An Expedient Regio- and Diastereoselective Synthesis of Hybrid Frameworks with Embedded Spiro[9,10]dihydroanthracene [9,3′]-pyrrolidine and Spiro[oxindole-3,2′-pyrrolidine] Motifs via an Ionic Liquid-Mediated Multicomponent Reaction
Abstract
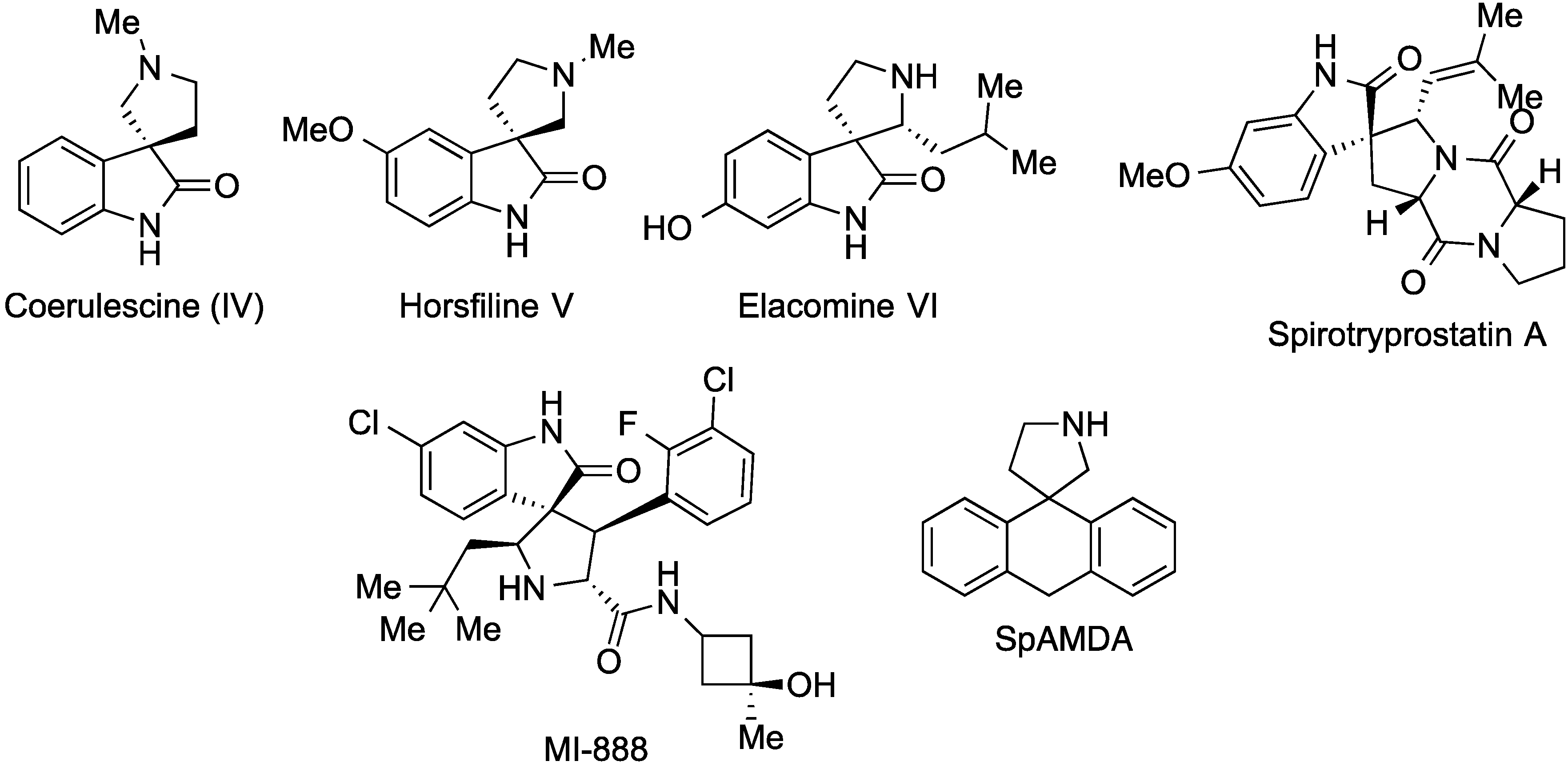
:1. Introduction

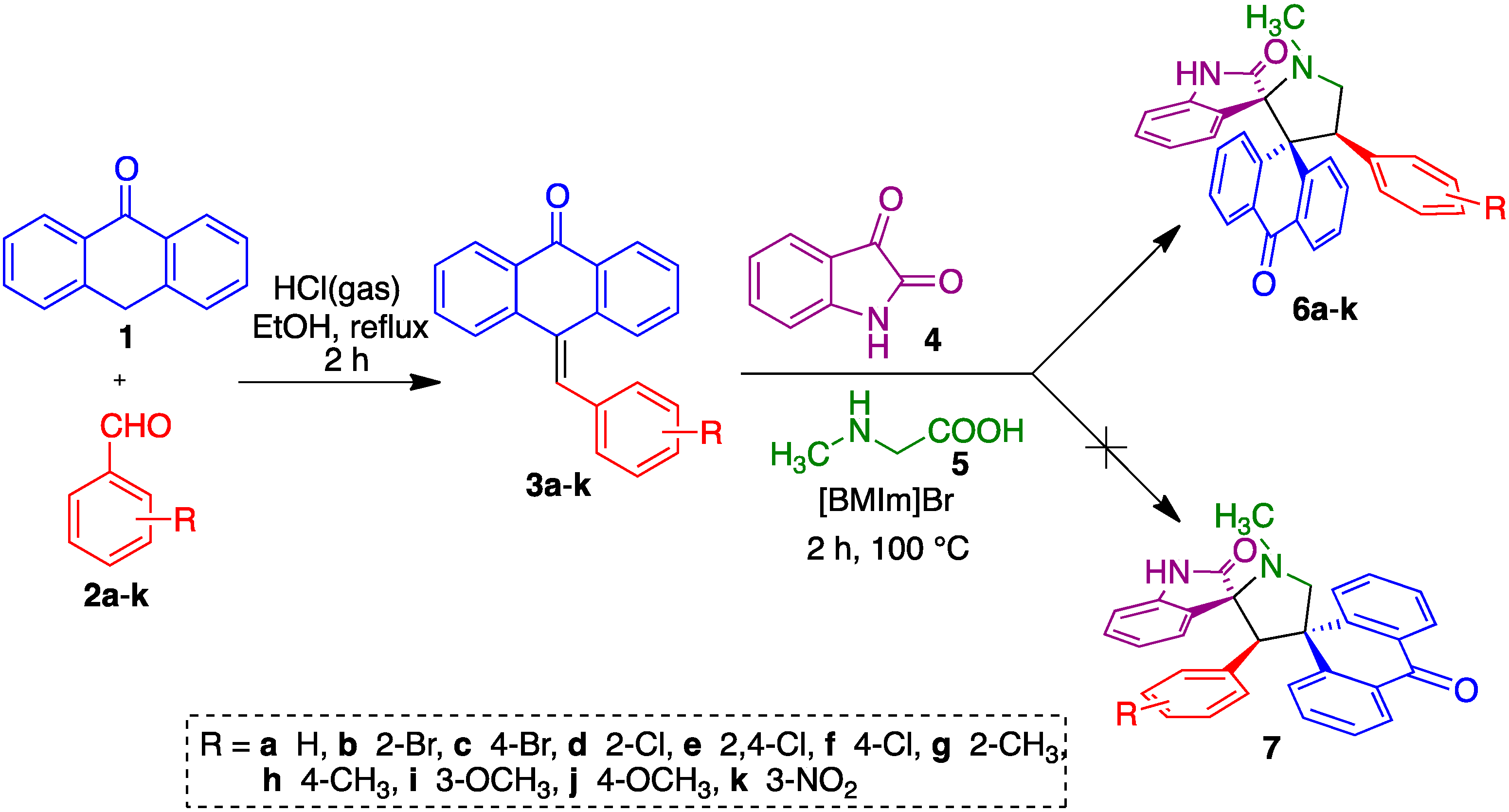
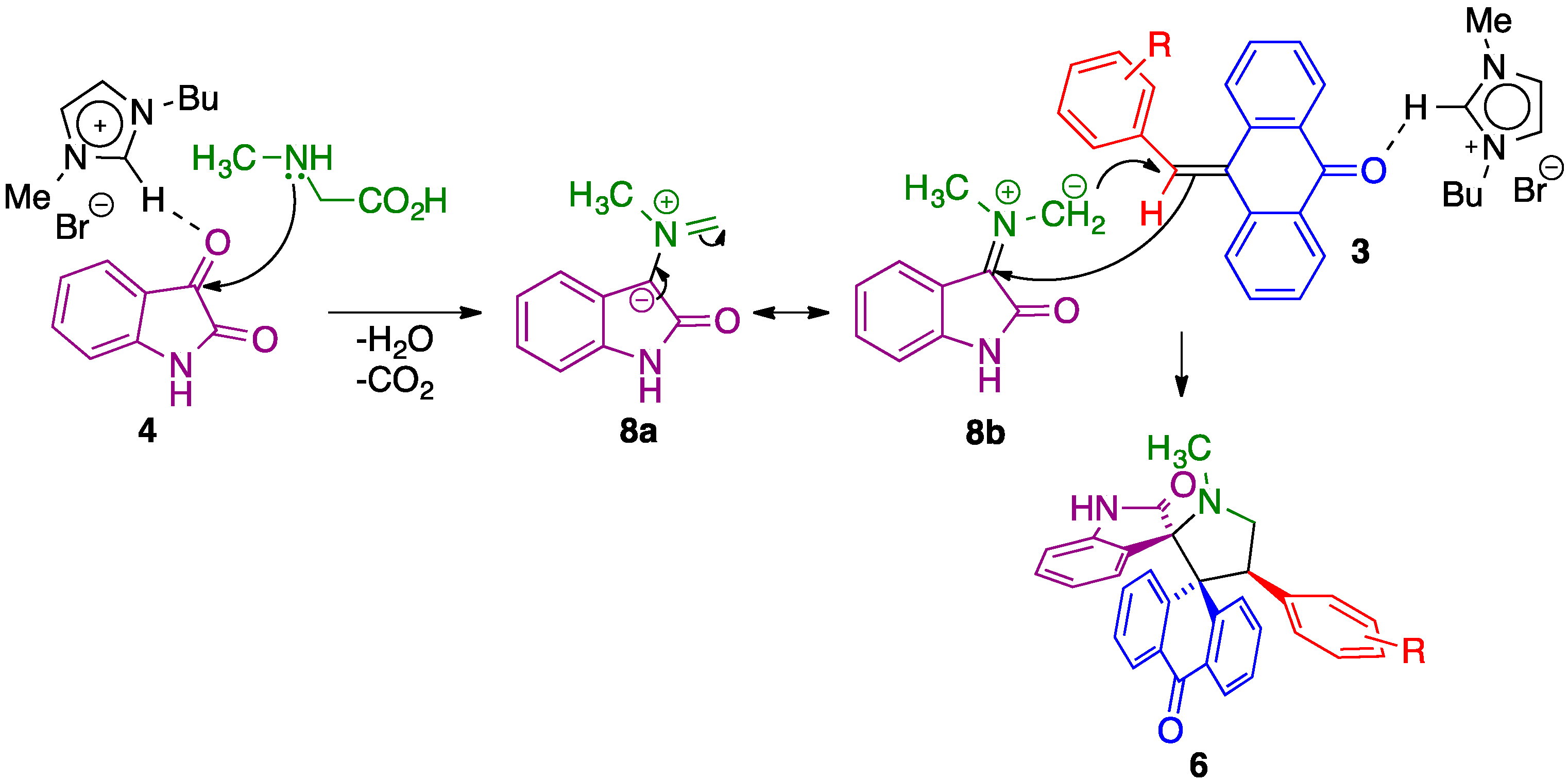
2. Results and Discussion
| Entry | Solvent | Temp, °C | Time, h | Yield, % b |
|---|---|---|---|---|
| 1 | DMF | 60 | 4 | 45 |
| 2 | DMF | 80 | 4 | 50 |
| 3 | DMF | 100 | 3 | 65 |
| 4 | [BIMm]Br | 100 | 2 | 89 |
| 5 | [BIMm]Br | 100 | 2 | 89 |
| 6 | [BIMm]Br | 100 | 2 | 87 |
