High Milk-Clotting Activity Expressed by the Newly Isolated Paenibacillus spp. Strain BD3526
Abstract
:1. Introduction
2. Results and Discussions
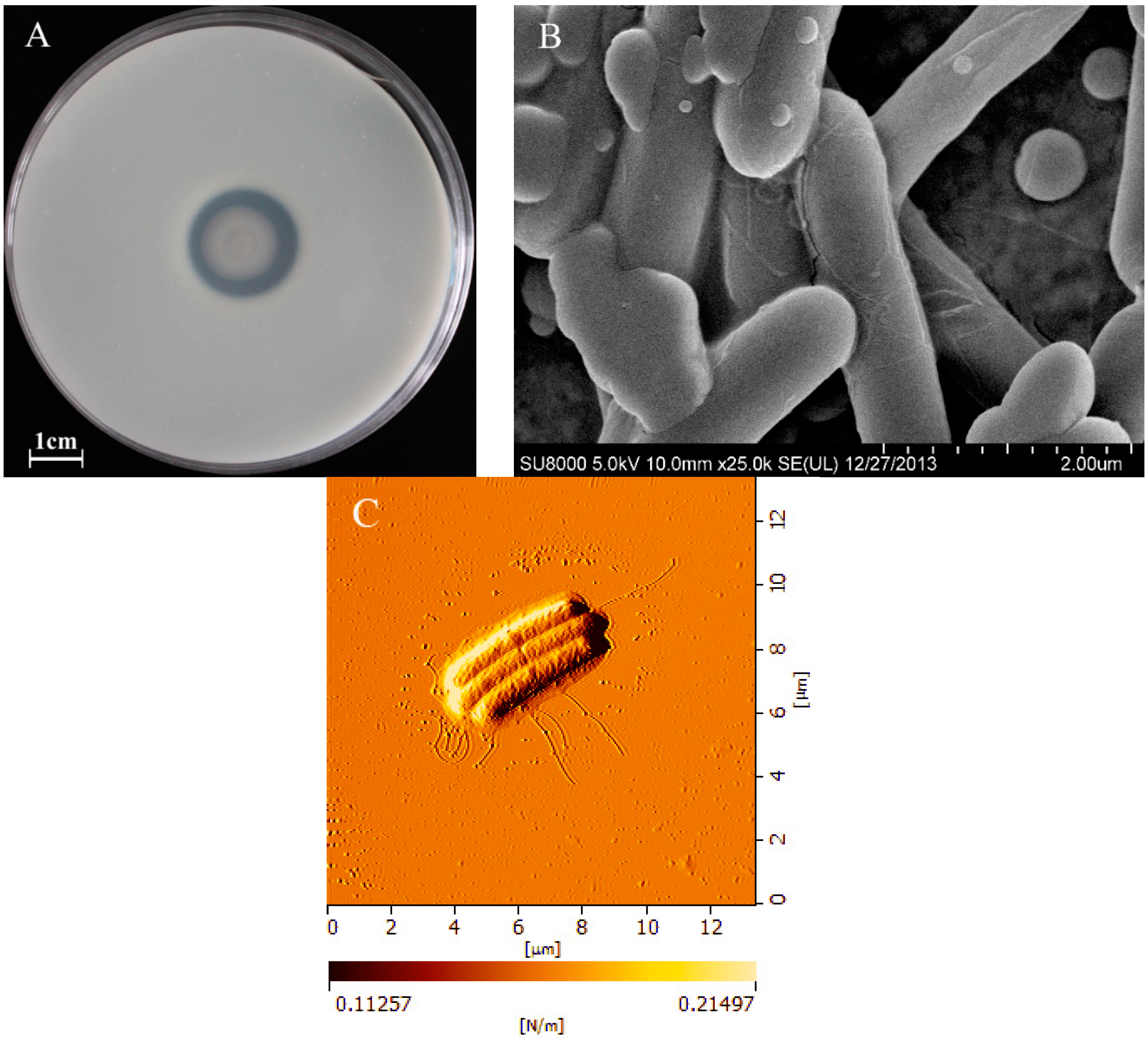
2.1. Morphological Characteristics of BD3526
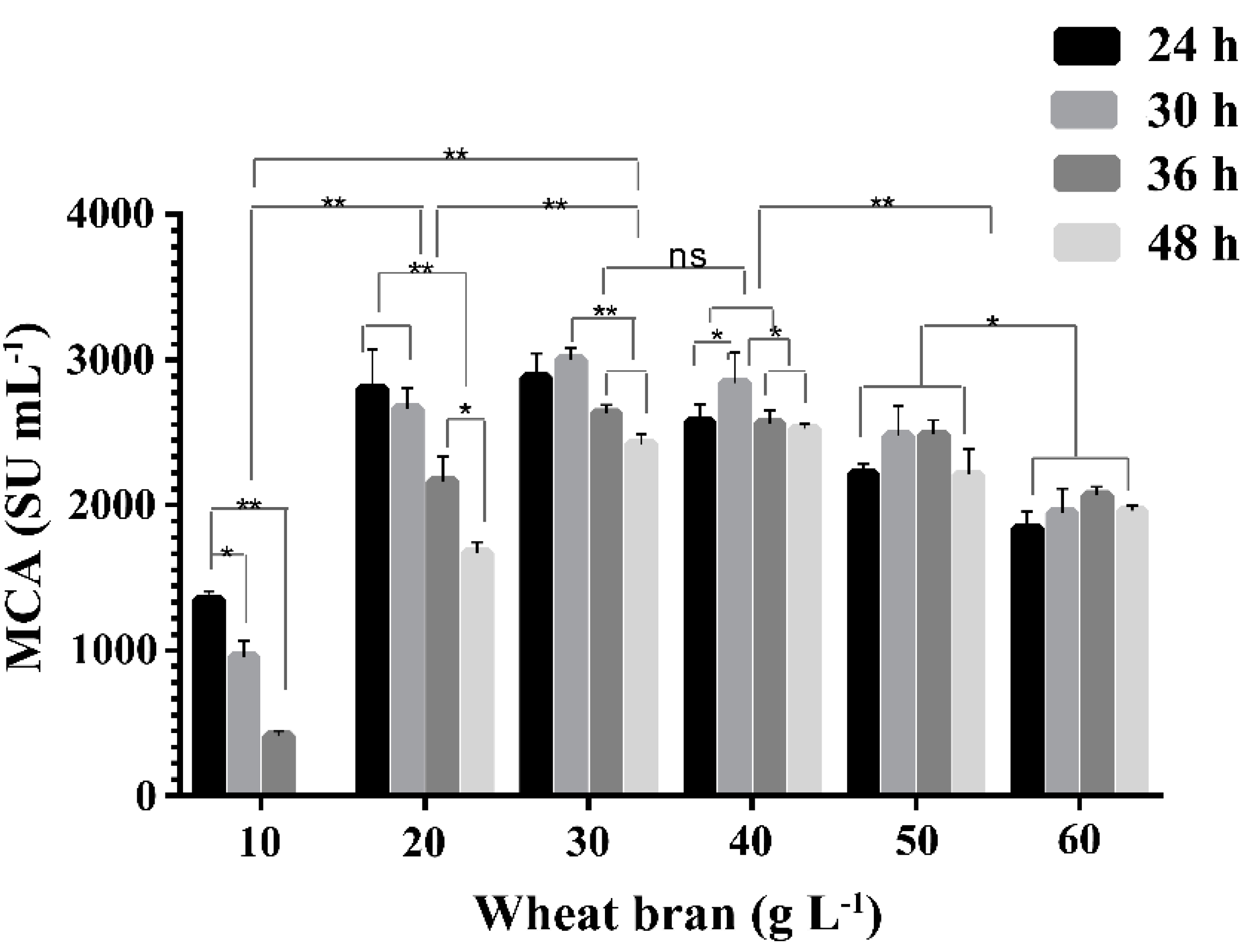
2.2. Effect of Concentration of Wheat Bran on the Expression of MCA by BD3526
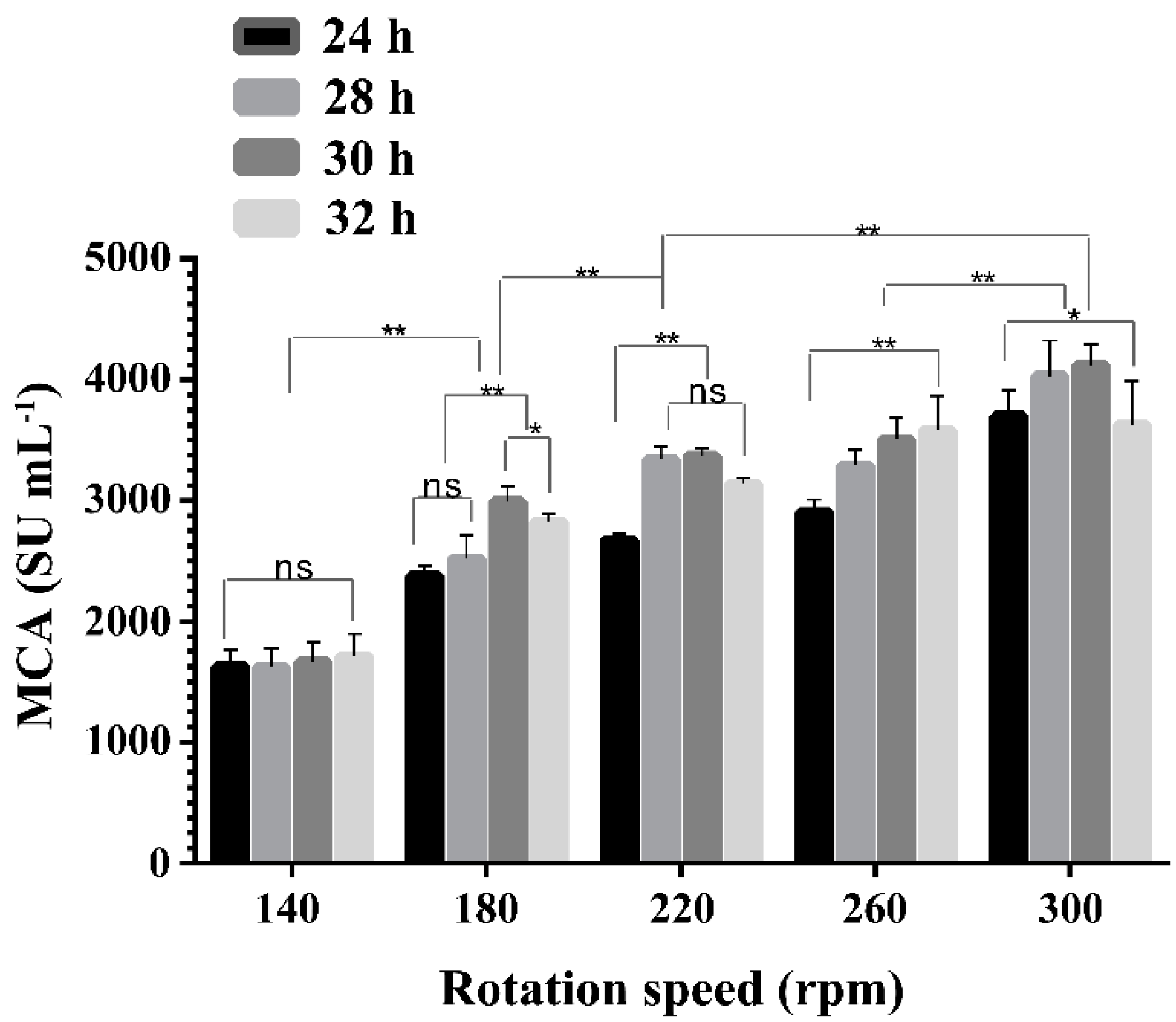
2.3. Effect of Rotation Speeds on the Expression of MCA by BD3526
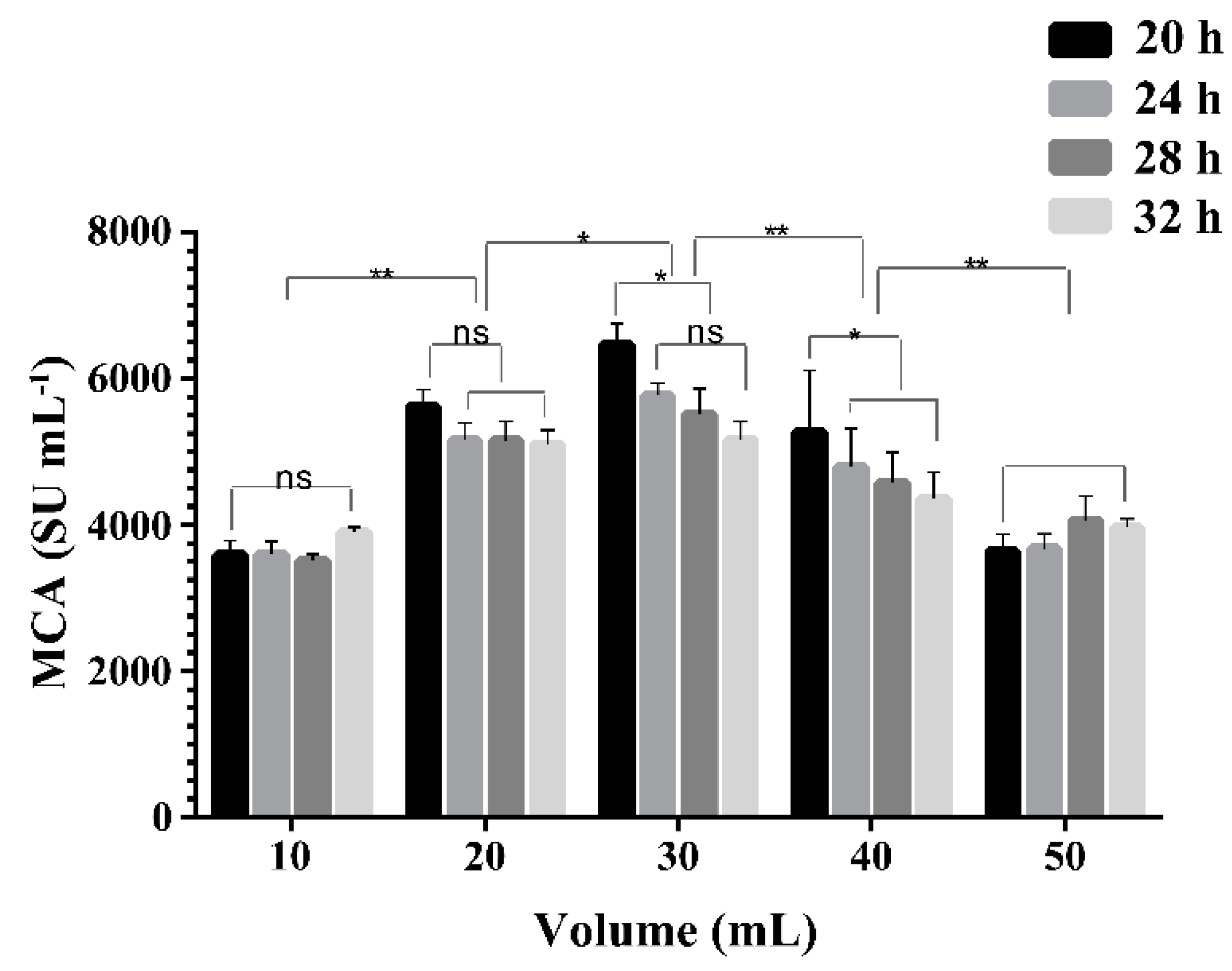
2.4. Effect of Liquid Volume on the Expression of MCA by BD3526
| Microorganism | Enzyme Production | Type | MW (kDa) | Reference |
|---|---|---|---|---|
| R. miehei NRRL 3420 | 1200 SU mL−1 | AP | - | [38] |
| R. pusillus | 1026 SU mg−1 protein | AP | 49 | [43] |
| M.mucedo DSM 809 | 130 SU mL−1 | AP | 32.7 | [33,44] |
| Thermomucor indicae-seudaticae N31 | 60.5 SU mL−1 | AP | - | [23] |
| Amylomyces rouxii | 1.9 SU mL−1 | AP | 40 | [45,46] |
| B. amyloliquefaciens D4 | 2683 SU mL−1 | MP | 58.2 | [16,35] |
| B. amyloliquefaciens JNU002 | 6590.41 SU mL−1 | MP | 28 | [17,18] |
| B. subtilis natto | 685.7 SU mL−1 | - | - | [28] |
| B. subtilis B1 | 1129.05 SU mL−1 | - | - | [15] |
| B. subtilis YB-3 | 200 SU mL−1 | MP | 42 | [27] |
| B. subtilis MTCC 10422 | - | - | 27 | [47] |
| B. sphaericus NRC 24 | 1212 SU mL−1 | SP | - | [39] |
| B. licheniformis USC13 | 45 U mL−1 * | SP | 34 | [29] |
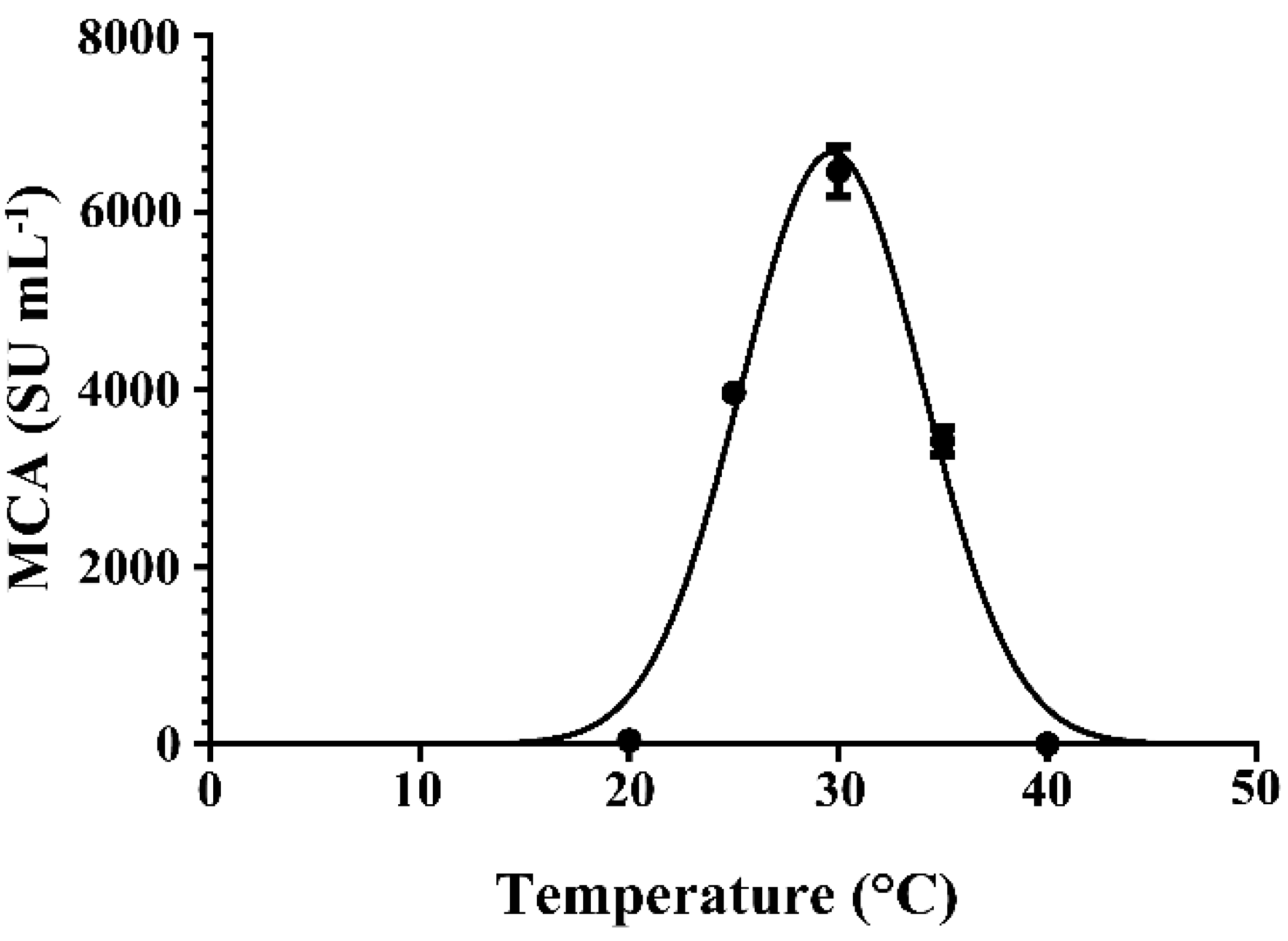
2.5. Effect of Cultivation Temperature on the Expression of MCA by BD3526
2.6. Characterization of BD3526 Protease
| Observed Mass | Calculated Mass | Δppm | Missed Cleavages | Peptide Sequences | n |
|---|---|---|---|---|---|
| 2464.3154 | 2464.3173 | 1.14 | 0 | M.AIPPKKNQDKTEIPTINTIASGE.P | 11 |
| 4394.2116 | 4394.2228 | 2.54 | 0 | M.AIPPKKNQDKTEIPTINTIASGEPTSTPTTEAVESTVATLED.S | 9 |
2.7. Comparison of the BD3526 Protease with Commercial Coagulants
| Coagulants | Tyrosine Concentration (µmol·mL−1) | ||||
|---|---|---|---|---|---|
| 10 min | 20 min | 40 min | 60 min | 90 min | |
