The Scavenging of DPPH, Galvinoxyl and ABTS Radicals by Imine Analogs of Resveratrol
Abstract
:1. Introduction
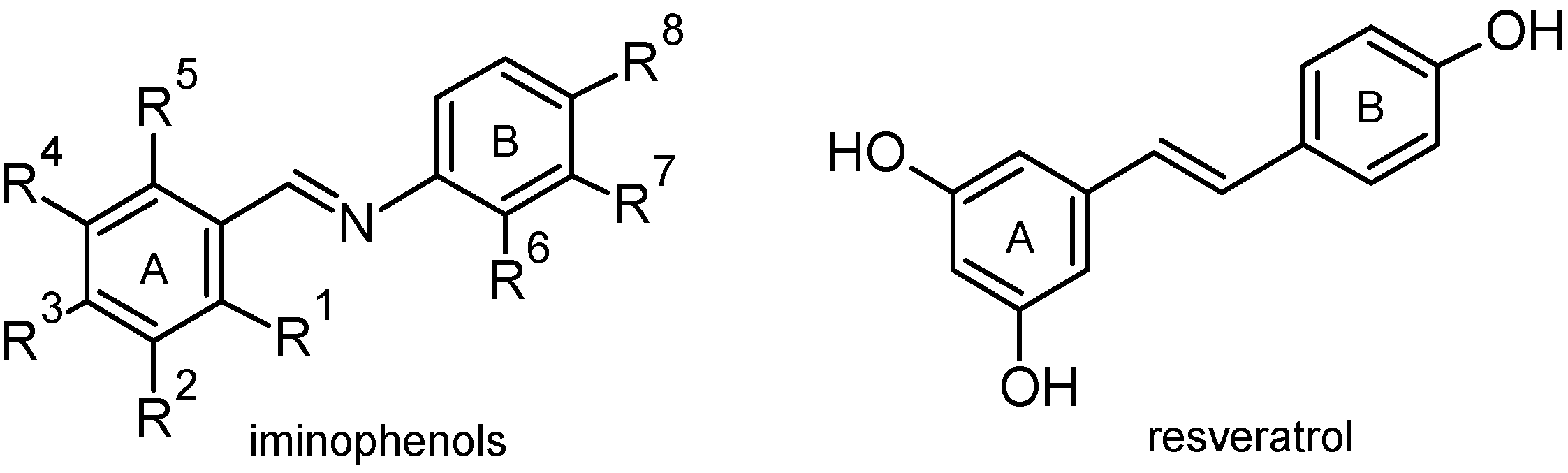
2. Results and Discussion
2.1. Chemistry
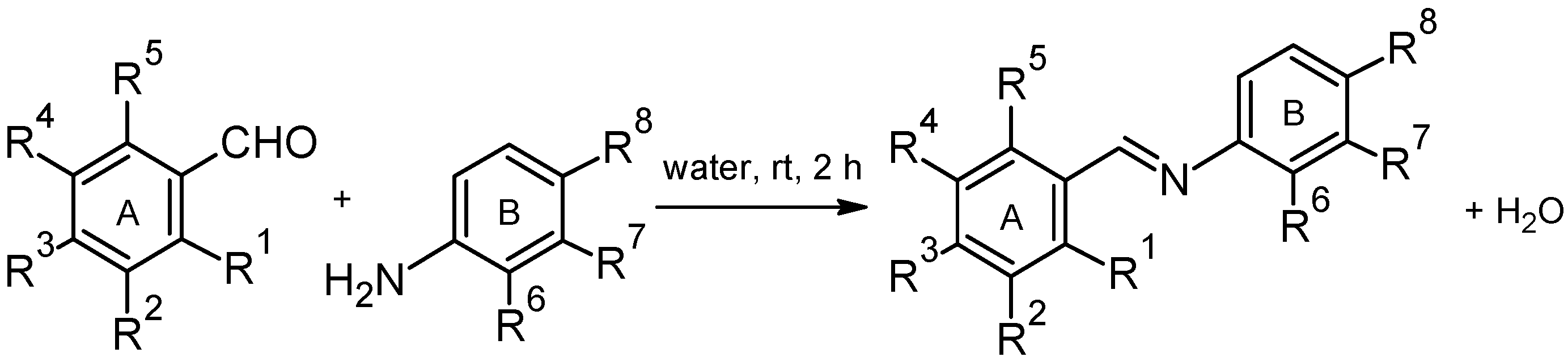
2.2. Antioxidant Activity
| Compound | DPPH SC50/r2 (μmol/dm3) | GOR SC50/r2 (μmol/dm3) | ABTS SC50/r2 (μmol/dm3) | PA + ETE in Methanol (kJ/mol) | PA + ETE in Water (kJ/mol) |
|---|---|---|---|---|---|
| 1 | 27.90/0.951 | 184/0.985 | 11.64/0.969 | 546.8 | 562.2 |
| 2 | 38.26/0.963 | 48.27/0.998 | 8.50/0.809 | 550.9 | 566.0 |
| 3 | 560/0.893 | 3075/0.809 | 8.50/0.928 | 547.7 | 562.2 |
| 4 | 967/0.976 | 415/0.995 | 3.54/0.993 | 565.4 | 580.7 |
| 5 | 383/0.936 | 393/0.903 | 6.74/0.869 | 547.1 | 562.1 |
| 6 | 88.64/0.974 | 251/0.999 | 3.86/0.994 | 550.1 | 564.4 |
| 7 | 53.98/0.937 | 184/0.979 | 6.57/954 | 547.4 | 562.8 |
| 8 | 21.00/0.997 | 127/0.971 | 14.39/0.972 | 543.1 | 551.9 |
| 9 | 43.00/0.991 | 39.96/0.945 | 18.16/0.922 | 520.8 | 537.4 |
| 10 | 19.00/0.994 | 2300/0.987 | 6.4/0.988 | 539.6 | 554.7 |
| 11 | 83.00/0.947 | 73.78/0.998 | 3.05/0.847 | 554.6 | 569.9 |
| 12 | 24.00/0.970 | 456/0.995 | 3.31/0.968 | 541.8 | 554.0 |
| 13 | 18.00/0.988 | 23.34/0.997 | 6.40/0.977 | 542.7 | 557.6 |
| 14 | 12.52/0.964 | 102/0.865 | 5.83/0.988 | 547.5 | 573.9 |
| 15 | 42.00/0.986 | 55.43/0.727 | 3.74/0.918 | 540.3 | 555.4 |
| 16 | 149/0.979 | 173/0.988 | 2.53/0.962 | 554.9 | 570.2 |
| 17 | 22.05/0.988 | 25.24/0.989 | 2.01/0.989 | 537.4 | 552.7 |
| 18 | 12.60/0.996 | 27.67/0.993 | 2.83/0.956 | 525.8 | 540.7 |
| 19 | 9.05/0.988 | 72.10/0.958 | 2.46/0.977 | 531.1 | 546.5 |
| 20 | 18.05/0.988 | 23.92/0.985 | 2.92/0.9997 | 548.4 | 563.1 |
| 21 | 8.77/0.943 | 15.39/0.994 | 1.98/0.994 | 528.5 | 544.0 |
| Resveratrol | 26.37/0.849 | 72.66/0.910 | 1.43/0.959 | 548.6 | 563.5 |

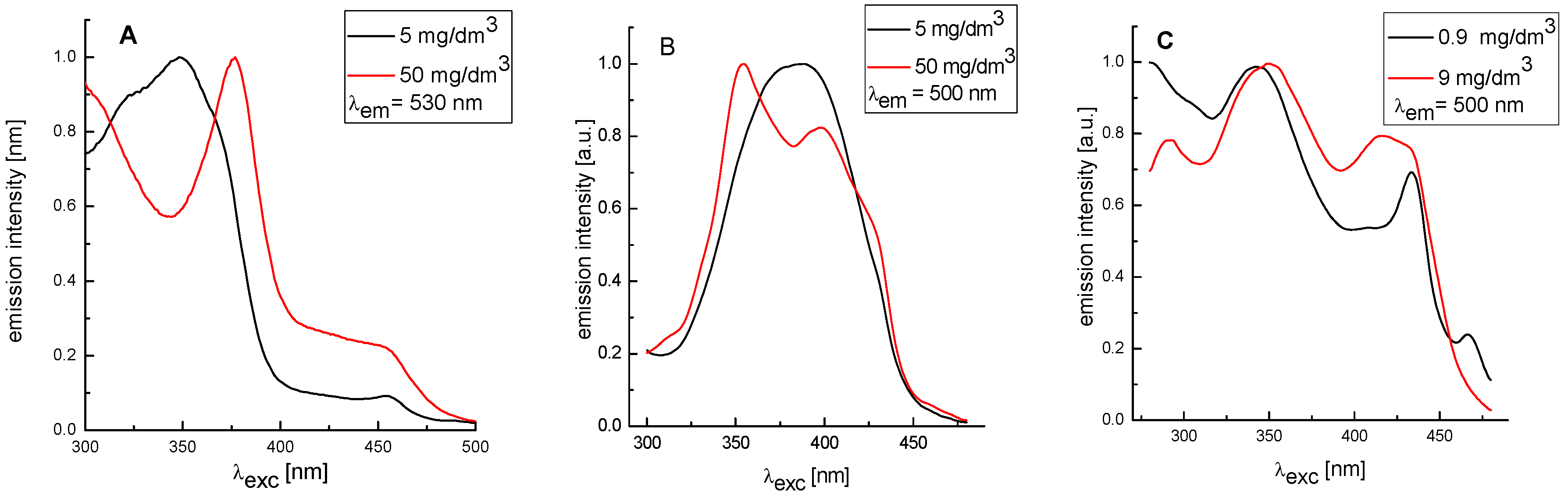
3. Experimental Section
3.1. General Information
3.2. Synthesis
General Procedure for the Synthesis of (Hydroxyphenyliminomethyl)phenols
3.3. DPPH Assay
3.4. Scavenging of Galvinoxyl Radicals
3.5. ABTS Assay
3.6. Molecular Calculations
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| ABTS | 2,2′-azino-bis(3-ethylbenzothiazoline)-6-sulphonic acid |
| AP | aminophenol |
| BDE | bond dissociation enthalpy |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| ETE | electron transfer enthalpy |
