Antibacterial Activity of Alkaloid Fractions from Berberis microphylla G. Forst and Study of Synergism with Ampicillin and Cephalothin
Abstract
:1. Introduction
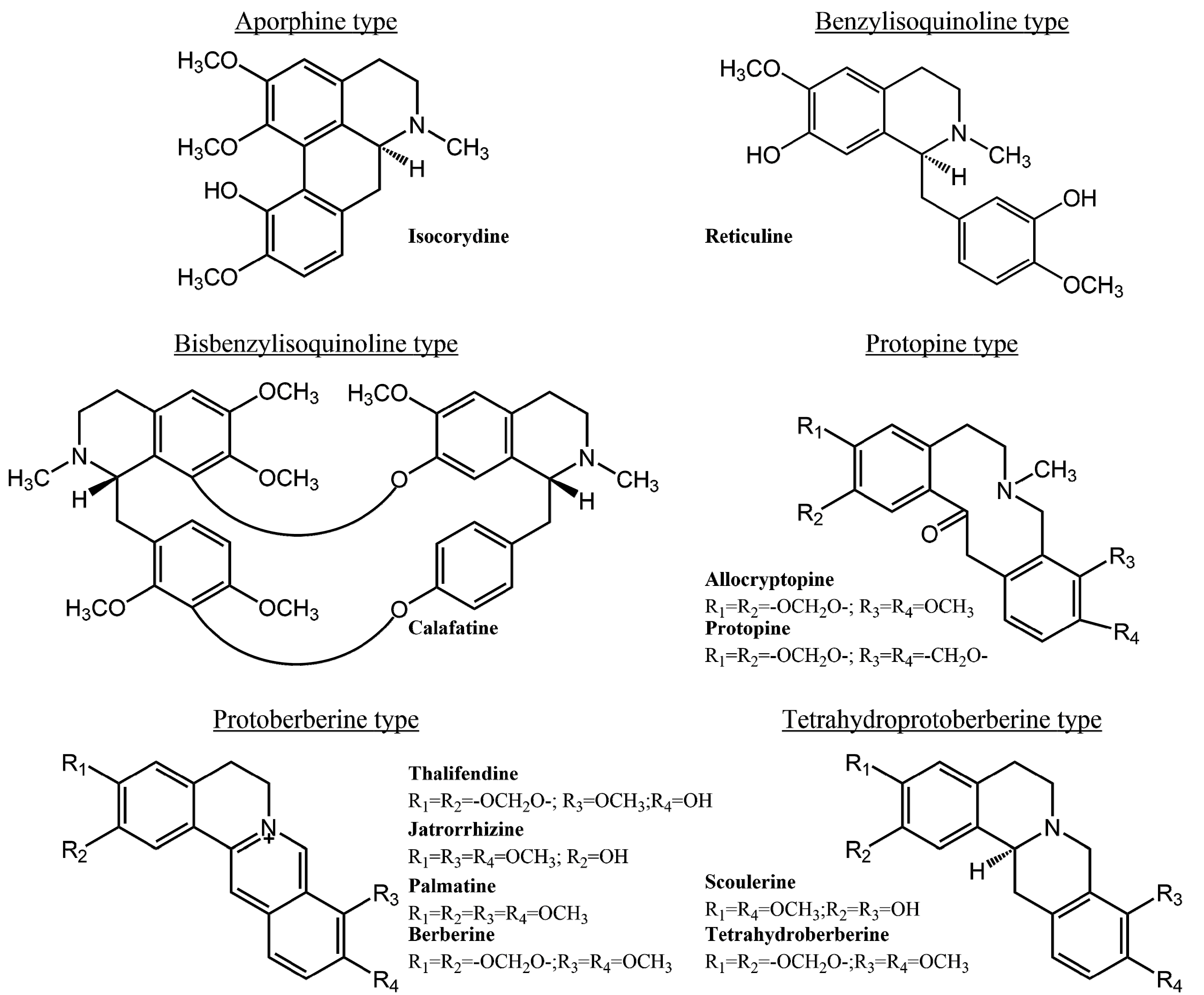
2. Results and Discussion
2.1. Antibacterial Activity of Alkaloid Extracts of B. microphylla
| Samples | Microorganisms | |||||||
|---|---|---|---|---|---|---|---|---|
| Gram-Negative | Gram-Positive | |||||||
| E. aerogenes ATCC 13084 | E.coli ATCC 25922 | L. monocytogenes ATCC 13932 | S. typhimurium ATCC 13311 | S. aureus ATCC 25923 | B. cereus ATCC 11778 | S. epidermidis ATCC 12228 | B. subtilis ATCC 6633 | |
| Plant extracts | ||||||||
| Leaves | ||||||||
| 500 µg/disc | i | i | i | i | 2.9 ± 0.2 h | 2.8 ± 0.2 f | 6.4 ± 0.5 e | 3.8 ± 0.2 g |
| 1000 µg/disc | i | i | i | i | 4.7 ± 0.6 g | 4.7 ± 0.5 e | 7.7 ± 0.6 e | 4.9 ± 0.2 ef |
| 2000 µg/disc | i | i | i | i | 7.7 ± 0.2 e | 6.9 ± 0.2 c | 9.7 ± 0.6 d | 5.7 ± 0.6 e |
| Stems | ||||||||
| 500 µg/disc | i | i | i | i | 5.7 ± 0.2 f | 3.9 ± 0.2 e | 7.00 ± 0.0 e | 3.8 ± 0.4 fg |
| 1000 µg/disc | i | i | i | i | 8.9 ± 0.2 d | 5.7 ± 0.6 d | 10.7 ± 0.6 d | 5.3 ± 0.6 e |
| 2000 µg/disc | i | i | i | i | 9.7 ± 0.6 d | 7.0 ± 0.5 c | 13.0 ± 0.5 c | 6.9 ± 0.2 cd |
| Roots | ||||||||
| 500 µg/disc | i | i | i | i | 5.7 ± 0.6 f | 11.0 ± 0.0 b | 9.7 ± 0.2 d | 3.8 ± 0.4 fg |
| 1000 µg/disc | i | i | i | i | 8.9 ± 0.2 d | 12.0 ± 0.5 b | 13.0 ± 0.6 c | 5.8 ± 0.3 de |
| 2000 µg/disc | i | i | i | i | 11.1 ± 0.1 c | 13.9 ± 0.2 a | 15.3 ± 0.6 b | 7.9 ± 0.20 c |
| Berberine | ||||||||
| 500 µg/disc | i | i | i | i | 6.9± 0.2 e | i | 12.7± 0.2 c | i |
| 1000 µg/disc | i | i | i | i | 7.7± 0.2 e | i | 14.3± 0.6 b | i |
| 2000 µg/disc | i | i | i | i | 9.7± 0.6 d | i | 14.9± 0.2 b | i |
| Antibiotics | ||||||||
| Ampicillin 10 µg/disc | 10.0 ± 0.0 | 21.0 ± 0.0 | 30.0 ± 0.0 | 19.0 ± 0.0 | 34.0 ± 0.5 b | 11.0 ± 0.06 b | 16.7 ± 0.5 b | 27.0 ± 0.06 b |
| Cephalothin 30 µg/disc | 20.0 ± 0.0 | 16.0 ± 0.0 | 20.0 ± 0.0 | 18.0 ± 0.0 | 35.6 ± 0.06 a | 2.6 ± 0.0 f | 25.9 ± 0.2 a | 36.0 ± 0.06 a |
| Samples | Gram-Positive Bacteria | |||||||
|---|---|---|---|---|---|---|---|---|
| S. aureus ATCC 25923 | B. cereus ATCC 11778 | S. epidermidis ATCC 12228 | B. subtilis ATCC 6633 | |||||
| MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | |
| Plant Extract | ||||||||
| Leaves | 250 ± 0 | 750 ± 0 | 333 ± 118 | 717 ± 118 | 125 ± 0 | 250 ± 0 | 333 ± 118 | 717 ± 118 |
| Stems | 167 ± 50 | 334 ± 100 | 125 ± 0 | 250 ± 0 | 83 ± 30 | 167 ± 60 | 250 ± 0 | 500 ± 0 |
| Roots | 83 ± 30 | 167 ± 60 | 125 ± 0 | 250 ± 0 | 83 ± 30 | 167 ± 60 | 167 ± 50 | 334 ± 100 |