| 7 | [BIMm]Br | 100 | 2 | 87 |
| Entry | Compound | R | Yield, % a |
|---|---|---|---|
| 1 | 6a | H | 81 |
| 2 | 6b | 2-Br | 80 |
| 3 | 6c | 4-Br | 85 |
| 4 | 6d | 2-Cl | 87 |
| 5 | 6e | 2,4-Cl2 | 84 |
| 6 | 6f | 4-Cl | 88 |
| 7 | 6g | 2-Me | 80 |
| 8 | 6h | 4-Me | 89 |
| 9 | 6i | 3-OMe | 81 |
| 10 | 6j | 4-OMe | 79 |
| 11 | 6k | 3-NO2 | 77 |
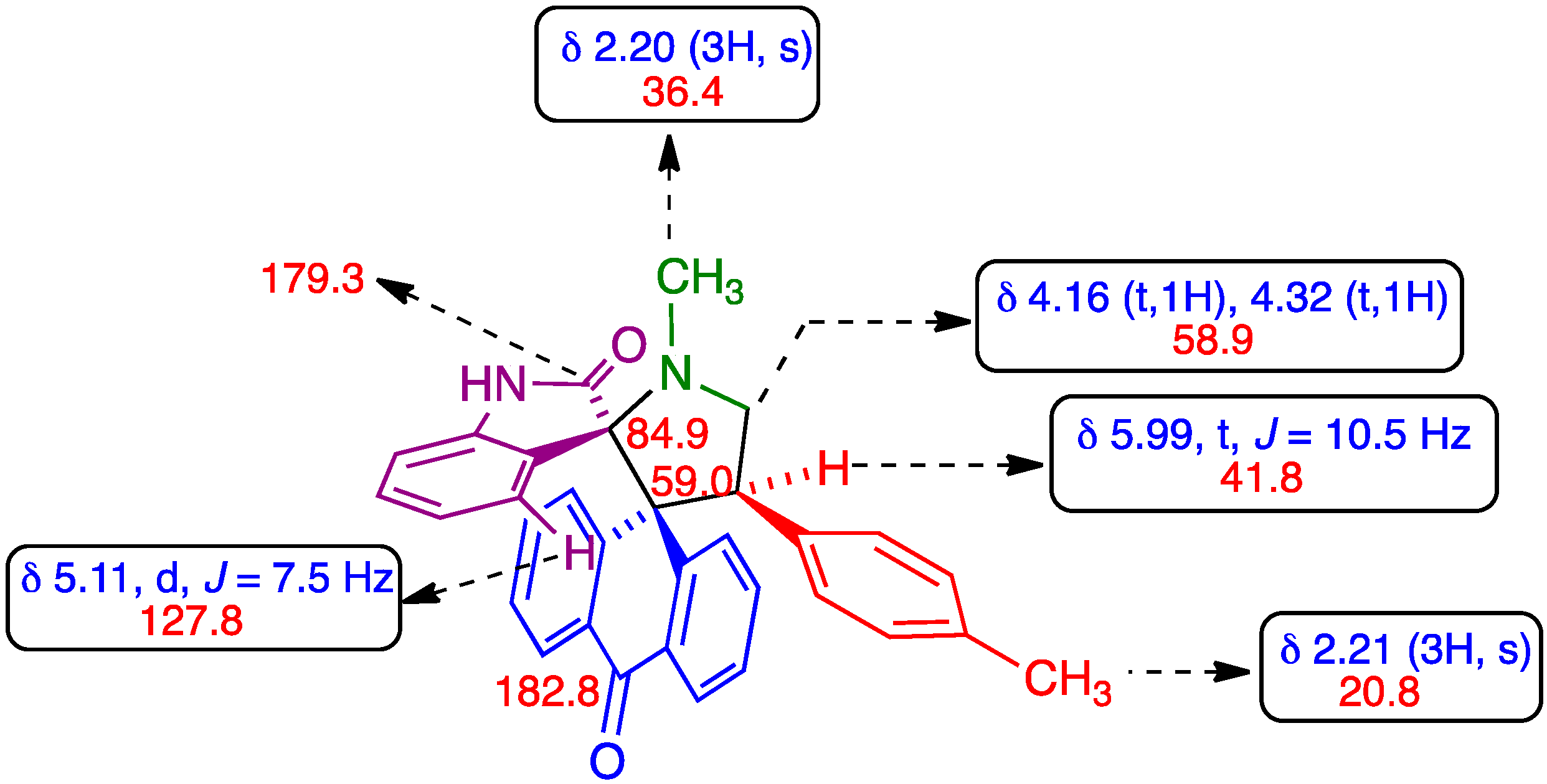
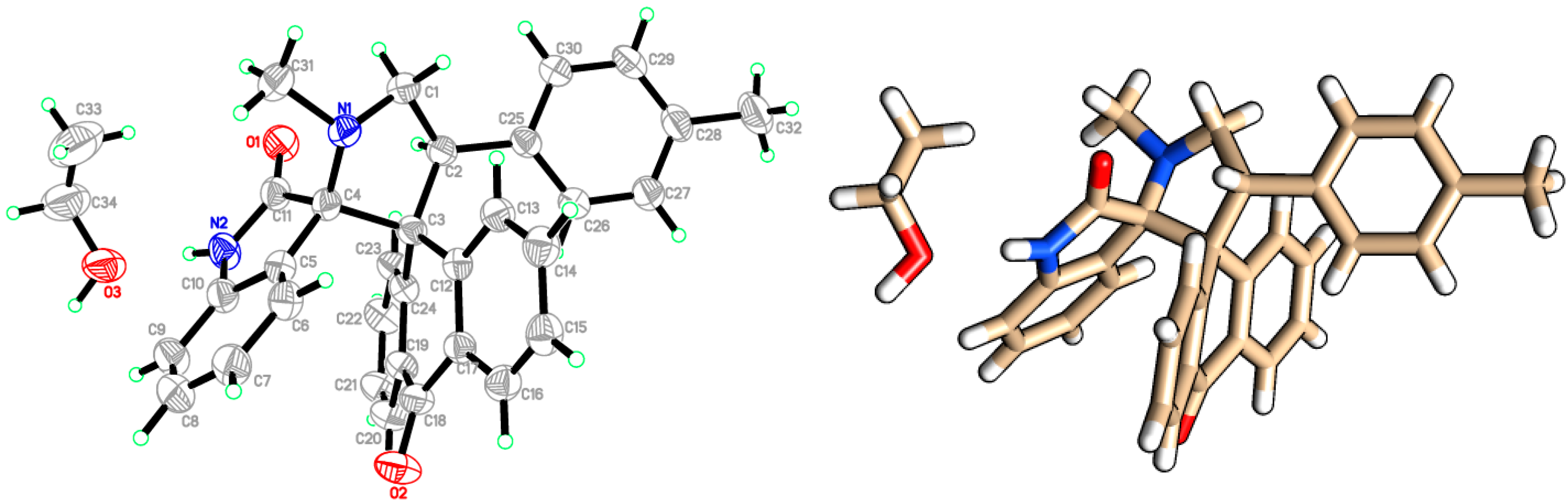
3. Experimental Section
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References and Notes
- Cerulli, V.; Banfi, L.; Basso, A.; Rocca, V.; Riva, R. Diversity oriented and chemoenzymatic synthesis of densely functionalized