Isolation and Functional Characterization of a Phenylalanine Ammonia-Lyase Gene (SsPAL1) from Coleus (Solenostemon scutellarioides (L.) Codd)
Abstract
:1. Introduction
2. Results and Discussion
2.1. Cloning and Characterization of the Full-Length SsPAL1 cDNA Sequence

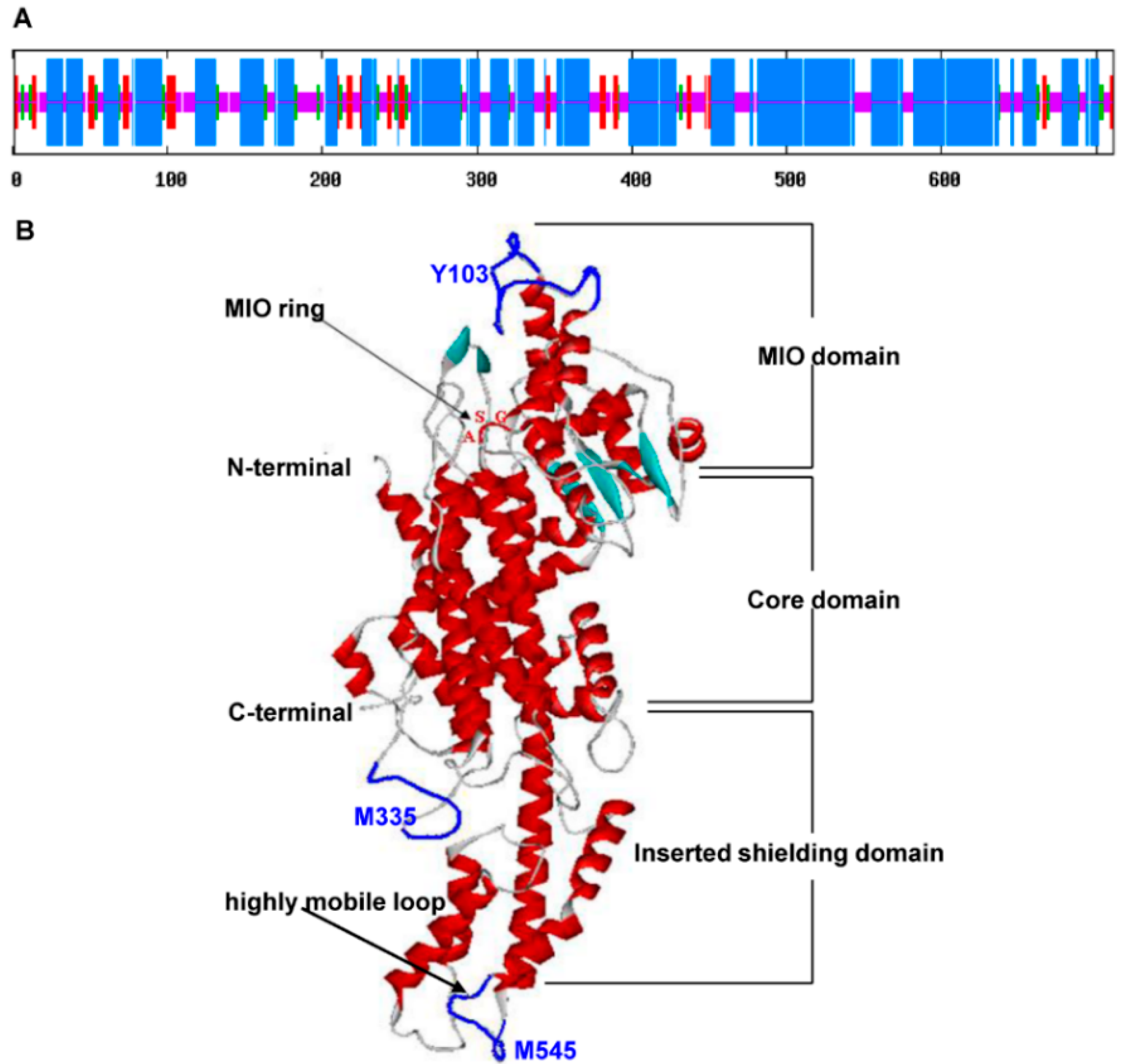
2.2. Characterization of the Deduced SsPAL1 Protein
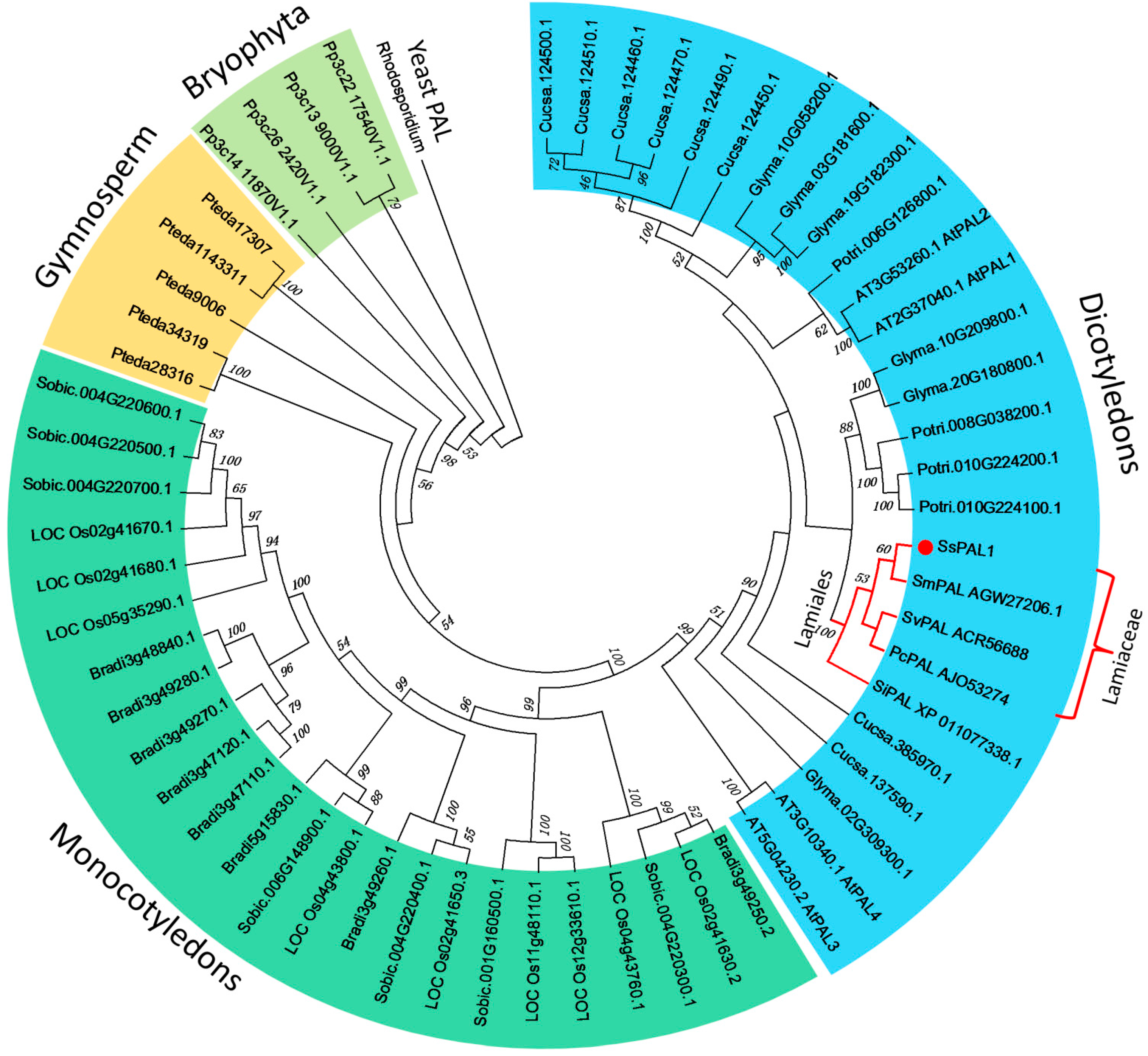
2.3. Phylogenetic Analysis of SsPAL1
2.4. Genomic Organization of SsPAL1

2.5. Analysis of the Upstream Region of SsPAL1
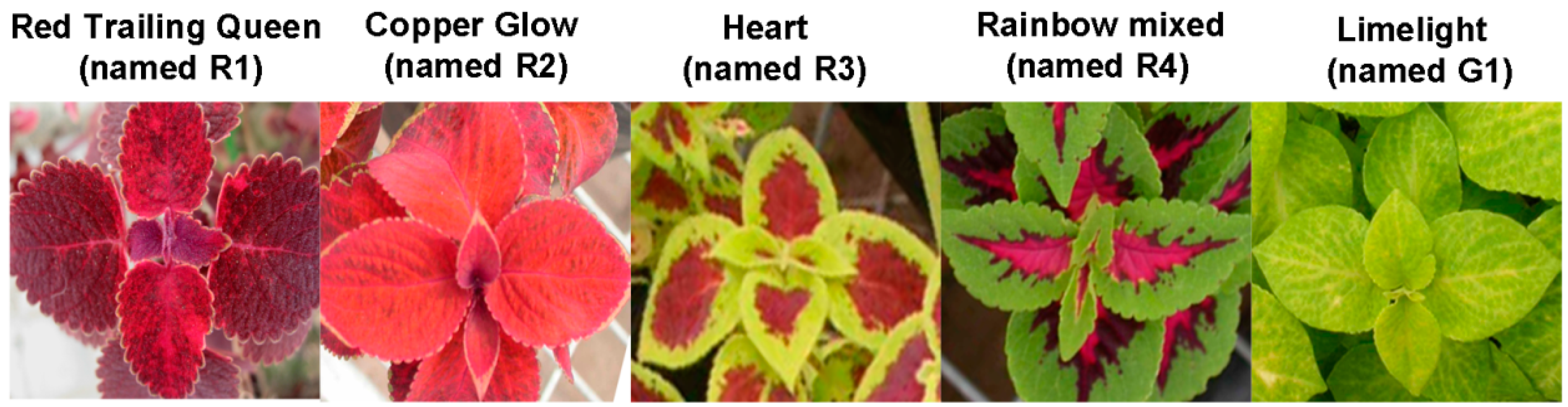
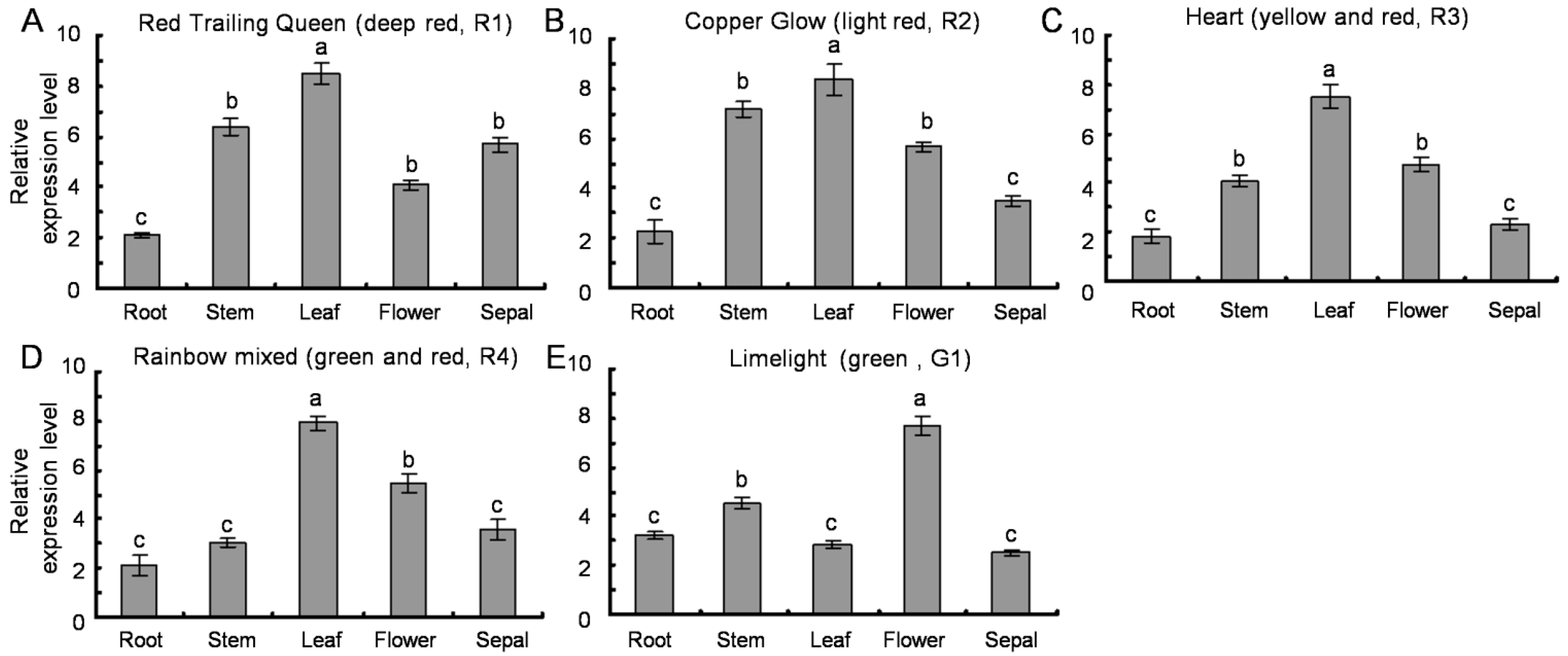
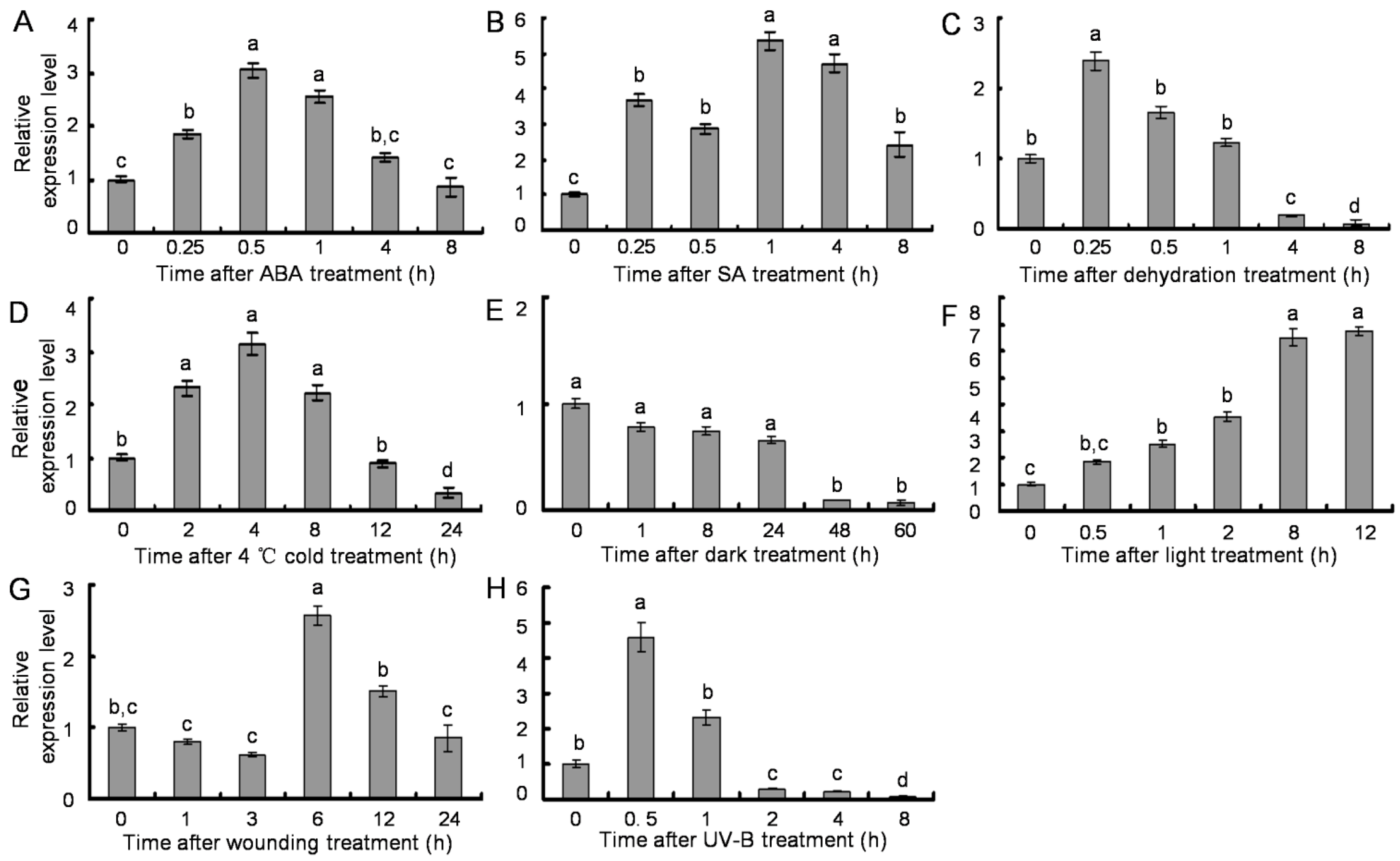
2.6. Transcription Profiles of SsPAL1
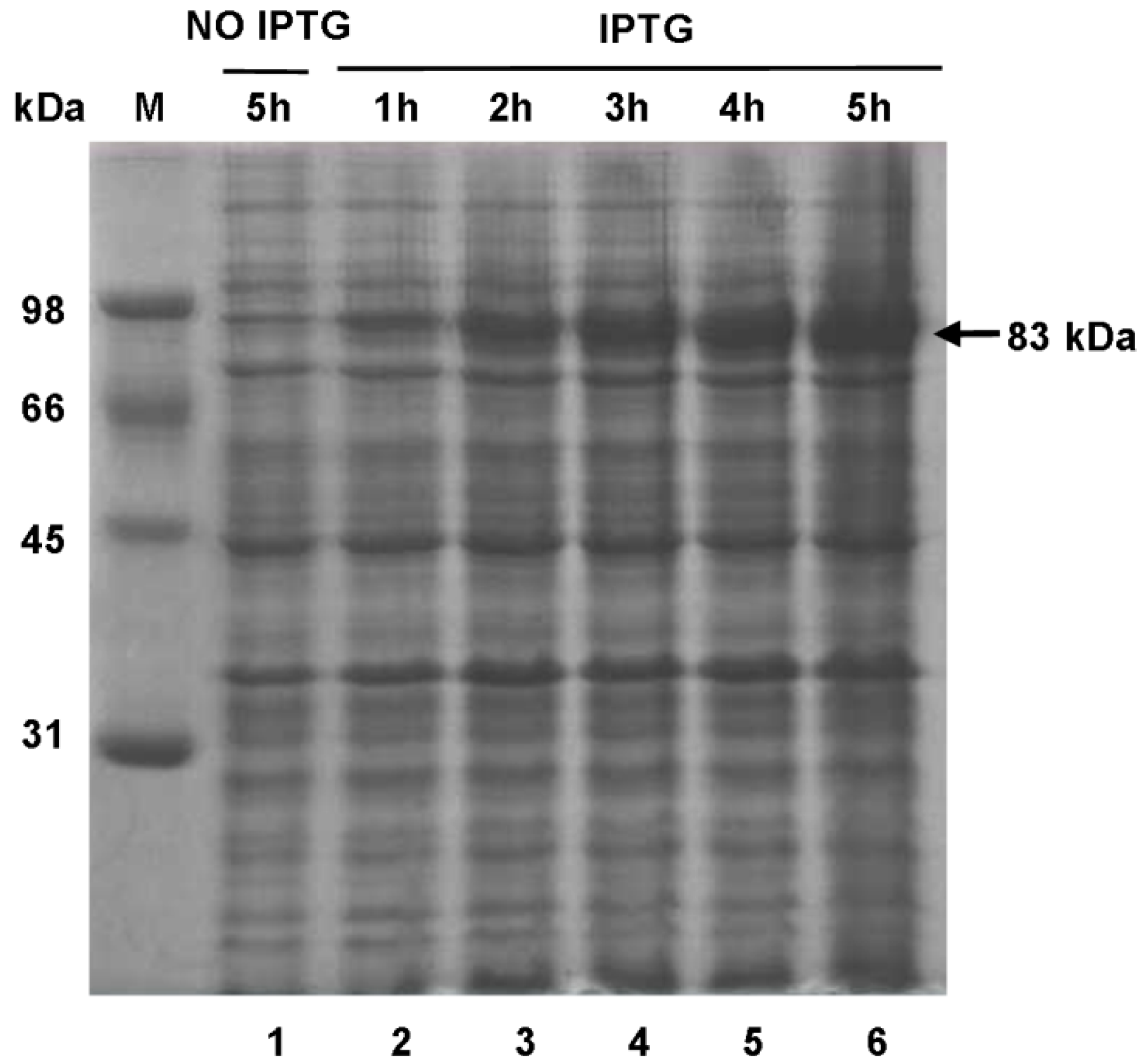
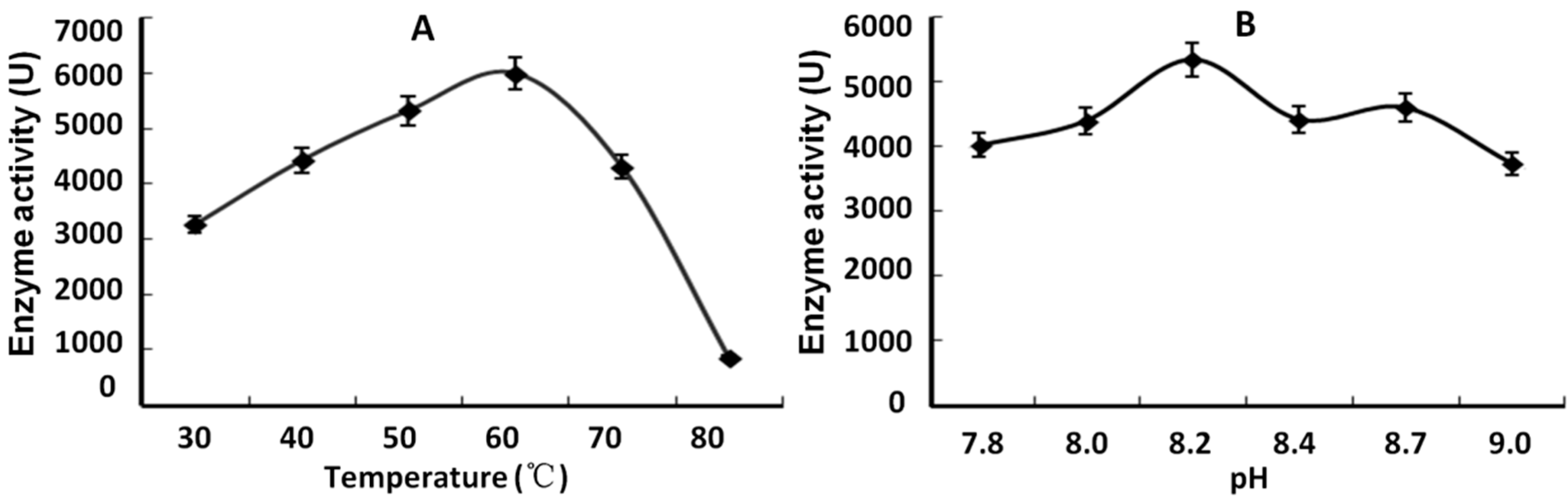
2.7. Expression of SsPAL1 in E. coli
2.8. Correlation Analysis between PAL Activity and Anthocyanin Accumulation in Coleus

3. Experimental Section
3.1. Plant Material and Treatments
3.2. Cloning of the Full-Length SsPAL1
3.3. Isolation of the 5′-Flanking Region of SsPAL1
3.4. Bioinformatics Analysis
3.5. Expression Analysis of SsPAL1 by Real-Time Quantitative RT-PCR
3.6. Expression of SsPAL1 in Escherichia coli BL21 (DE3)
3.7. Enzyme Activity Assay for SsPAL1
3.8. PAL Activity and Anthocyanin Concentration in Coleus Cultivars
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Natasa, B.; Dunja, L.L.; Snjezana, M.; Sibila, J. Genetic transformation of Coleus blumei. Food Technol. Biotechnol. 2002, 40, 163–169. [Google Scholar]
- Petersen, M.; Hausler, E.; Karwatzki, B.; Meinhard, J. Proposed biosynthetic pathway for rosmarinic acid in cell cultures of Coleus blumei Benth. Planta 1993, 189, 10–14. [Google Scholar] [CrossRef]
- Lebowitz, R.J. The genetics and breeding of coleus. Plant Breed. Rev. 1985, 3, 343–360. [Google Scholar]