| calf rennet | 0.87 ± 0.02 | 0.94 ± 0.03 | 0.92 ± 0.03 | .90 ± 0.02 | 0.90 ± 0.03 |
| recombinant chymosin (Chy-MAX®) | 0.93 ± 0.03 | 0.91 ± 0.05 | 0.96 ± 0.06 | 0.96 ± 0.03 | 0.95 ± 0.04 |
| R.miehei (Marzyme® 150 MG) | 0.95 ± 0.03 | 0.96 ± 0.04 | 0.96 ± 0.05 | 1.00 ± 0.04 | 1.04 ± 0.03 |
| BD3526 | 0.90 ± 0.03 | 0.90 ± 0.04 | 0.96 ± 0.04 | 1.01 ± 0.04 | 1.02 ± 0.05 |
3. Experimental Section
3.1. Materials
3.2. Strain Screening and Culture Conditions
3.3. Morphological Observations and Characterization
3.4. Preparation of Inoculums
3.5. Wheat Bran Broth and Cultivation Conditions
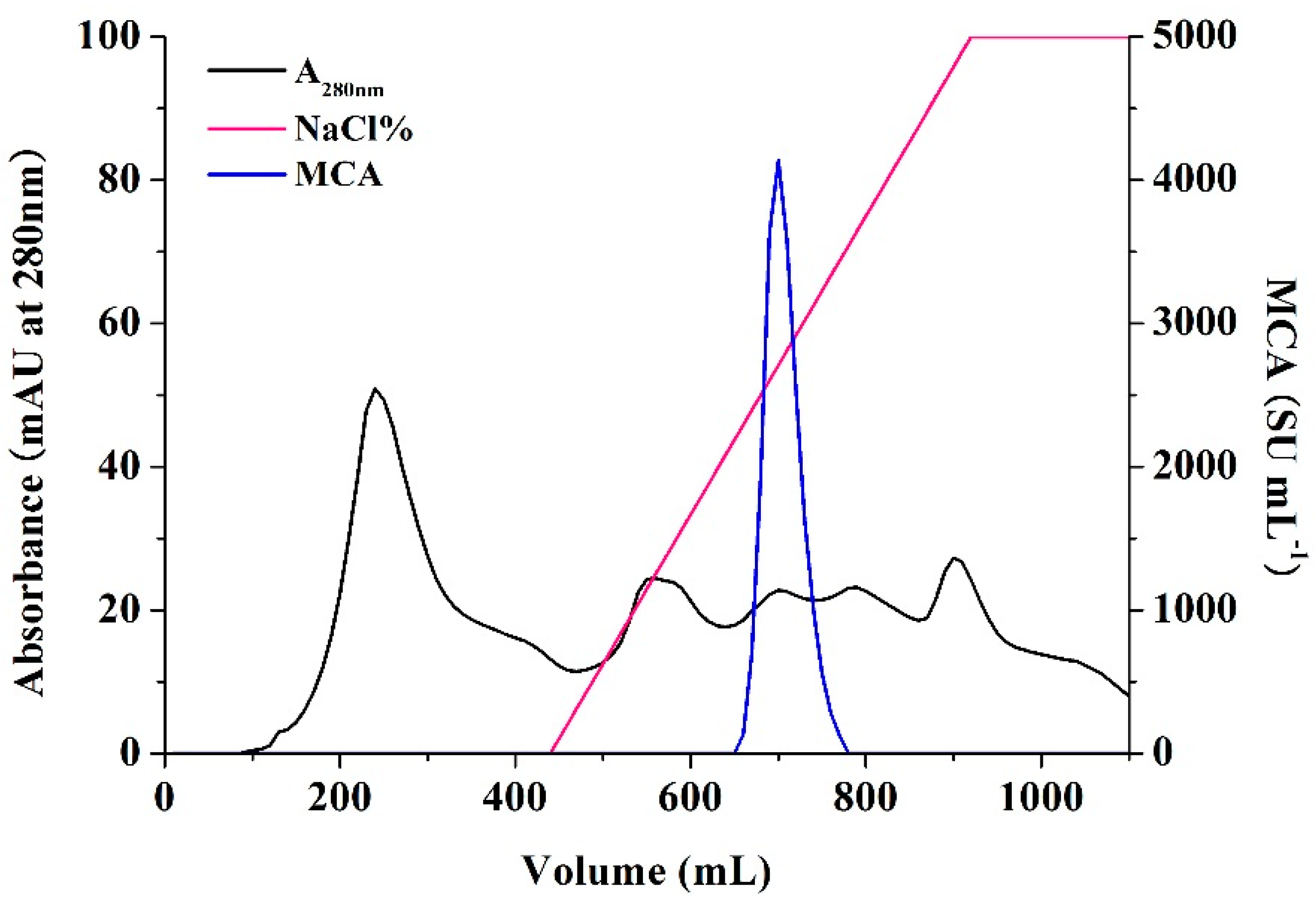
3.6. Enzyme Preparation and Purification
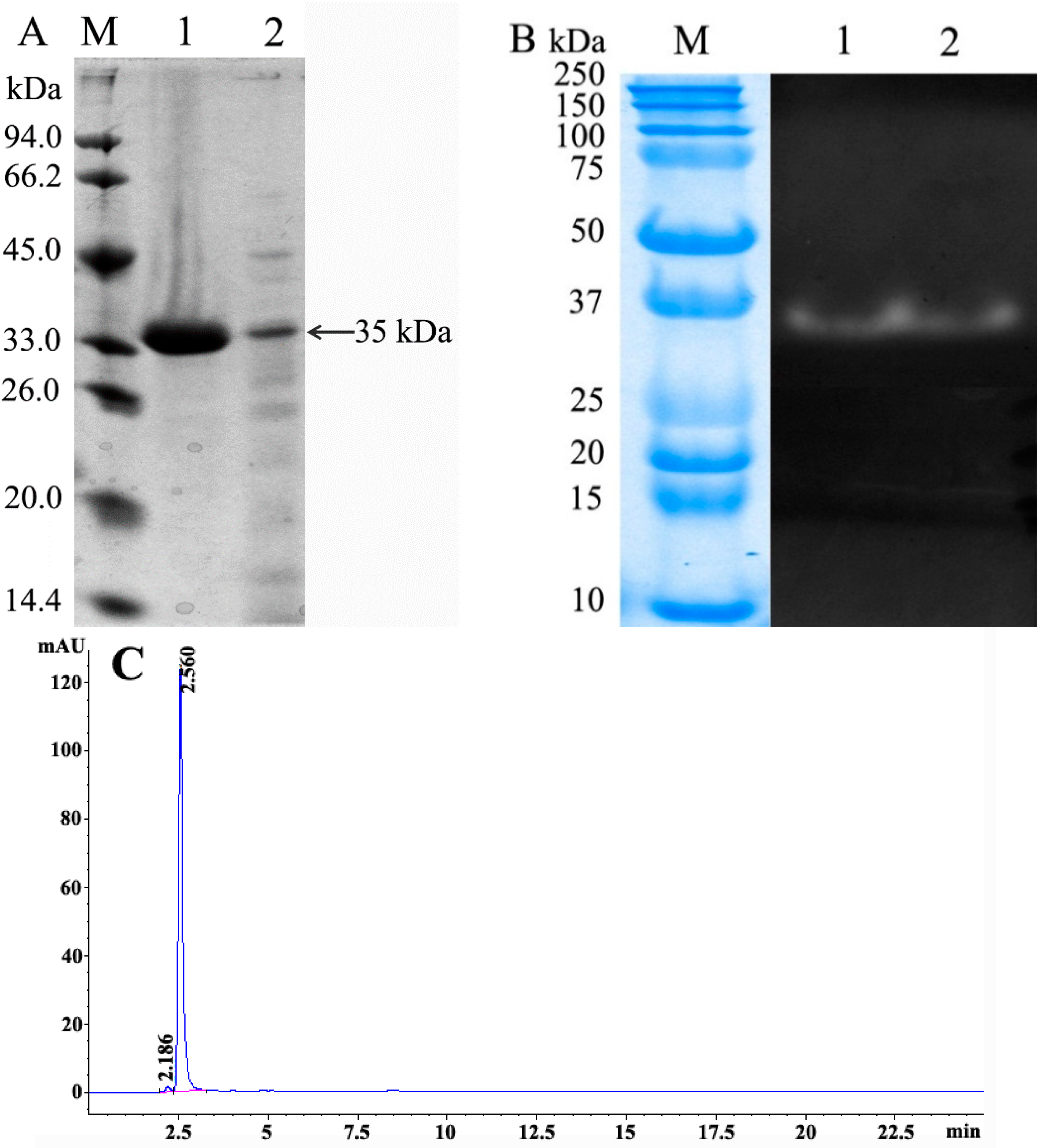
3.7. Gel Electrophoresis
3.8. Hydrophilic Interaction Liquid Chromatography
3.9. Determination of Milk-clotting Activity and Proteolytic Activity
3.10. Evaluation of Protein Hydrolysis of the BD3526 Coagulant
3.11. Statistical Analysis
4. Conclusions
Acknowledgements
Author Contributions
Conflicts of Interest
References
- Roseiro, L.B.; Barbosa, M.; Ames, J.M.; Wilbey, R.A. Cheesemaking with vegetable coagulants—The use of Cynara L. for the production of ovine milk cheeses. Int. J. Dairy Technol. 2003, 56, 76–85. [Google Scholar] [CrossRef]
- Møller, K.K.; Rattray, F.P.; Sørensen, J.C.; Ardö, Y. Comparison of the hydrolysis of bovine κ-casein by camel and bovine chymosin: A kinetic and specificity study. J. Agric. Food Chem. 2012, 60, 5454–5460. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, A.K.; Mukhopadhyay, U.K.; Kaushik, J.K.; Grover, S.; Batish, V.K. Isolation, purification and characterization of chymosin from riverine buffalo (Bubalus bubalis). J. Dairy Res. 2003, 70, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Rossano, R.; Piraino, P.; D’Ambrosio, A.; O’Connell, O.F.; Ungaro, N.; McSweeney, P.L.H.; Riccio, P. Proteolysis in miniature cheddar-type cheeses manufactured using extracts from the crustacean Munida as coagulant. J. Biotechnol. 2005, 120, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, I.A.M.; Morishima, I.; Babiker, E.E.; Mori, N. Characterisation of partially purified milk-clotting enzyme from Solanum dubium Fresen seeds. Food Chem. 2009, 116, 395–400. [Google Scholar] [CrossRef]