| GOR | galvinoxyl radical |
| HBA | hydroxybenzaldehyde |
| IP | ionization potential |
| IR | infrared |
| MS | mass spectrometry |
| NMR | nuclear magnetic resonance |
| PA | proton affinity |
References
- Celotti, E.; Ferrarini, R.; Zironi, R.; Conte, L.S. Resveratrol content of some wines obtained from dried Valpolicella grapes: Recioto and Amarone. J. Chromatogr. A 1996, 730, 47–52. [Google Scholar] [CrossRef]
- Soleas, G.J.; Diamandis, E.P.; Goldberg, D.M. Resveratrol: A molecule whose time has come? And gone? Clin. Biochem. 1997, 30, 91–113. [Google Scholar] [CrossRef]
- Gu, X.L.; Chub, Q.Y.; O’Dwyer, M.; Zeece, M. Analysis of resveratrol in wine by capillary electrophoresis. J. Chromatogr. A 2000, 881, 471–481. [Google Scholar] [CrossRef]
- Kaeberlein, K.; McDonagh, T.; Heltweg, B.; Hixon, J.; Westman, E.A.; Caldwell, S.D.; Napper, A.; Curtis, R.; DiStefano, P.S.; Fields, S.; et al. Substrate-specific activation of sirtuins by resveratrol. J. Biol. Chem. 2005, 280, 17038–17045. [Google Scholar] [CrossRef] [PubMed]
- Renaud, S.; de Lorgeril, M. Wine, alcohol, platelets, and the French paradox for coronary heart disease. Lancet 1992, 339, 1523–1526. [Google Scholar] [CrossRef]
- Santos, J.A.; de Carvaho, G.S.; Oliveira, V.; Raposo, N.R.; da Silva, A.D. Resveratrol and analogues: A review of antioxidant activity and applications to human health. Recent Pat. Food Nutr. Agric. 2013, 5, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.J. Positive oxidative stress in aging and aging-related disease tolerance. Redox Biol. 2014, 2C, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Ghanim, H.; Sia, C.L.; Korzeniewski, K.; Lohano, T.; Abuaysheh, S.; Marumganti, A.; Chaudhuri, A.; Dandona, P. A resveratrol and polyphenol preparation suppresses oxidative and inflammatory stress response to a high-fat, high-carbohydrate meal. J. Clin. Endocrinol. Metab. 2011, 96, 1409–1414. [Google Scholar]
- Hubbard, B.P.; Sinclair, D.A. Small molecule SIRT1 activators for the treatment of aging and age-related diseases. Trends Pharmacol. Sci. 2014, 35, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Xu, X.; Wang, X.J.; Pan, Y. Imine resveratrol analogues: Molecular design, NRF2 activation and SAR analysis. PLoS ONE 2014, 9, e101455. [Google Scholar]