- Petersen, M.; Abdullah, Y.; Benner, J.; Eberle, D.; Gehlen, K.; Hücherig, S.; Janiak, V.; Kim, K.H.; Sande, M.; Weitzel, C.; et al. Evolution of rosmarinic acid biosynthesis. Phytochemistry 2009, 70, 1663–1679. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Janiak, V.; Petersen, M. Purification, cloning and functional expression of hydroxyphenylpyruvate reductase involved in rosmarinic acid biosynthesis in cell cultures of Coleus blumei. Plant Mol. Biol. 2004, 54, 311–323. [Google Scholar] [CrossRef] [PubMed]
- Eberle, D.; Ullmann, P.; Werck-Reichhart, D.; Petersen, M. cDNA cloning and functional characterisation of CYP98A14 and NADPH, cytochrome P450 reductase from Coleus blumei involved in rosmarinic acid biosynthesis. Plant Mol. Biol. 2009, 69, 239–253. [Google Scholar] [CrossRef] [PubMed]
- Weitzel, C.; Petersen, M. Cloning and characterisation of rosmarinic acid synthase from Melissa officinalis L. Phytochemistry 2011, 72, 572–578. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Mu, L.; Yan, G.L.; Liang, N.N.; Pan, Q.H.; Wang, J.; Reeves, M.J.; Duan, C.Q. Biosynthesis of anthocyanins and their regulation in colored grapes. Molecules 2010, 15, 9057–9091. [Google Scholar] [CrossRef] [PubMed]
- Vogt, T. Phenylpropanoid biosynthesis. Mol. Plant 2010, 3, 2–20. [Google Scholar] [CrossRef] [PubMed]
- Chon, S.; Boo, H.; Heo, B.G.; Gorinstein, S. Anthocyanin content and the activities of polyphenol oxidase, peroxidase and phenylalanine ammonia-lyase in lettuce cultivars. Int. J. Food Sci. Nutr. 2012, 6, 45–48. [Google Scholar] [CrossRef] [PubMed]
- Minami, E.; Ozeki, Y.; Matsuoka, M.; Koiruka, N.; Tanaka, Y. Structure and some characterization of the gene for phenylalanine ammonia-lyase from rice plants. Eur. J. Biochem. 1989, 185, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Wanner, L.A.; Li, G.; Ware, D.; Somssich, I.E.; Davis, K.R. The phenylalanine ammonialyase gene family in Arabidopsis thaliana. Plant Mol. Biol. 1995, 27, 327–338. [Google Scholar] [CrossRef] [PubMed]