pyrrolidines through a highly diastereoselective Ugi multicomponent reaction. Org. Biomol. Chem. 2012, 10, 1255–1274. [Google Scholar] [CrossRef] [PubMed]
- Sore, H.F.; Blackwell, D.T.; MacDonald, S.J.F.; Spring, D.R. Diversity-oriented synthesis of disubstituted alkenes using masked silanols. Org. Lett. 2010, 12, 2806–2809. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, S.L. Target-oriented and diversity-oriented organic synthesis in drug discovery. Science 2000, 287, 1964–1969. [Google Scholar] [CrossRef] [PubMed]
- Spring, D.R. Diversity-oriented synthesis; a challenge for synthetic chemists. Org. Biomol. Chem. 2003, 1, 3867–3870. [Google Scholar] [CrossRef] [PubMed]
- Burke, M.D.; Schreiber, S.L. A planning strategy for diversity-oriented synthesis. Angew. Chem. Int. Ed. 2004, 43, 46–58. [Google Scholar] [CrossRef] [PubMed]
- Domling, A. Isocyanide based multi component reactions in combinatorial chemistry. Comb. Chem. High Throughput Screen. 1998, 1, 1–22. [Google Scholar] [PubMed]
- Mironov, M.A. Multicomponent reactions and combinatorial chemistry. Russ. J. Gen. Chem. 2010, 80, 2628–2646. [Google Scholar] [CrossRef]
- Hulme, C.; Gore, V. “Multi-component reactions: Emerging chemistry in drug discovery” “from xylocain to crixivan”. Curr. Med. Chem. 2003, 10, 51–80. [Google Scholar] [CrossRef] [PubMed]
- Weber, L. The application of multi-component reactions in drug discovery. Curr. Med. Chem. 2003, 9, 2085–2093. [Google Scholar] [CrossRef]
- Kalinski, C.; Umkehrer, M.; Weber, L.; Kolb, J.; Burdack, C.; Ross, G. On the industrial applications of MCRs: Molecular diversity in drug discovery and generic drug synthesis. Mol. Divers. 2010, 14, 513–522. [Google Scholar] [CrossRef] [PubMed]
- Freemantle, M. Ionic liquids may boost clean technology development. Chem. Eng. News 1998, 76, 32–37. [Google Scholar] [CrossRef]
- Ionic Liquids in Synthesis, 2nd ed.; Wasserscheid, P.; Welton, T (Eds.) Wiley: Hoboken, NJ, USA, 2007.
