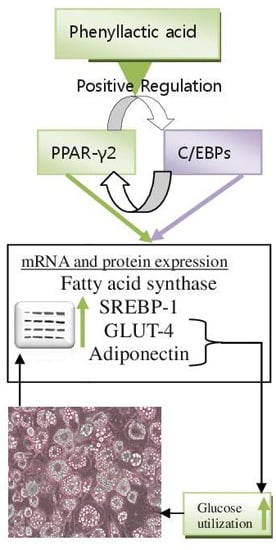
Phenyllactic Acid from Lactobacillus plantarum PromotesAdipogenic Activity in 3T3-L1 Adipocyte via Up-Regulationof PPAR-γ2
Abstract
:1. Introduction
2. Results and Discussion
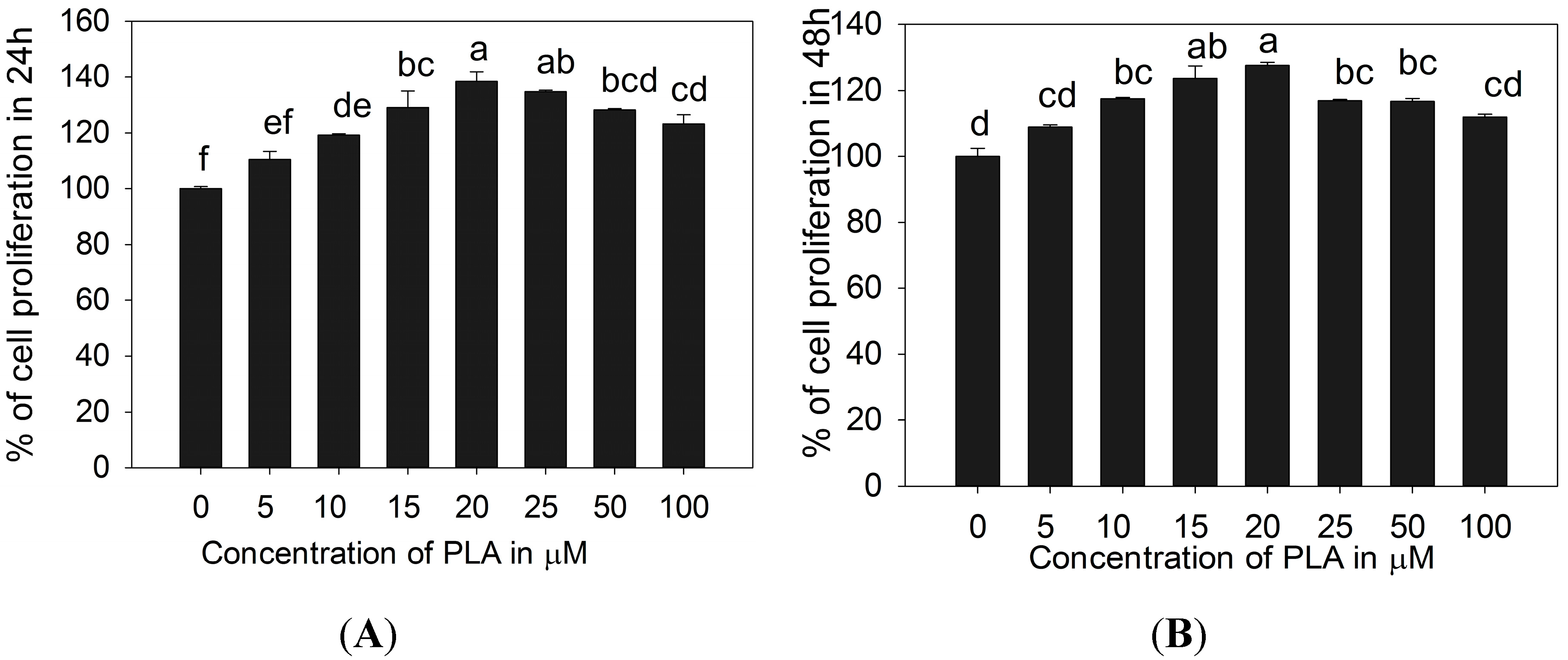