| Berberine | 167 ± 50 | 334 ± 100 | i | i | 167 ± 50 | 334 ± 100 | i | i |
2.2. Fractional Inhibitory Concentration Index
3. Experimental Section
3.1. Plant Material
3.2. Alkaloid Extraction
| Samples | Gram-Positive | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S. aureus ATCC 25923 | B. cereus ATCC 11778 | S. epidermidis ATCC 12228 | B. subtilis ATCC 6633 | |||||||||||||||||
| MICa | MICb | FIC | FICI | Effect | MICa | MICb | FIC | FICI | Effect | MICa | MICb | FIC | FICI | Effect | MICa | MICb | FIC | FICI | Effect | |
| Leaves extracts (µg/mL) | ||||||||||||||||||||
| Leaves | 250 | 125 | 0.5 | 1 | I | 358 | 89.5 | 0.25 | 0.5 | S | 124 | 31.0 | 0.25 | 0.5 | S | 333 | 83.25 | 0.25 | 0.5 | S |
| Ampicillin | 0.06 | 0.03 | 0.5 | 4.0 | 1.0 | 0.25 | 1.6 | 0.4 | 0.25 | 0.03 | 0.0075 | 0.25 | ||||||||
| Stems extracts (µg/mL) | ||||||||||||||||||||
| Stems | 167 | 41.7 | 0.25 | 0.5 | S | 125 | 125 | 1 | 2 | I | 83 | 41.5 | 0.5 | 1 | I | 274 | 68.5 | 0.25 | 0.5 | S |
| Ampicillin | 0.06 | 0.015 | 0.25 | 4.0 | 4.0 | 1 | 1.6 | 0.8 | 0.5 | 0.03 | 0.0075 | 0.25 | ||||||||
| Roots extracts (µg/mL) | ||||||||||||||||||||
| Roots | 83 | 41.5 | 0.5 | 1 | I | 125 | 125 | 1 | 2 | I | 83 | 41.5 | 0.5 | 1 | I | 187 | 46.7 | 0.25 | 0.5 | S |
| Ampicillin | 0.06 | 0.03 | 0.5 | 4.0 | 4.0 | 1 | 1.6 | 0.8 | 0.5 | 0.03 | 0.0075 | 0.25 | ||||||||
| Samples | Gram-Positive | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S. aureus ATCC 25923 | B. cereus ATCC 11778 | S. epidermidis ATCC 12228 | B. subtilis ATCC 6633 | |||||||||||||||||
| MICa | MICb | FIC | FICI | Effect | MICa | MICb | FIC | FICI | Effect | MICa | MICb | FIC | FICI | Effect | MICa | MICb | FIC | FICI | Effect | |
| Leaves extracts (µg/mL) | ||||||||||||||||||||
| Leaves | 250 | 62.5 | 0.25 | 0.5 | S | 358 | 89.5 | 0.25 | 0.5 | S | 124 | 31.0 | 0.25 | 0.5 | S | 333 | 166.5 | 0.5 | 1 | I |
| Cephalothin | 0.06 | 0.015 | 0.25 | 50 | 12.5 | 0.25 | 0.2 | 0.05 | 0.25 | 0.01 | 0.005 | 0.5 | ||||||||
| Stems extracts (µg/mL) | ||||||||||||||||||||
| Stems | 167 | 41.7 | 0.25 | 0.5 | S | 125 | 62.5 | 0.5 | 1 | I | 83 | 41.5 | 0.5 | 1 | I | 274 | 68.5 | 0.25 | 0.5 | S |
| Cephalothin | 0.06 | 0.015 | 0.25 | 50 | 25 | 0.5 | 0.2 | 0.1 | 0.5 | 0.01 | 0.0025 | 0.25 | ||||||||
| Roots extracts (µg/mL) | ||||||||||||||||||||
| Roots | 83 | 41.5 | 0.5 | 1 | I | 125 | 31.2 | 0.25 | 0.5 | S | 83 | 20.7 | 0.25 | 0.5 | S | 187 | 93.5 | 0.5 | 1 | I |
| Cephalothin | 0.06 | 0.03 | 0.5 | 50 | 12.5 | 0.25 | 0.2 | 0.05 | 0.25 | 0.01 | 0.005 | 0.5 | ||||||||
3.3. Microorganism Strains and Antibiotics
3.4. Antibacterial Assays