- Bao, W.; Wang, Z. An effective synthesis of bromoesters from aromatic aldehydes using tribromide ionic liquid based on l-prolinol as reagent and reaction medium under mild conditions. Green Chem. 2006, 8, 1028–1033. [Google Scholar] [CrossRef]
- Dupont, J.; de Souza, R.F.; Suarez, P.A.Z. Ionic liquid (Molten Salt) phase organometallic catalysis. Chem. Rev. 2002, 102, 3667–3692. [Google Scholar] [CrossRef] [PubMed]
- Earle, M.J.; Ktdare, S.P.; Seddon, K.R. Paradigm Confirmed: The first use of ionic liquids to dramatically influence the outcome of chemical reactions. Org. Lett. 2004, 6, 707–710. [Google Scholar] [CrossRef] [PubMed]
- Kamal, A.; Chouhan, G. A task-specific ionic liquid [bmim]SCN for the conversion of alkyl halides to alkyl thiocyanates at room temperature. Tetrahedron Lett. 2005, 46, 1489–1491. [Google Scholar] [CrossRef]
- Isambert, N.; Duque, M.M.S.; Plaquevent, J.C.; Genisson, Y.; Rodriguez, J.; Constantieux, T. Multicomponent reactions and ionic liquids: A perfect synergy for eco-compatible heterocyclic synthesis. Chem. Soc. Rev. 2011, 40, 1347–1357. [Google Scholar] [CrossRef] [PubMed]
- Michael, S.; Balasubramainan, R.; Bala, D.; Perumal, S. Multi-component, 1,3-dipolar cycloaddition reactions for the chemo-, regio- and stereoselective synthesis of novel hybrid spiroheterocycles in ionic liquid. Tetrahedron Lett. 2012, 53, 5367–5371. [Google Scholar] [CrossRef]
- Arumugam, N.; Almansour, A.I.; Suresh Kumar, R.; Perumal, S.; Ghabbour, H.A.; Fun, H.K. A 1,3-dipolar cycloaddition–annulation protocol for the expedient regio-, stereo- and product-selective construction of novel hybrid heterocycles comprising seven rings and seven contiguous stereocentres. Tetrahedron Lett. 2013, 55, 2515–2519. [Google Scholar] [CrossRef]
- Almansour, A.I.; Arumugam, N.; Suresh Kumar, R.; Periyasami, G.; Ghabbour, H.A.; Fun, H.K. A novel one-pot green synthesis of dispirooxindolo-pyrrolidines via 1,3-dipolar cycloaddition reactions of azomethine ylides. Molecules 2015, 20, 780–791. [Google Scholar] [CrossRef] [PubMed]
- Suresh Kumar, R.; Almansour, A.I.; Arumugam, N.; Menéndez, J.C.; Osman, H.; Ranjith Kumar, R. Dipolar cycloaddition-based multicomponent reactions in ionic Liquids: A green, fully stereoselective synthesis of novel polycyclic cage systems with the generation of two new azaheterocyclic rings. Synthesis 2015, 47. [Google Scholar] [CrossRef]
- Dubreuil, J.F.; Bazureau, J.P. Rate accelerations of 1,3-dipolar cycloaddition reactions in ionic liquids. Tetrahedron Lett. 2000, 41, 7351–7354. [Google Scholar] [CrossRef]
- Jain, R.; Sharma, K.; Kumar, D. Ionic liquid mediated 1,3-dipolar cycloaddition of azomethine ylides: A facile and green synthesis of novel dispiro heterocycles. Tetrahedron Lett. 2012, 53, 1993–1997. [Google Scholar] [CrossRef]
- Cui, C.B.; Kakeya, H.; Okada, G.; Onose, R.; Osada, H. Novel mammalian cell cycle inhibitors, tryprostatins A, B and other diketopiperazines produced by Aspergillus fumigatus. I. Taxonomy, fermentation, isolation and biological properties. J. Antibiot. 1996, 49, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.B.; Kakeya, H.; Osada, H. Novel mammalian cell cycle inhibitors, spirotryprostatins A and B, produced by Aspergillus fumigatus, which inhibit mammalian cell cycle at G2/M phase. Tetrahedron 1996, 52, 12651–12666. [Google Scholar] [CrossRef]