- Cochrane, F.C.; Davin, L.B.; Lewis, N.G. The Arabidopsis phenylalanine ammonia lyase gene family, kinetic characterization of the four PAL isoforms. Phytochemistry 2004, 65, 1557–1564. [Google Scholar] [CrossRef]
- Whetten, R.W.; Sederoff, R.R. Phenylalanine ammonia-lyase from loblolly pine, purification of the enzyme and isolation of complementary DNA clones. Plant Physiol. 1992, 98, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Wang, Z. Molecular cloning, expression and characterization of a phenylalanine ammonia-lyase gene (SmPAL1) from Salvia miltiorrhiza. Mol. Biol. Rep. 2008, 36, 939–952. [Google Scholar] [CrossRef]
- Jiang, Y.; Xia, N.; Li, X.; Shen, W.; Liang, L.; Wang, C.; Wang, R.; Peng, F.; Xia, B. Molecular cloning and characterization of a phenylalanine ammonia-lyase gene (LrPAL) from Lycoris radiata. Mol. Biol. Rep. 2011, 38, 1935–1940. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, L.S.; Hsieh, Y.L.; Yeh, C.S.; Cheng, C.Y.; Yang, C.C.; Lee, P.D. Molecular characterization of a phenylalanine ammonialyase gene (BoPAL1) from Bambusa oldhamii. Mol. Biol. Rep. 2010, 38, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Deng, G.; Cheng, S.; Zhang, W.; Huang, X.; Li, L.; Cheng, H.; Rong, X.; Li, J. Molecular cloning, characterization and expression of the phenylalanine ammonia-lyase gene from Juglans Regia. Molecules 2012, 17, 7810–7823. [Google Scholar] [CrossRef] [PubMed]
- Weitzel, C.; Petersen, M. Enzymes of phenylpropanoid metabolism in the important medicinal plant Melissa officinalis L. Planta 2010, 232, 731–742. [Google Scholar] [CrossRef] [PubMed]