3.5. Antimicrobial Activity (Disc Diffusion Assay)
3.6. Minimum Inhibitory Concentration (MIC)
3.7. Minimum Bactericidal Concentration (MBC)
3.8. Determination of the Fractional Inhibitory Concentration (FIC) Index
3.9. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Landrum, L.R. Revision of berberis (Berberidaceae) in Chile and adjacent Southern Argentina. Ann. MO. Bot. Gard. 1999, 86, 793–834. [Google Scholar] [CrossRef]
- Bottini, M.; Greizerstein, E.J.; Aulicino, M.B.; Poggio, L. Relationships among genome size, environmental conditions and geographical distribution in natural populations of NW Patagonian species of Berberis L. (Berberidaceae). Ann. Bot. 2000, 86, 565–573. [Google Scholar] [CrossRef]
- Landrum, L.R. Berberidaceae. Marticorena, C., Rodríguez, R., Eds.; In Flora de Chile; Editorial Universidad de Concepción: Concepción, Chile, 2003; Volume 2, pp. 1–23. [Google Scholar]
- Zin, J.; Weiss, C. La Salud por Medio de Plantas Medicinales, 8th ed.; Don Bosco: Santiago, Chile, 1998; p. 148. [Google Scholar]
- Domínguez, E.; Aguilera, O.; Villa-Martínez, R.; Aravena, J.C.; Henríquez, J.M. Ethnobotanical suvey of Kalau Island, ancestral Kawesqar territory, Magallanes Región, Chile. Inst. Patagon. Ann. 2012, 40, 19–35. [Google Scholar] [CrossRef]
- Manosalva, L.; Mutis, A.; Díaz, J.; Urzúa, A.; Fajardo, V.; Quiroz, A. Identification of isoquinoline alkaloids from Berberis microphylla by HPLC ESI-MS/MS. Bol. Latinoam. Caribe Plant. Med. Aromat. 2014, 13, 323–334. [Google Scholar]
- Odds, F.C. Synergy, antagonism, and what the chequerboard puts between them. J. Antimicrob. Chemother. 2003, 52. [Google Scholar] [CrossRef] [PubMed]
- Denyer, S.P.; Maillard, J.Y. Cellular impermeability and uptake of biocides and antibiotics in Gram-negative bacteria. J. Appl. Microbiol. 2002, 92, 35S–45S. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Srivastava, S.; Rawat, A. Antimicrobial activities of Indian Berberis species. Fitoterapia 2007, 78, 574–576. [Google Scholar] [CrossRef] [PubMed]
- Gulfraz, M.; Fatima, N.; Parveen, Z.; Mahmood, S. Antimicrobial activity of Berberis lyceum Royle against different microorganisms. Can. J. Pure Appl. Sci. 2007, 1, 15–20. [Google Scholar]
- Bhandari, D.K.; Nath, G.; Ray, A.B.; Tewari, P.V. Antimicrobial activity of crude extracts from Berberis asiatica stem bark. Pharm. Biol. 2000, 38, 254–257. [Google Scholar] [CrossRef]
- Iwasa, K.; Kamigauchi, M.; Ueki, M.; Taniguchi, M. Antibacterial activity and structure-activity relationships of berberine analogs. Eur. J. Med. Chem. 1996, 31, 469–478. [Google Scholar] [CrossRef]