- Barros, R.; Malcata, F. Molecular characterization of peptides released from β-lactoglobulin and α-lactalbumin via cardosins A and B. J. Dairy Sci. 2006, 89, 483–494. [Google Scholar] [CrossRef]
- Brutti, C.B.; Pardo, M.F.; Caffini, N.O.; Natalucci, C.L. Onopordum acanthium L. (Asteraceae) flowers as coagulating agent for cheesemaking. LWT Food Sci. Technol. 2012, 45, 172–179. [Google Scholar] [CrossRef]
- Néstor, G.-M.; Dely Rubí, C.-G.; Héctor, J.-C. Exploring the milk-clotting properties of a plant coagulant from the berries of S. elaeagnifolium var. Cavanilles. J. Food Sci. 2012, 77, C89–C94. [Google Scholar] [CrossRef] [PubMed]
- Piero, A.R.L.; Puglisi, I.; Petrone, G. Characterization of the purified actinidin as a plant coagulant of bovine milk. Eur. Food Res. Technol. 2011, 233, 517–524. [Google Scholar] [CrossRef]
- Reis, P.C.M.; Lourenço, P.L.; Domingos, A.; Clemente, A.F.; Salomé Pais, M.; Malcata, X. Applicability of extracts from Centaurea calcitrapa in ripening of bovine cheese. Int. Dairy J. 2000, 10, 775–780. [Google Scholar] [CrossRef]
- Silva, S.V.; Malcata, F.X. Action of cardosin A from Cynara humilis on ovine and caprine caseinates. J. Dairy Res. 2000, 67, 449–454. [Google Scholar] [CrossRef] [PubMed]
- Cavalli, S.V.; Silva, S.V.; Cimino, C.; Malcata, F.X.; Priolo, N. Hydrolysis of caprine and ovine milk proteins, brought about by aspartic peptidases from Silybum marianum flowers. Food Chem. 2008, 106, 997–1003. [Google Scholar] [CrossRef]
- Kumar, A.; Grover, S.; Sharma, J.; Batish, V.K. Chymosin and other milk coagulants: Sources and biotechnological interventions. Crit. Rev. Biotechnol. 2010, 30, 243–258. [Google Scholar] [CrossRef] [PubMed]
- Jacob, M.; Jaros, D.; Rohm, H. Recent advances in milk clotting enzymes. Int. J. Dairy Technol. 2011, 64, 14–33. [Google Scholar] [CrossRef]