- Mahesh, V.; Rakotomalala, J.J.; le Gal, L.; Vigne, H.; de Kochko, A.; Hamon, S.; Noirot, M.; Campa, C. Isolation and genetic mapping of a Coffea canephora phenylalanine ammonia-lyase gene (CcPAL1) and its involvement in the accumulation of caffeoyl quinic acids. Plant Cell Rep. 2006, 25, 986–992. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, M.J.; D’Cunha, G.B. A modern view of phenylalanine ammonia lyase. Biochem. Cell Biol. 2007, 85, 273–282. [Google Scholar] [CrossRef]
- Allwood, E.G.; Davies, D.R.; Gerrish, C.; Ellis, B.E.; Bolwell, G.P. Phosphorylation of phenylalanine ammonia-lyase, evidence for a novel protein kinase and identification of the phosphorylated residue. FEBS Lett. 1999, 457, 47–52. [Google Scholar] [CrossRef]
- Ritter, H.; Schulz, G.E. Structural basis of the entrance into the phenylpropanoid metabolism catalyzed by phenylalanine ammonia-lyase. Plant Cell 2004, 16, 3426–3436. [Google Scholar] [CrossRef] [PubMed]
- Geourjon, C.; Deleage, G. SOPMA, significant improvements in protein secondary structure prediction by consensus prediction from multiple alignments. Comput. Appl. Biosci. 1995, 11, 681–684. [Google Scholar] [CrossRef] [PubMed]
- Arnold, K.; Bordoli, L.; Kopp, J.; Schwede, T. The SWISS-MODEL workspace, a web-based environment for protein structure homology modelling. Bioinformatics 2006, 22, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Lister, C.E.; Lancaster, J.E.; Walker, J.R.L. Developmental changes in enzymes of flavonoid biosynthesis in the skins of red and green apple cultivars. J. Sci. Food Agric. 1996, 71, 313–320. [Google Scholar] [CrossRef]
- Wu, Z.H.; Gui, S.T.; Wang, S.Z.; Ding, Y. Molecular evolution and functional characterisation of an ancient phenylalanine ammonia-lyase gene (NnPAL1) from Nelumbo nucifera: Novel insight into the evolution of the PAL family in angiosperms. BMC Evol. Biol. 2014, 14. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, O.F.; Oerum, H. Analysis of the gene for phenylalanine ammonia-lyase from Rhodosporidium toruloides. DNA Seq. 1991, 1, 207–211. [Google Scholar] [PubMed]