- Franco, D.C.Z.; de Carvalho, G.S.G.; Rocha, P.R.; da Silva Teixeira, R.; da Silva, A.D.; Raposo, N.R.B. Inhibitory effects of resveratrol analogs on mushroom tyrosinase activity. Molecules 2012, 17, 11816–11825. [Google Scholar] [CrossRef] [PubMed]
- Chalal, M.; Klinguer, A.; Echairi, A.; Meunier, P.; Vervandier-Fasseur, D.; Adrian, M. Antimicrobial activity of resveratrol analogues. Molecules 2014, 19, 7679–7688. [Google Scholar] [CrossRef] [PubMed]
- Alexander, V. Design and synthesis of macrocyclic ligands and their complexes of lanthanides and actinides. Chem. Rev. 1995, 95, 273–342. [Google Scholar] [CrossRef]
- Westheimer, F.H.; Taguchi, K. Catalysis by molecular sieves in the preparation of ketimines and enamines. J. Org. Chem. 1971, 36, 1570–1572. [Google Scholar] [CrossRef]
- Castellano, J.A.; Goldmacher, J.E.; Barton, L.A.; Kane, J.S. Liquid crystals. II. Effects of terminal group substitution on the mesomorphic behavior of some benzylideneanilines. J. Org. Chem. 1968, 33, 3501–3504. [Google Scholar] [CrossRef]
- Tanaka, K.; Shiraishi, R. Clean and efficient condensation reactions of aldehydes and amines in a water suspension medium. Green Chem. 2000, 2, 272–273. [Google Scholar] [CrossRef]
- Van den Ancker, T.R.; Cave, G.W.V.; Raston, C.L. Benign approaches for the synthesis of bis-imine Schiff bases. Green Chem. 2006, 8, 50–53. [Google Scholar] [CrossRef]
- Koldas, S.; Demitras, I.; Ozen, T.; Demirici, M.; Behcet, L. Phytochemical screening, anticancer and antioxidant activities of Origanum vulgare L. ssp viride (Boiss.) Hayek, a plant of traditional usage. J. Sci. Food Agric. 2015, 95, 786–798. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, F.; Saary, N.S.; Hashim, R.; Sulaiman, O.; Hashida, K.; Otsuka, Y.; Nakamura, M.; Ohara, S. Subcritical water extraction of low-molecular-weight phenolic compounds from oil palm biomass. Jpn. Agr. Res. Q. 2014, 48, 355–362. [Google Scholar] [CrossRef]
- Choudhary, A.; Mittal, A.K.; Radhika, M.; Tripathy, D.; Chatterjee, A.; Banerjee, U.C.; Singh, I.P. Two new stereoisomeric antioxidant triterpenes from Potentilla fulgens. Fitoterapia 2013, 91, 290–297. [Google Scholar]
- Asikin, Y.; Takahashi, M.; Mishima, T.; Mizu, M.; Takara, K.; Wada, K. Antioxidant activity of sugarcane molasses against 2,2′-azobis(2-amidinopropane) dihydrochloride-induced peroxyl radicals. Food Chem. 2013, 141, 466–472. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Jimenes, G.C.; Vargas-Garcia, A.; Espinoza-Pérez, D.J.; Salgado-Cervantes, M.A.; Robles-Olvera, V.J. García-Alvarado, M.A. Mass transfer during vanilla pods solid liquid extraction: Effect of extraction method. Food Bioprocess Technol. 2013, 6, 2640–2650. [Google Scholar] [CrossRef]