- Ding, Z.; Liu, S.; Gu, Z.; Zhang, L.; Zhang, K.; Shi, G. Production of milk-clotting enzyme by Bacillus subtilis B1 from wheat bran. African J. Biotechnol. 2011, 10, 9370–9378. [Google Scholar]
- He, X.; Ren, F.; Guo, H.; Zhang, W.; Song, X.; Gan, B. Purification and properties of a milk-clotting enzyme produced by Bacillus amyloliquefaciens D4. Korean J. Chem. Eng. 2011, 28, 203–208. [Google Scholar] [CrossRef]
- Ding, Z.; Wang, W.; Wang, B.; Ouyang, A.; Xiao, S.; Wang, Y.; Liu, S.; Ding, M.; Zhang, L.; Shi, G. Production and characterization of milk-clotting enzyme from Bacillus amyloliquefaciens JNU002 by submerged fermentation. Eur. Food Res. Technol. 2012, 234, 415–421. [Google Scholar] [CrossRef]
- Ding, Z.; Ai, L.; Ouyang, A.; Ding, M.; Wang, W.; Wang, B.; Liu, S.; Gu, Z.; Zhang, L.; Shi, G. A two-stage oxygen supply control strategy for enhancing milk-clotting enzyme production by Bacillus amyloliquefaciens. Eur. Food Res. Technol. 2012, 234, 1043–1048. [Google Scholar] [CrossRef]
- An, Z.; He, X.; Gao, W.; Zhao, W.; Zhang, W. Characteristics of miniature cheddar-type cheese made by microbial rennet from Bacillus amyloliquefaciens: A comparison with commercial calf rennet. J. Food Sci. 2014, 79, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Lemes, A.C.; Pavón, Y.; Lazzaroni, S.; Rozycki, S.; Brandelli, A.; Kalil, S.J. A new milk-clotting enzyme produced by Bacillus sp. P45 applied in cream cheese development. LWT Food Sci. Technol. 2016, 66, 217–224. [Google Scholar] [CrossRef]
- Fu, L.; Jiang, B.; Liu, J.; Zhao, X.; Liu, Q.; Hu, X. Genome sequence analysis of a flocculant-producing bacterium, Paenibacillus shenyangensis. Biotechnol. Lett. 2015, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Dutt, K.; Gupta, P.; Saran, S.; Misra, S.; Saxena, R.K. Production of milk-clotting protease from Bacillus subtilis. Appl. Biochem. Biotechnol. 2009, 158, 761–772. [Google Scholar] [CrossRef] [PubMed]
- Silva, B.; Geraldes, F.; Murari, C.; Gomes, E.; Da-Silva, R. Production and characterization of a milk-clotting protease produced in submerged fermentation by the thermophilic fungus Thermomucor indicae-seudaticae N31. Appl. Biochem. Biotechnol. 2014, 172, 1999–2011. [Google Scholar] [CrossRef] [PubMed]