- Tegos, G.; Stermitz, F.R.; Lomovskaya, O.; Lewis, K.K. Multidrug pump inhibitors uncover remarkable activity of plant antimicrobials. Antimicrob. Agents Chemother. 2002, 46, 3133–3141. [Google Scholar] [CrossRef] [PubMed]
- Karou, D.; Savadogo, A.; Canini, A.; Yameogo, S.; Montesano, C.; Simpore, J.; Colizzi, V.; Traore, A.S. Antibacterial activity of alkaloids from Sida acuta. Afr. J. Biotechnol. 2006, 5, 195–200. [Google Scholar]
- Slobodníková, L.; Kost'álová, D.; Labudová, D.; Kotulová, D.; Kettmann, V. Antimicrobial activity of Mahonia aquifolium crude extract and its major isolated alkaloids. Phytother. Res. 2004, 18, 674–676. [Google Scholar]
- Su, Y.; Li, S.; Li, N.; Chen, L.; Jiwen, C.; Zhang, J.; Wang, J. Seven alkaloids and their antibacterial activity from Hypecoum erectum L. J. Med. Plant. Res. 2011, 5, 5428–5432. [Google Scholar]
- Zuo, G.-Y.; Li, Y.; Wang, T.; Han, J.; Wang, G.-C.; Zhang, Y.-L.; Pan, W.-D. Synergistic Antibacterial and Antibiotic effect of Bisbenzylisoquinoline alkaloids on clinical isolates of Methicillin-Resistant Staphylococcus aureus (MRSA). Molecules 2011, 16, 9819–9826. [Google Scholar] [CrossRef] [PubMed]
- Paulo, M.; Barbosa-Filho, J.; Lima, E.; Maia, R.; Barbosa, R.; Kaplan, M.A. Antimicrobial activity of benzylisoquinoline alkaloids from Annona salzmanni D.C. J. Ethnopharm. 1992, 36, 39–41. [Google Scholar] [CrossRef]
- Stefanovic, O.; Comic, L. Inhibitory effect of Cytisus nigricans L. and Cytisus capitatus Scop. on growth of bacteria. Afr. J. Microbiol. Res. 2011, 5, 4725–4730. [Google Scholar] [CrossRef]
- Konaté, K.; Mavoungou, J.F.; Lepengué, A.N.; Aworet-Samseny, R.R.R.; Hilou, A.; Souza, A.; Mamoudou, H.; Dicko, M.H.; M’Batchi, B. Antibacterial activity against β-lactamase producing methicillin and ampicillin-resistant’s Staphylococcus aureus: Fractional Inhibitory Concentration Index (FICI) determination. Ann. Clin. Microbiol. Antimicrob. 2012, 11, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Chung, P.; Navaratnam, P.; Chung, L. Synergistic antimicrobial activity between pentacyclic triterpenoids and antibiotics against Staphylococcus aureus strains. Ann. Clin. Microbiol. Antimicrob. 2011, 10, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Olajuyigbe, O.O.; Afolayan, A.J. Synergistic interactions of methanolic extract of Acacia mearnsii de wild. With antibiotics against bacteria of clinical relevance. Int. J. Mol. Sci. 2012, 13, 8915–8932. [Google Scholar] [CrossRef] [PubMed]