- Xu, F.; Cai, R.; Cheng, S.Y.; Du, H.W.; Wang, Y.; Cheng, S.H. Molecular cloning: Characterization and expression of phenylalanine ammonia-lyase gene from Ginkgo biloba. Afr. J. Biotechnol. 2008, 7, 721–729. [Google Scholar]
- Raes, J.; Rohde, A.; Christensen, J.H.; van de Peer, Y.; Boerjan, W. Genome-wide characterization of the lignification toolbox in Arabidopsis. Plant Physiol. 2003, 133, 1051–1071. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Gu, M.; Lai, Z.; Fan, B.; Shi, K.; Zhou, Y.H.; Yu, J.Q.; Chen, Z. Functional analysis of the Arabidopsis PAL gene family in plant growth, development, and response to environmental stress. Plant Physiol. 2010, 153, 1526–1538. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Zhang, S.; Cai, F.; Zheng, X.; Lin, N.; Qin, X.; Ou, Y.; Gu, X.; Zhu, X.; Xu, Y.; Chen, F. Characterization and expression profile of a phenylalanine ammonia lyase gene from Jatropha curcas L. Mol. Biol. Rep. 2012, 39, 3443–3452. [Google Scholar] [CrossRef] [PubMed]
- Terauchi, R.; Kahl, G. Rapid isolation of promoter sequence by TAIL-PCR, the 5′-flanking regions of Pal and Pgi genes from yams (Dioscorea). Mol. Gen. Genet. 2000, 263, 554–560. [Google Scholar] [CrossRef] [PubMed]
- Tamagnone, L.; Merida, A.; Parr, A.; Mackay, S.; Culianez-Macia, F.A.; Roberts, K.; Martin, C. The AmMYB308 and AmMYB330 transcription factors from antirrhinum regulate phenylpropanoid and lignin biosynthesis in transgenic tobacco. Plant Cell 1998, 10, 135–154. [Google Scholar] [CrossRef] [PubMed]