- Sathya, R.; Pradeep, B.; Angayarkanni, J.; Palaniswamy, M. Production of milk clotting protease by a local isolate of Mucor circinelloides under SSF using agro-industrial wastes. Biotechnol. Bioprocess Eng. 2009, 14, 788–794. [Google Scholar] [CrossRef]
- Alvarez, V.; von der Weid, I.; Seldin, L.; Santos, A. Influence of growth conditions on the production of extracellular proteolytic enzymes in Paenibacillus peoriae NRRL BD-62 and Paenibacillus polymyxa SCE2. Lett. Appl. Microbiol. 2006, 43, 625–630. [Google Scholar] [CrossRef] [PubMed]
- Sato, S.; Tokuda, H.; Koizumi, T.; Nakanishi, K. Purification and characterization of an extracellular proteinase having milk-clotting activity from Enterococcus faecalis TUA2495L. Food Sci. Technol. Res. 2004, 10, 44–50. [Google Scholar] [CrossRef]
- Li, Y.; Liang, S.; Zhi, D.; Chen, P.; Su, F.; Li, H. Purification and characterization of Bacillus subtilis milk-clotting enzyme from Tibet Plateau and its potential use in yak dairy industry. Eur. Food Res. Technol. 2012, 234, 733–741. [Google Scholar] [CrossRef]
- Shieh, C.J.; Phan Thi, L.A.; Shih, I.L. Milk-clotting enzymes produced by culture of Bacillus subtilis natto. Biochem. Eng. J. 2009, 43, 85–91. [Google Scholar] [CrossRef]
- Ageitos, J.; Vallejo, J.; Sestelo, A.; Poza, M.; Villa, T. Purification and characterization of a milk-clotting protease from Bacillus licheniformis strain USC13. J. Appl. Microbiol. 2007, 103, 2205–2213. [Google Scholar] [CrossRef] [PubMed]
- Tubesha, Z.; Al-Delaimy, K. Rennin-like milk coagulant enzyme produced by a local isolate of Mucor. Int. J. Dairy Technol. 2003, 56, 237–241. [Google Scholar] [CrossRef]
- Hashem, A.M. Optimization of milk-clotting enzyme productivity by Penicillium oxalicum. Bioresour. Technol. 1999, 70, 203–207. [Google Scholar] [CrossRef]