2.1. 3T3-L1 Preadipocytes Proliferation Activity of PLA
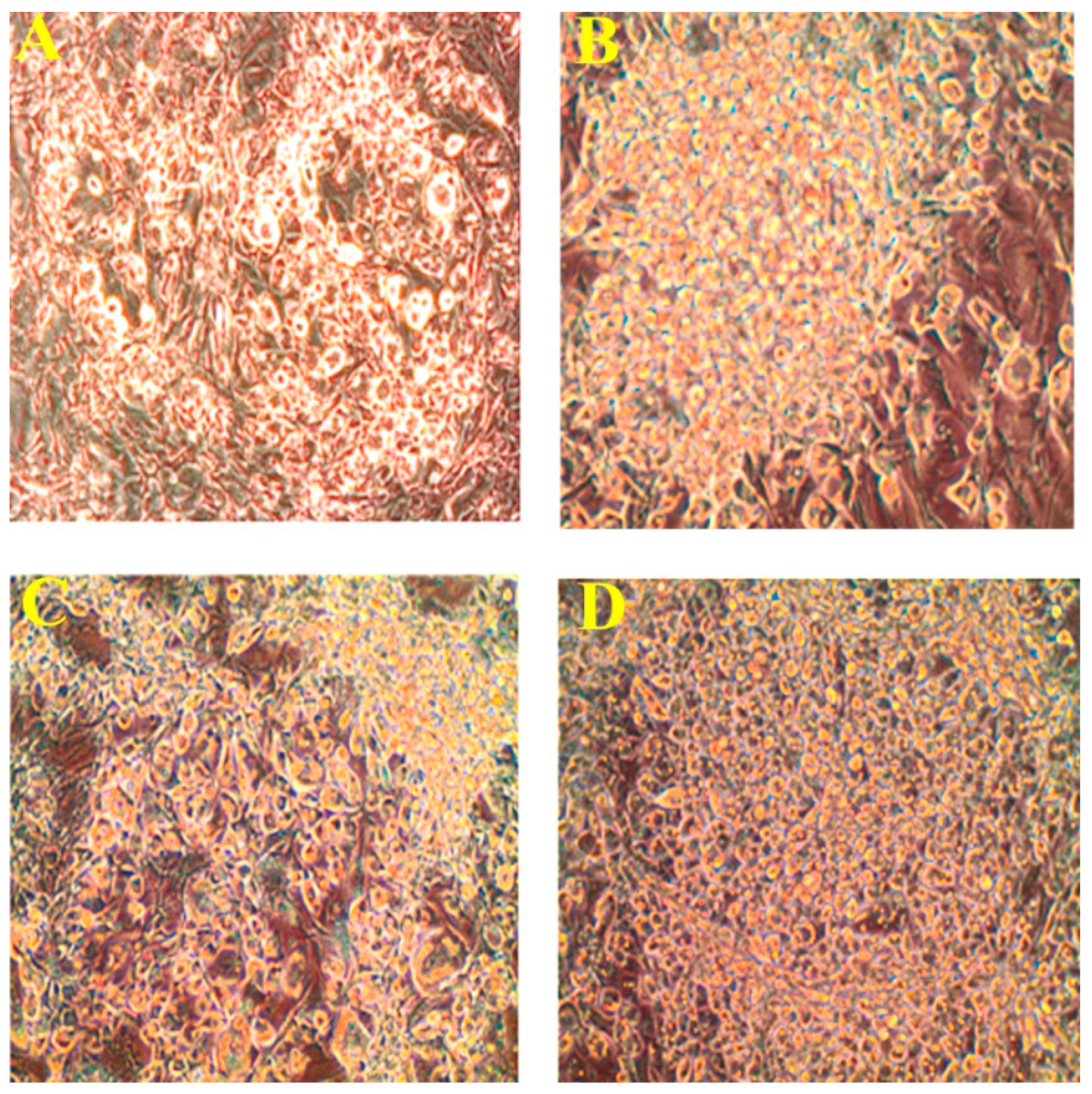
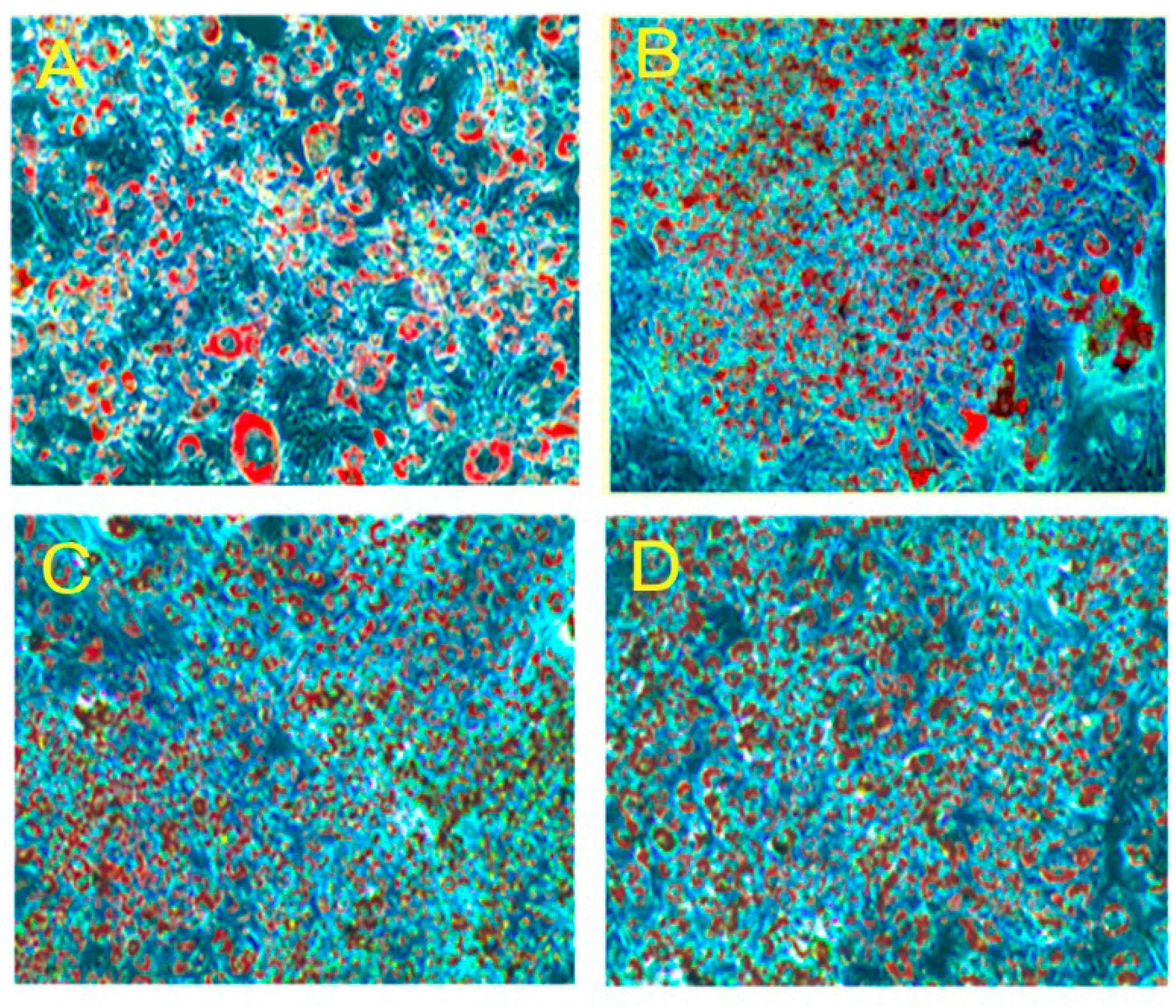
