
v/
v). Firstly, we analyzedthe inhibition effects of the extract from
C. coreana Uyeki flos on methicillin resistant
Staphylococcus aureus 693E (MRSA 693E) through the disk diffusion method and found that extract displayed antimicrobial activity (
Table 5). The 100% ethanolic extract showed the best antimicrobial activity. In
Table 4, quercetin was used as control. Quercetin showed antimicrobial activity against MRSA 693E with 400 μg of quercetin loading on the disc. Accordinh to
Figure 2, the ethanolic extract contains very low levels of quercetin. After purifying unknown antimicrobial compounds, which is now in progress, we will study the antimicrobial effect of the extract in detail in relation to their corresponding reference compounds. Secondly, a HPLC method was applied to analyze the six samples. The average amounts (%wt) of bergenin, quercetin, quercitrin and isosalipurposide are presented in
Figure 2. The amount of the four compounds in the 100% ethanolic extract was higher than that of in the 0%–80% ethanolic extracts. According to these results, a 100% ethanol solution was selected as the most effective extraction solvent.
Table 5.
Antimicrobial activity of extract from C. coreana Uyeki flos.
Table 5.
Antimicrobial activity of extract from C. coreana Uyeki flos.
| Ethanolic Extract (Ethanol %, v/v) | Zone of Inhibition ± SD (cm, Diameter of disc:0.8) |
|---|
| Vancomycin (10 μg/disc) | 1.3 ± 0.1 |
| Quercetin (400 μg/disc) | 1.07 ± 0.06 |
| 0 | 0.8 |
| 20 | 0.8 |
| 40 | 0.93 ± 0.06 |
| 60 | 1.07 ± 0.06 |
| 80 | 1.20 ± 0.1 |
| 100 | 1.23 ± 0.06 |
Figure 2.
Concentration of bergenin, quercetin, quercitrin and isosalipurposide in ethanolic extract from C. coreana Uyeki flos. Each value was the mean ± SD (n = 3).
Figure 2.
Concentration of bergenin, quercetin, quercitrin and isosalipurposide in ethanolic extract from C. coreana Uyeki flos. Each value was the mean ± SD (n = 3).
2.4. Antioxidant Activity and Total Phenolic Contents of C. coreana Uyeki Flos Extracts
The antioxidant potential of various extracts of
C. coreana Uyeki flos was determined by the 2,2-diphenyl-1-picrylhydrazyl (DPPH) and reducing power assays. Plants have been reported to be plentiful sources of phytochemicals such as phenolics and various health benefits like antioxidant activity have been suggested [
18]. In addition, Cho
et al. [
19] reported that the various antioxidant activities of natural resources were significantly correlated with their major compound contents, such as polyphenols. Therefore, we determined the total phenolic contents of extracts obtained from
C. coreana Uyeki flos.
The DPPH scavenging effect is a widely used method to evaluate the free radical scavenging ability of different samples, including plant extracts. The measured DPPH radical scavenging activity is shown in
Table 5. A low IC
50 value indicates strong antioxidant activity in a sample. The scavenging effect from IC
50 data on the DPPH radical increased in the order: water extract (182.3 ± 0.004 µg/mL) > 20% ethanol extract (139.3 ± 0.005 µg/mL) > 40% ethanol extract (100 ± 0.07 µg/mL) > 60% ethanol extract (90 ± 0.001 µg/mL) > 100% ethanol extract (56.1 ± 0.004 µg/mL) > 80% ethanol extract (30.6 ± 0.01 µg/mL).
Fe3+ was transformed into Fe2+ in the presence of extracts to measure the reductive capability. Eighty % etanolic extract had highest activity among the six extracts. At 100 µg/mL, the values, which were expressed as vitamin C equivalents, decreased in the order: 80% ethanol extract (64.33 ± 5.05 µg/mL) > 60% ethanol extract (62.93 ± 3.56 µg/mL) > 40% ethanol extract (62.63 ± 1.39 µg/mL) > 20% ethanol extract (42.21 ± 2.53 µg/mL) > water extract (37.38 ± 1.26 µg/mL) > 100% ethanol extract (25.58 ± 6.65 µg/mL).
Total phenol compounds, as determined by the Folin Ciocalteu method [
20], is reported as gallic acid equivalents by reference to standard curve (
r2 = 0.999). The total phenolic content of extracts is shown in
Table 6. The amount of the phenolic compounds in the 80% ethanolic extract was higher than that of in the 0%–60% and 100% ethanolic extracts. Eighty percentage ethanolic extract showed the best DPPH radical scavenging activity, reducing power and phenolic contents. Antioxidant and phenolic contents of 100% ethanolic extract was less than 80% ethanolic extract.
Table 6.
Antioxidant activity and total phenolic contents of extracts of C. coreana flos.
Table 6.
Antioxidant activity and total phenolic contents of extracts of C. coreana flos.