- Vishwanatha, K.; Rao, A.A.; Singh, S.A. Acid protease production by solid-state fermentation using Aspergillus oryzae MTCC 5341: Optimization of process parameters. J. Ind. Microbiol. Biotechnol. 2010, 37, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Yegin, S.; Fernandez-Lahore, M.; Guvenc, U.; Goksungur, Y. Production of extracellular aspartic protease in submerged fermentation with Mucor mucedo DSM 809. African J. Biotechnol. 2010, 9, 6380–6386. [Google Scholar]
- Escobar, J.; Barnett, S.M. Effect of agitation speed on the synthesis of Mucor miehei acid protease. Enzym. Microb. Technol. 1993, 15, 1009–1013. [Google Scholar] [CrossRef]
- He, X.; Zhang, W.; Ren, F.; Gan, B.; Guo, H. Screening fermentation parameters of the milk-clotting enzyme produced by newly isolated Bacillus amyloliquefaciens D4 from the Tibetan Plateau in China. Ann. Microb. 2012, 62, 357–365. [Google Scholar] [CrossRef]
- Liu, Y.S.; Wu, J.Y.; Ho, K.P. Characterization of oxygen transfer conditions and their effects on Phaffia rhodozyma growth and carotenoid production in shake-flask cultures. Biochem. Eng. J. 2006, 27, 331–335. [Google Scholar] [CrossRef]
- Cavalcanti, M.; Martinez, C.; Furtado, V.; Neto, B.; Teixeira, M.; Lima Filho, J.; Porto, A. Milk-clotting protease production by Nocardiopsis sp. in an inexpensive medium. World J. Microbiol. Biotechnol. 2005, 21, 151–154. [Google Scholar] [CrossRef]
- De Lima, C.; Cortezi, M.; Lovaglio, R.B.; Ribeiro, E.; Contiero, J.; de Araújo, E. Production of rennet in submerged fermentation with the filamentous fungus Mucor miehei NRRL 3420. World Appl. Sci. J. 2008, 4, 578–585. [Google Scholar]
- El-Bendary, M.A.; Moharam, M.E.; Ali, T.H. Purification and characterization of milk clotting enzyme produced by Bacillus sphaericus. J. Appl. Sci. Res. 2007, 3, 695–699. [Google Scholar]
- Jiang, T.; Chen, L.; Xue, L.; Chen, L. Study on milk-clotting mechanism of rennet-like enzyme from glutinous rice wine: Proteolytic property and the cleavage site on κ-casein. J. Dairy Sci. 2007, 90, 3126–3133. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Chen, H.; Chen, W. Fermentation parameter and partial biochemical characterisation of milk clotting enzyme from Chinese distiller’s yeast. Ann. Microbiol. 