- Razafintsalama, V.; Sarter, S.; Mambu, L.; Randrianarivo, R.; Petit, T.; Rajaonarison, J.F.; Mertz, C.; Rakoto, D.; Jeannoda, V. Antimicrobial activities of Dilobeia thouarsii Roemer and Schulte, a traditional medicinal plant from Madagascar. S. Afr. J. Bot. 2013, 87, 1–3. [Google Scholar] [CrossRef]
- Friedman, M.; Henika, P.R.; Mandrell, R.E. Antibacterial Activities of phenolic benzaldehydes and benzoic acids against Campylobacter jejuni, Escherichia coli, Listeria monocytogenes, and Salmonella enterica. J. Food Prot. 2003, 66, 1811–1821. [Google Scholar] [PubMed]
- Mangas, J.; Rodriguez, R.; Moreno, J.; Suarez, B.; Blanco, D. Evolution of aromatic and furanic congeners in the maturation of cider brandy: A contribution to its characterization. J. Agric. Food Chem. 1996, 44, 3303–3307. [Google Scholar] [CrossRef]
- Bountagkidou, O.G.; Ordoudi, S.A.; Tsimidou, M.Z. Structure–antioxidant activity relationship study of natural hydroxybenzaldehydes using in vitro assays. Food Res. Int. 2010, 43, 2014–2019. [Google Scholar] [CrossRef]
- Nenadis, N.; Tsimidou, M.Z. Contribution of DFT computed molecular descriptors in the study of radical scavenging activity trend of natural hydroxybenzaldehydes and corresponding acids. Food Res. Int. 2012, 48, 538–543. [Google Scholar] [CrossRef]
- Li, S.Y.; Wang, X.B.; Kong, L.Y. Design, synthesis and biological evaluation of imine resveratrol derivatives as multi-targeted agents against Alzheimer’s disease. Eur. J. Med. Chem. 2014, 71, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Li, C.; Chai, Y.F.; Yang, D.Y.; Sun, C.R. The antioxidant effect of imine resveratrol analogues. Bioorg. Med. Chem. Lett. 2012, 22, 5744–5747. [Google Scholar] [CrossRef] [PubMed]
- Šeršeň, F.; Walko, M.; Loos, D. Antioxidative effect of some hydroxy substituted aromatic bisimines. Gen. Physiol. Biophys. 2009, 28, 210–214. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Khan, M.S.Y. Synthesis of novel tetrahydroimidazole derivatives and studies for their biological properties. Eur. J. Med. Chem. 2001, 36, 651–658. [Google Scholar] [CrossRef]