- Basri, D.F.; Xian, L.W.; Shukor, N.I.A.; Latip, J. Bacteriostatic antimicrobial combination: antagonistic interaction between epsilon-viniferin and vancomycin against methicillin-resistant Staphylococcus aureus. Biomed. Res. Int. 2014. [Google Scholar] [CrossRef] [PubMed]
- Teethaisong, Y.; Autarkool, N.; Sirichaiwetchakoon, K.; Krubphachaya, P.; Kupittayanant, S.; Eumkeb, G. Synergistic activity and mechanism of action of Stephania suberosa Forman extract and ampicillin combination against ampicillin-resistant Staphylococcus aureus. J. Biomed. Sci. 2014, 21, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Wojtyczka, R.; Dziedzic, A.; Kępa, M.; Kubina, R.; Kabała-Dzik, A.; Mularz, T.; Idzik, D. Berberine enhances the antibacterial activity of selected antibiotics against coagulase-negative Staphylococcus strains in vitro. Molecules 2014, 19, 6583–6596. [Google Scholar] [CrossRef] [PubMed]
- Cabezas, N.J.; Urzúa, A.M.; Niemeyer, H.M. Translocation of isoquinoline alkaloids to the hemiparasite, Tristerix verticillatus from its host, Berberis montana. Biochem. Syst. Ecol. 2009, 37, 225–227. [Google Scholar] [CrossRef]
- Ericsson, H.; Sherris, J.C. Antibiotic sensitivity testing. Report of an international collaborative study. Acta Pathol. Microbiol. Scand. Sect. B 1971, 217, 1–90. [Google Scholar]
- Stefanović, O.D.; Stanojević, D.D.; Comić, L.R. Synergistic antibacterial activity of Salvia officinalis and Cichorium intybus extracts and antibiotics. Acta Pol. Pharm. 2012, 69, 457–463. [Google Scholar] [PubMed]
- Fuchs, P.C.; Barry, A.L.; Brown, S.D. In vitro bactericidal activity of daptomycin against staphylococci. J. Antimicrob. Chemother. 2002, 49, 467–470. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of calafatine, berberine, jatrorrhizine and palmatine are available from the authors.
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manosalva, L.; Mutis, A.; Urzúa, A.; Fajardo, V.; Quiroz, A. Antibacterial Activity of Alkaloid Fractions from Berberis microphylla G. Forst and Study of Synergism with Ampicillin and Cephalothin. Molecules 2016, 21, 76. https://doi.org/10.3390/molecules21010076
Manosalva L, Mutis A, Urzúa A, Fajardo V, Quiroz A. Antibacterial Activity of Alkaloid Fractions from Berberis microphylla G. Forst and Study of Synergism with Ampicillin and Cephalothin. Molecules. 2016; 21(1):76. https://doi.org/10.3390/molecules21010076
Chicago/Turabian StyleManosalva, Loreto, Ana Mutis, Alejandro Urzúa, Victor Fajardo, and Andrés Quiroz. 2016. "Antibacterial Activity of Alkaloid Fractions from Berberis microphylla G. Forst and Study of Synergism with Ampicillin and Cephalothin" Molecules 21, no. 1: 76. https://doi.org/10.3390/molecules21010076