2008, 58, 717–722. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, C.; Yao, Z.; Zhao, J.; Lu, F.; Yu, G.; Lan, W.; Lu, Z. Isolation and identification of a fungal strain QY229 producing milk-clotting enzyme. Eur. Food Res. Technol. 2011, 232, 861–866. [Google Scholar] [CrossRef]
- Preetha, S.; Boopathy, R. Influence of culture conditions on the production of milk-clotting enzyme from Rhizomucor. World J. Microbiol. Biotechnol. 1994, 10, 527–530. [Google Scholar] [CrossRef] [PubMed]
- Yegin, S.; Goksungur, Y.; Fernandez-Lahore, M. Purification, structural characterization, and technological properties of an aspartyl proteinase from submerged cultures of Mucor mucedo DSM 809. Food Chem. 2012, 133, 1312–1319. [Google Scholar] [CrossRef]
- Yu, P.J.; Chou, C.C. Factors Affecting the growth and production of milk-clotting enzyme by Amylomyces rouxii in rice liquid medium. Food Technol. Biotechnol. 2005, 43, 283–288. [Google Scholar]
- Marcial, J.; Pérez de los Santos, A.; Fernández, F.; Díaz-Godínez, G.; Montiel-González, A.; Tomasini, A. Characterization of an aspartic protease produced by Amylomyces rouxii. Rev. Mex. Ing. Quím. 2011, 10, 9–16. [Google Scholar]
- Narwal, R.K.; Bhushan, B.; Pal, A.; Panwar, A.; Malhotra, S. Purification, physico-chemico-kinetic characterization and thermal inactivation thermodynamics of milk clotting enzyme from Bacillus subtilis MTCC 10422. LWT Food Sci. Technol. 2016, 65, 652–660. [Google Scholar] [CrossRef]
- Vishwanatha, K.S.; Appu, R.A. G.; Singh, S.A. Production and characterization of a milk-clotting enzyme from Aspergillus oryzae MTCC 5341. Appl. Microbiol. Biotechnol. 2010, 85, 1849–1859. [Google Scholar] [CrossRef] [PubMed]
- Thakur, M.; Karanth, N.; Nand, K. Production of fungal rennet by Mucor miehei using solid state fermentation. Appl. Microbiol. Biotechnol. 1990, 32, 409–413. [Google Scholar] [CrossRef]