- Cigáň, M.; Jakusová, K.; Donovalová, J.; Filo, J.; Horváth, M.; Gáplovský, A. Fluorescence of isatin N-phenylsemicarbazones: Aggregation and hydrazide–hydrazonol tautomerism. J. Phys. Org. Chem. 2015, 28, 337–346. [Google Scholar] [CrossRef]
- Gobec, M.; Tomašič, T.; Markovič, T.; Mlinarič-Raščan, I.; Dolenc, M.S.; Jakopin, Ž. Antioxidant and anti-inflammatory properties of 1,2,4-oxadiazole analogs of resveratrol. Chem. Biol. Interact. 2015, 240, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Šeršeň, F.; Mučaji, P.; Grančai, D.; Nagy, M. Antioxidative properties of methanol extracts from leaves and fruits of Ligustrum vulgare L. Acta Facult. Pharm. Univ. Comen. 2005, 52, 204–209. [Google Scholar]
- Kurin, E.; Mučaji, P.; Nagy, M. In vitro antioxidant activities of three red wine polyphenols and their mixtures: An interaction study. Molecules 2012, 17, 14336–14348. [Google Scholar] [CrossRef] [PubMed]
- Stewart, J.J.P. Optimization of parameters for semiempirical methods V: Modification of NDDO approximations and application to 70 elements. J. Mol. Model. 2007, 13, 1173–1213. [Google Scholar] [CrossRef] [PubMed]
- MOPAC, version 2012; Stewart Computational Chemistry: Colorado Springs, CO, USA, 2012.
- Klamt, A.; Schüümann, G. COSMO: A new approach to dielectric screening in solvents with explicit expressions for the screening energy and its gradient. J. Chem. Soc. Perkin Trans. 1993, 2, 799–805. [Google Scholar] [CrossRef]
- MOPAC Manual. Available online: http://OpenMOPAC.net/Manual (accessed on 10 January 2015).
- Sample Availability: Samples of the compounds are not available from the authors.
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kotora, P.; Šeršeň, F.; Filo, J.; Loos, D.; Gregáň, J.; Gregáň, F. The Scavenging of DPPH, Galvinoxyl and ABTS Radicals by Imine Analogs of Resveratrol. Molecules 2016, 21, 127. https://doi.org/10.3390/molecules21010127
Kotora P, Šeršeň F, Filo J, Loos D, Gregáň J, Gregáň F. The Scavenging of DPPH, Galvinoxyl and ABTS Radicals by Imine Analogs of Resveratrol. Molecules. 2016; 21(1):127. https://doi.org/10.3390/molecules21010127
Chicago/Turabian StyleKotora, Peter, František Šeršeň, Juraj Filo, Dušan Loos, Juraj Gregáň, and Fridrich Gregáň. 2016. "The Scavenging of DPPH, Galvinoxyl and ABTS Radicals by Imine Analogs of Resveratrol" Molecules 21, no. 1: 127. https://doi.org/10.3390/molecules21010127