- Edmonson, S.; Danishefsky, S.J.; Sepp-Lorenzino, L.; Rosen, N. Total Synthesis of Spirotryprostatin A, Leading to the Discovery of Some Biologically Promising Analogues. J. Am. Chem. Soc. 1999, 121, 2147–2155. [Google Scholar] [CrossRef]
- Zhao, Y.; Yu, S.; Sun, W.; Liu, L.; Lu, J.; McEachern, D.; Shargary, S.; Bernard, D.; Li, X.; Zhao, T.; et al. A potent small-molecule inhibitor of the MDM2-p53 interaction (MI-888) achieved complete and durable tumor regression in mice. J. Med. Chem. 2013, 56, 5553–5561. [Google Scholar] [CrossRef] [PubMed]
- Peddi, S.; Roth, B.L.; Glennon, R.A.; Westkaemper, R.B. Spiro9,10-dihydroanthracene-9,3′-pyrrolidine-a structurally unique tetracyclic 5-HT2A receptor antagonist. Eur. J. Pharmacol. 2003, 482, 335–337. [Google Scholar] [CrossRef] [PubMed]
- Peddi, S.; Roth, B.L.; Glennon, R.A.; Westkaemper, R.B. Structural determinants for high 5-HT2A receptor affinity of spiro[9,10-dihydroanthracene]-9,3′-pyrrolidine (SpAMDA). Bioorg. Med. Chem. Lett. 2004, 14, 2279–2283. [Google Scholar] [CrossRef] [PubMed]
- Stevens, M.C.G.; Caron, H.N.; Biondi, A. Cancer in Children: Clinical Management, 6th ed.; Oxford University Press: Oxford, UK, 2012; Chapter 10. [Google Scholar]
- Arumugam, N.; Raghunathan, R.; Almansour, A.I.; Karama, U. An efficient synthesis of highly functionalized novel chromeno[4,3-b]pyrroles and indolizino[6,7-b]indoles as potent antimicrobial and antioxidant agents. Bioorg. Med. Chem. Lett. 2012, 22, 1375–1379. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, N.; Periyasami, G.; Raghunathan, R.; Kamalraj, S.; Muthumary, J. Synthesis and antimicrobial activity of highly functionalised novel β-lactam grafted spiropyrrolidines and pyrrolizidines. Eur. J. Med. Chem. 2011, 46, 600–607. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, N.; Raghunathan, R. Facile synthesis of β-lactam-grafted spirooxindolopyrrolidine through regioselective 1,3-dipolar cycloaddition reaction. Synth. Commun. 2011, 41, 2747–2755. [Google Scholar] [CrossRef]
- Arumugam, N.; Raghunathan, R. Synthesis of highly functionalized β-lactam substituted pyrroloisoquinoline and indolizinoindole system by sequential intermolecular 1,3-dipolar cycloaddition reaction and Pictet-Spengler cyclization. Tetrahedron 2010, 66, 969–975. [Google Scholar] [CrossRef]
- Arumugam, N.; Raghunathan, R.; Shanmugaiah, V.; Mathivanan, N. Synthesis of novel β-lactam fused spiroisoxazolidine chromanones and tetralones as potent antimicrobial agent for human and plant pathogens. Bioorg. Med. Chem. Lett. 2010, 20, 3698–3702. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, N.; Jayashankaran, J.; Rathna Durga, R.S.M.; Raghunathan, R. A novel access to highly functionalised β-lactams by regio- and stereoselective 1,3-dipolar cycloaddition reaction. Tetrahedron 2005, 61, 8512–8516. [Google Scholar] [CrossRef]
- 1,3-Dipolar Cycloaddition Chemistry; Padwa, A. (Ed.) Wiley: New York, NY, USA, 1984; Volumes 1 and 2.