- Sablowski, R.W.; Moyano, E.; Culianez-Macia, F.A.; Schuch, W.; Martin, C.; Bevan, M. A flower-specific Myb protein activates transcription of phenylpropanoid biosynthetic genes. EMBO J. 1994, 13, 128–137. [Google Scholar] [PubMed]
- Logemann, E.; Parniske, M.; Hahlbrock, K. Modes of expression and common structural features of the complete phenylalanine ammonia-lyase gene family in parsley. Proc. Natl Acad. Sci. USA 1995, 92, 5905–5909. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, L.; Rohfritsch, O.; Fritig, B.; Legrand, M. Phenylalanine ammonia-lyase in tobacco. Molecular cloning and gene expression during the hypersensitive reaction to tobacco mosaic virus and the response to a fungal elicitor. Plant Physiol. 1994, 106, 877–886. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.L.; Yang, Z.F.; Zhang, Q.Y.; Chen, L.T.; Liu, Y.G. Robust multi-type plasmid modification based on isothermal in vitro recombination. Gene 2014, 548, 39–42. [Google Scholar] [CrossRef] [PubMed]
- Rosler, J.; Krekel, F.; Amrhein, N.; Schmid, J. Maize phenylalanine ammonia-lyase has tyrosine ammonia-lyase activity. Plant Physiol. 1997, 113, 175–179. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, I.; Kubo, Y.; Sugiura, A.; Tomana, T. Changes in L-phenylalanine ammonia-lyase activity and anthocyanin synthesis during berry ripening of three grape cultivars. J. Jpn. Soc. Hort. Sci. 1983, 52, 273–279. [Google Scholar] [CrossRef]
- Given, N.K.; Venis, M.A.; Grierson, D. Phenylalanine ammonia-lyase activity and anthocyanin synthesis in ripening strawberry. J. Plant Physiol. 1988, 133, 25–30. [Google Scholar] [CrossRef]
- Ju, Z.G.; Yuan, Y.B.; Liu, C.L.; Xin, S.H. Relationship among phenylalanine ammonia-lyase activity, simple phenol concentrations and anthocyanin accumulation in apple. Sci. Hort. 1995, 61, 215–226. [Google Scholar] [CrossRef]