- Ayhan, F.; Çelebi, S.S.; Tanyolac, A. The effect of fermentation parameters on the production of Mucor miehei acid protease in a chemically defined medium. J. Chem. Technol. Biotechnol. 2001, 76, 153–160. [Google Scholar] [CrossRef]
- Liu, H.; Sun, W.B.; Liang, R.B.; Huang, L.; Hou, J.L.; Liu, J.H. iTRAQ-based quantitative proteomic analysis of Pseudomonas aeruginosa SJTD-1: A global response to n-octadecane induced stress. J. Proteomics 2015, 123, 14–28. [Google Scholar] [CrossRef] [PubMed]
- De Castro, R.J.S.; Nishide, T.G.; Sato, H.H. Production and biochemical properties of proteases secreted by Aspergillus niger under solid state fermentation in response to different agroindustrial substrates. Biocatal. Agric. Biotechnol. 2014, 3, 236–245. [Google Scholar] [CrossRef]
- Channe, P.; Shewale, J. Influence of culture conditions on the formation of milk-clotting protease by Aspergillus niger MC4. Appl. Microbiol. Biotechnol. 1998, 14, 11–15. [Google Scholar]
- Vishwanatha, K.; Appu Rao, A.; Singh, S.A. Characterisation of acid protease expressed from Aspergillus oryzae MTCC 5341. Food Chem. 2009, 114, 402–407. [Google Scholar] [CrossRef]
- Arima, K. Milk clotting enzyme from microorganisms. Agric. Biol. Chem. 1967, 31, 540–545. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are not available from the authors.
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hang, F.; Liu, P.; Wang, Q.; Han, J.; Wu, Z.; Gao, C.; Liu, Z.; Zhang, H.; Chen, W. High Milk-Clotting Activity Expressed by the Newly Isolated Paenibacillus spp. Strain BD3526. Molecules 2016, 21, 73. https://doi.org/10.3390/molecules21010073
Hang F, Liu P, Wang Q, Han J, Wu Z, Gao C, Liu Z, Zhang H, Chen W. High Milk-Clotting Activity Expressed by the Newly Isolated Paenibacillus spp. Strain BD3526. Molecules. 2016; 21(1):73. https://doi.org/10.3390/molecules21010073
Chicago/Turabian StyleHang, Feng, Peiyi Liu, Qinbo Wang, Jin Han, Zhengjun Wu, Caixia Gao, Zhenmin Liu, Hao Zhang, and Wei Chen. 2016. "High Milk-Clotting Activity Expressed by the Newly Isolated Paenibacillus spp. Strain BD3526" Molecules 21, no. 1: 73. https://doi.org/10.3390/molecules21010073