| Extract | DPPH Assay (IC50, μg/mL) | Reducing Power (Ascorbic Acid eq. μg/100 μg Extract) | Total Phenolic Content (Gallic Acid eq. mg/g) |
|---|
| Ascorbic acid | 4.25 ± 0.04 | | |
| Water | 182.3 ± 0.004 | 37.88 ± 1.26 | 111.47 ± 5.1 |
| 20% EtOH Ex | 139.3 ± 0.005 | 41.21 ± 2.53 | 138.13 ± 17.87 |
| 40% EtOH Ex | 100 ± 0.07 | 62.63 ± 1.39 | 220 ± 14.7 |
| 60% EtOH Ex | 90 ± 0.001 | 62.93 ± 3.56 | 245.07 ± 23.17 |
| 80% EtOH Ex | 30.6 ± 0.01 | 64.33 ± 5.05 | 269.37 ± 98.72 |
| 100% EtOH Ex | 56.1 ± 0.01 | 25.58 ± 6.65 | 140.53 ± 14.95 |
Kim,
et al reported that content of caffeic acid derivatives from
Ligularia fischeri increased when extracted with hot water (116 μg/mL). But 50% and 100% ethanolic extracts, contents of caffeic acid derivatives were 72 and 11 μg/mL [
21]. Similarly, we previously reported that extraction efficiency of caffeic acid from pear pomace decreased with increasing percentage of ethanol. Besides, the extraction efficiency of chlorogenic acid from pear pomace increased with increasing amount of ethanol [
22]. We concluded that phenolic extraction maybe affected by solvent combinations. In our case, 80% ethanol was a more efficient solvent in the extraction of phenolic compounds from
C. coreana.
From the ethanolic extracts from
C. coreana flos, we identified phenolic compounds such as bergenin, quercetin, quercitrin. They are phenolic compounds widely found in food products derived from plant sources, and they have been shown to possess antioxidant activities [
23,
24,
25].
2.5. C. coreana Uyeki Flos: A Potential Natural Source for the Treatment of Infectious Arthritis
Septic arthritis is an acute or chronic infectious disease that mainly occurs in a native or prosthetic joint. Timely and appropriate treatment can effectively reduce morbidity [
26]. American studies estimated an occurrence of 20,000 cases of suppurative arthritis per year (7.8 individuals per 100,000/year). In Denmark from 2003 to 2004
Streptococcal infections occurred at a rate of 2.6 cases per 100,000 people [
27]. To date, interest in the use of antibiotics in arthritis treatment has been motivated by two factors: (1) in some forms of chronic arthritis, microbial antigens persist in the synovial membrane; and (2) the increasing knowledge of the anti-inflammatory and immunosuppressive effects of antibiotics. Several studies have reported a beneficial effect of antibiotics such as tetracycline and ciprofloxacin on rheumatoid arthritis and reactive arthritis [
28,
29]. The adverse effects seem to be mild, however the long-term efficacy and safety of tetracycline as a disease-modifying anti rheumatic drug remain to be demonstrated. In particular the eradication of methicillin resistant
Staphylococcus aureus (MRSA) that can produce extended-spectrum β-lactamases (ESBL) and carbapenemases is urgent [
30,
31].
Recently there has been great effort to find candidates from natural products to effectively control infectious strains. For instance, flower extracts of
Calotropis procera [
32] and
Delonix regia [
33] have been reported to exhibit antimicrobial activity against infectious strains. Extracts of various plants containing flavonoids and isocoumarins have also been previously reported to possess antimicrobial activity. The flavonoids quercetin and quercitrin have antimicrobial activities against general infectious bacteria such as
Staphylococcus aureus,
Staphylococcus epidemidis,
Streptococcus pyrogenes and
Pseudomonas aeruginosa [
34]. The isocoumarin bergenin also has antimicrobial activities against the
Candida species and some
Aspergillus species [
35]. Recent reports show that phenolic compounds have curative effects on septic arthritis. For example, chlorogenic acid is known as one of the most abundant polyphenols in the human diet. It has activity against arthritis caused by
Candida albicans [
36]. Gentamicin in combination with ascorbic acid regulates the severity of
Staphylococcus aureus infection-induced septic arthritis in mice [
37].
From the HPLC analysis, we found that the amount of bergenin was the highest among the four identified compounds in the extract from C. coreana Uyeki flos, with isosalipurposide being the second. Quercitrin and quercetin were minor compounds in the extract. Based on the analytical data, we focused on the anti-microbial, antioxidant and anti-inflammatory activities of the two flavonoids and one isocoumarin but not isosalipurposide.
First, we focused on bergenin. One hundred percent ethanolic extract of
C. coreana Uyeki flos contained bergenin with a concentration of 17.5% (
w/
w). Luis
et al. found that contents of bergenin were 15.7% and 20% (
w/
w) in 50% ethanolic and hot water extract from
Endopleura uchi [
11]. Our result indicate that extracts
C. coreana Uyeki flos are a rich source of bergenin similar to the extract from
Endopleura uchi and there are no prior reports about the presence of bergenin in
C. coreana Uyeki flos.
Bergenin was reported to have anti-inflammatory activity [
38,
39]. Additionally, bergenin showed antinociceptive properties in models of inflammatory pain and did not show any apparent systemic toxicity [
31]. Thus, bergenin may be a useful compound for the treatment of arthritis. We suggest that bergenin and extracts containing bergenin may be good candidates for the treatment of arthritis caused by microbial infection.
Secondly we considered quercetin and quercitrin. Guardia,
et al. found that quercetin has anti-inflammatory effects in rat adjuvant arthritis [
40] and can reduce the production of macrophage inflammatory mediators in the adjuvant-induced arthritis mouse model [
41]. Quercetin has minor side effects. In clinical study phase I, the recommended dose of quercetin is 1400 mg/m
2, which corresponds to approximately 2.5 g for a 70 kg individual [
42]. For a 4 g single-dose oral administration or 500 mg twice daily, no side effects were found in through the repeated dosing study [
43]. In the present study, quercetin and quercitrin where found in a ratio of 1:32 in the 100% ethanolic extract of
C. coreana Uyeki flos. The extract shows antimicrobial activity and contains four pharmacologically active compounds. We examined the pharmacological activities of two flavonoids (quercetin and quercitrin) and an isocoumarin (bergenin) and the mild side effects. We conclude that the validation method for
C. coreana Uyeki flos is quite valuable and this plant is a promising antimicrobial and anti-inflammatory drug source candidate.