- Wang, H.Q.; Arakawa, O.; Motomura, Y. Influence of maturity and bagging on the relationship between anthocyanin accumulation and phenylalanine ammonia-lyase (PAL) activity in “Jonathan” apples. Postharvest Biol. Technol. 2000, 19, 123–128. [Google Scholar] [CrossRef]
- Wang, H.C.; Huang, X.M.; Hu, G.B.; Huang, H.B. Studies on the relationship between anthocyanin biosynthesis and related enzymes in litchi pericarp. Sci. Agric. Sin. 2004, 37, 2028–2032. [Google Scholar]
- Albert, N.W.; Lewis, D.H.; Zhang, H.B.; Irving, L.J.; Jameson, P.E.; Davies, K.M. Light-induced vegetative anthocyanin pigmentation in Petunia. J. Exp. Bot. 2009, 60, 2191–2202. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, P.; Cin, V.D. The role of light on foliage colour development in coleus (Solenostemon scutellarioides (L.) Codd). Plant Physiol. Biochem. 2009, 47, 934–945. [Google Scholar] [CrossRef] [PubMed]
- Doyle, J.; Doyle, J.L. A rapid DNA isolation procedure from small quantities of fresh leaf tissue. Phytochem. Bull. 1987, 19, 11–15. [Google Scholar]
- Liu, Y.G.; Chen, Y.L. High-efficiency thermal asymmetric interlaced PCR for amplification of unknown flanking sequences. BioTechniques 2007, 43, 649–656. [Google Scholar] [CrossRef] [PubMed]
- NCBI BLAST Server. Available online: http://blast.ncbi.nlm.nih.gov/Blast.cgi (accessed on 2 September 2015).
- ExPASy Molecular Biology Server. Available online: http://www.expasy.org/ (accessed on 2 September 2015).
- Higo, K.; Ugawa, Y.; Iwamoto, M.; Korenaga, T. Plant cis-acting regulatory DNA elements (PLACE) database. Nucleic Acids Res. 1999, 27, 297–300. [Google Scholar] [CrossRef] [PubMed]
- Lescot, M.; Déhais, P.; Thijs, G.; Marchal, K.; Moreau, Y.; van de Peer, Y.; Rouzé, P.; Rombauts, S. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002, 30, 325–327. [Google Scholar] [CrossRef] [PubMed]