- Tsuge, O.; Kanemasa, S. Advances in Heterocyclic Chemistry; Katritzky, A.R., Ed.; Academic: San Diego, CA, USA, 1989; Volume 45, pp. 231–252. [Google Scholar]
- Gothelf, K.V.; Jørgensen, K.A. Asymmetric 1,3-Dipolar Cycloaddition Reactions. Chem. Rev. 1998, 98, 863–909. [Google Scholar] [CrossRef] [PubMed]
- Harju, K.; Yli-Kauhaluoma, J. Recent advances in 1,3-dipolar cycloaddition reactions on solid supports. Mol. Divers. 2005, 9, 187–207. [Google Scholar] [CrossRef] [PubMed]
- Prinz, H.; Ishii, Y.; Hirano, T.; Stoiber, T.; Camacho Gómez, J.A.; Schmidt, P.; Düssmann, H.; Burger, A.M.; Prehn, J.H.M.; Günther, E.G.; et al. Novel Benzylidene-9(10H)-anthracenones as Highly Active Antimicrotubule Agents. Synthesis, Antiproliferative Activity, and Inhibition of Tubulin Polymerization. J. Med. Chem. 2003, 46, 3382–3384. [Google Scholar] [CrossRef] [PubMed]
- Crystallographic data (including structure factors) for compound 6h have been deposited with the Cambridge Crystallographic Data Centre as supplementary publication number CCDC. Copies of the data can be obtained, free of charge, on application to CCDC 1054307, 12 Union Road, Cambridge CB2 1EZ, UK [fax: +44 (0)1223 336033 or e-mail: deposit@ccdc.cam.ac.uk].
- Sample Availability: Samples of the compounds are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arumugam, N.; Almansour, A.I.; Kumar, R.S.; Menéndez, J.C.; Sultan, M.A.; Karama, U.; Ghabbour, H.A.; Fun, H.-K. An Expedient Regio- and Diastereoselective Synthesis of Hybrid Frameworks with Embedded Spiro[9,10]dihydroanthracene [9,3′]-pyrrolidine and Spiro[oxindole-3,2′-pyrrolidine] Motifs via an Ionic Liquid-Mediated Multicomponent Reaction. Molecules 2015, 20, 16142-16153. https://doi.org/10.3390/molecules200916142
Arumugam N, Almansour AI, Kumar RS, Menéndez JC, Sultan MA, Karama U, Ghabbour HA, Fun H-K. An Expedient Regio- and Diastereoselective Synthesis of Hybrid Frameworks with Embedded Spiro[9,10]dihydroanthracene [9,3′]-pyrrolidine and Spiro[oxindole-3,2′-pyrrolidine] Motifs via an Ionic Liquid-Mediated Multicomponent Reaction. Molecules. 2015; 20(9):16142-16153. https://doi.org/10.3390/molecules200916142
Chicago/Turabian StyleArumugam, Natarajan, Abdulrahman I. Almansour, Raju Suresh Kumar, J. Carlos Menéndez, Mujeeb A. Sultan, Usama Karama, Hazem A. Ghabbour, and Hoong-Kun Fun. 2015. "An Expedient Regio- and Diastereoselective Synthesis of Hybrid Frameworks with Embedded Spiro[9,10]dihydroanthracene [9,3′]-pyrrolidine and Spiro[oxindole-3,2′-pyrrolidine] Motifs via an Ionic Liquid-Mediated Multicomponent Reaction" Molecules 20, no. 9: 16142-16153. https://doi.org/10.3390/molecules200916142
APA StyleArumugam, N., Almansour, A. I., Kumar, R. S., Menéndez, J. C., Sultan, M. A., Karama, U., Ghabbour, H. A., & Fun, H.-K. (2015). An Expedient Regio- and Diastereoselective Synthesis of Hybrid Frameworks with Embedded Spiro[9,10]dihydroanthracene [9,3′]-pyrrolidine and Spiro[oxindole-3,2′-pyrrolidine] Motifs via an Ionic Liquid-Mediated Multicomponent Reaction. Molecules, 20(9), 16142-16153. https://doi.org/10.3390/molecules200916142