- Yan, Q.; Shi, M.; Ng, J.; Wu, J.Y. Elicitor-induced rosmarinic acid accumulation and secondary metabolism enzyme activities in Salvia miltiorrhiza hairy roots. Plant Sci. 2006, 170, 853–858. [Google Scholar] [CrossRef]
- Bariola, P.A.; MacIntosh, G.C.; Green, P.J. Regulation of S-like ribonuclease levels in arabopsis. Antisense inhibition of RNS1 or RNS2 elevates anthocyanin accumulation. Plant Physiol. 1999, 119, 331–342. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds are not avaliable.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Q.; Xie, X.; Lin, H.; Sui, S.; Shen, R.; Yang, Z.; Lu, K.; Li, M.; Liu, Y.-G. Isolation and Functional Characterization of a Phenylalanine Ammonia-Lyase Gene (SsPAL1) from Coleus (Solenostemon scutellarioides (L.) Codd). Molecules 2015, 20, 16833-16851. https://doi.org/10.3390/molecules200916833
Zhu Q, Xie X, Lin H, Sui S, Shen R, Yang Z, Lu K, Li M, Liu Y-G. Isolation and Functional Characterization of a Phenylalanine Ammonia-Lyase Gene (SsPAL1) from Coleus (Solenostemon scutellarioides (L.) Codd). Molecules. 2015; 20(9):16833-16851. https://doi.org/10.3390/molecules200916833
Chicago/Turabian StyleZhu, Qinlong, Xianrong Xie, Haoxiang Lin, Shunzhao Sui, Rongxin Shen, Zhongfang Yang, Kun Lu, Mingyang Li, and Yao-Guang Liu. 2015. "Isolation and Functional Characterization of a Phenylalanine Ammonia-Lyase Gene (SsPAL1) from Coleus (Solenostemon scutellarioides (L.) Codd)" Molecules 20, no. 9: 16833-16851. https://doi.org/10.3390/molecules200916833
APA StyleZhu, Q., Xie, X., Lin, H., Sui, S., Shen, R., Yang, Z., Lu, K., Li, M., & Liu, Y.-G. (2015). Isolation and Functional Characterization of a Phenylalanine Ammonia-Lyase Gene (SsPAL1) from Coleus (Solenostemon scutellarioides (L.) Codd). Molecules, 20(9), 16833-16851. https://doi.org/10.3390/molecules200916833