2.2. Effect of PLA on Lipid Accumulation and Glycerol Release

2.3. Quantification of Adipogenic mRNA and Their Proteins by qPCR and Western Blot

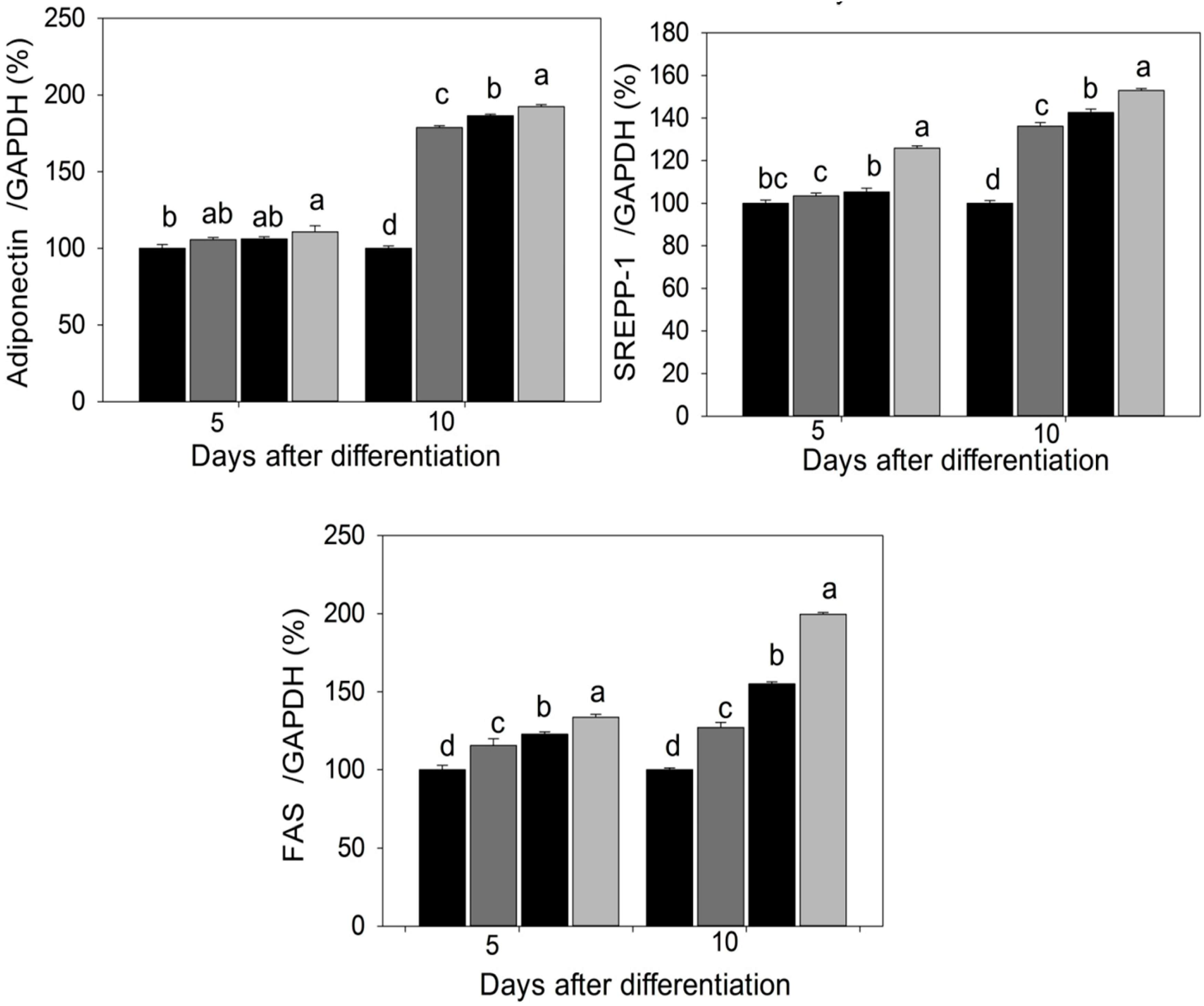

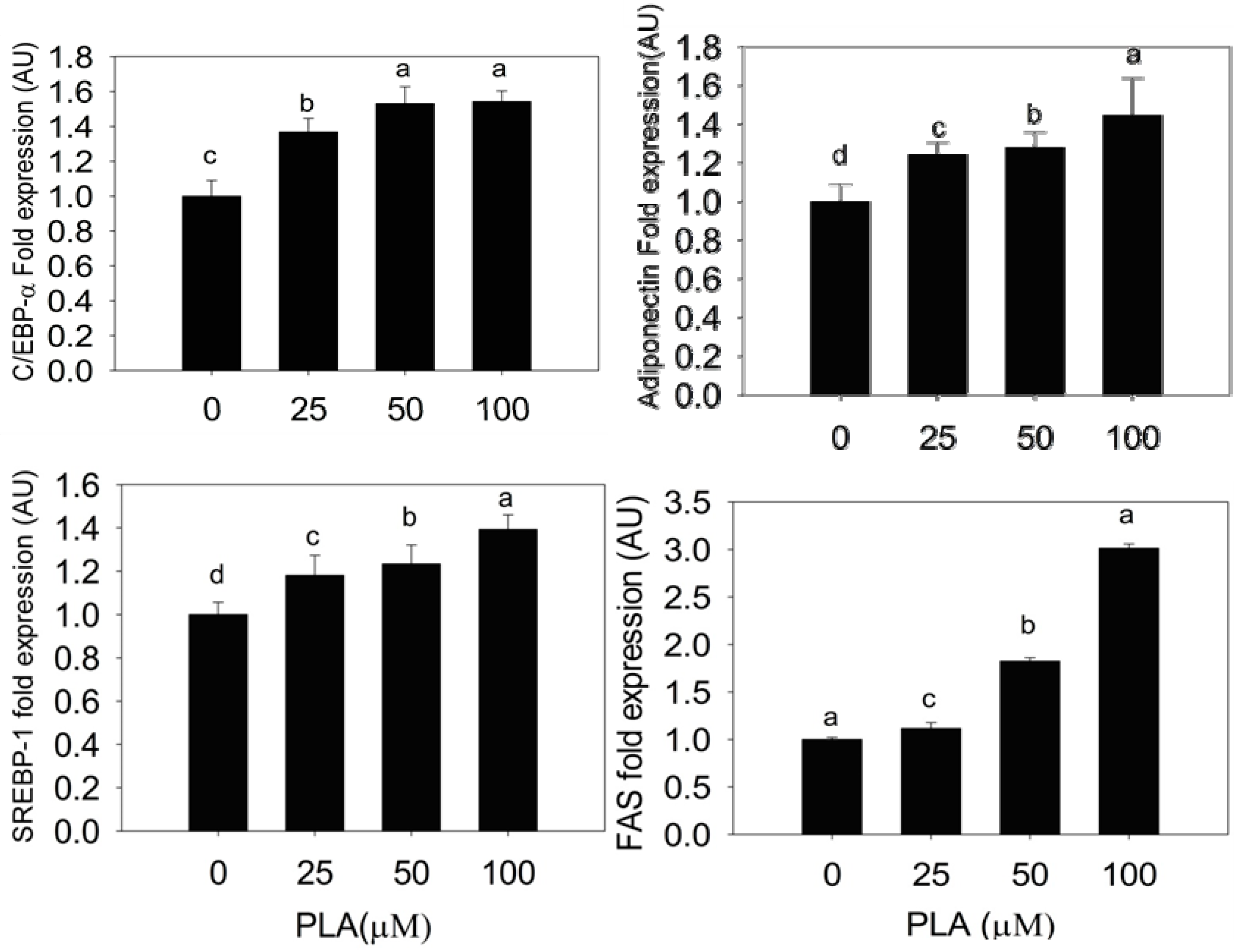

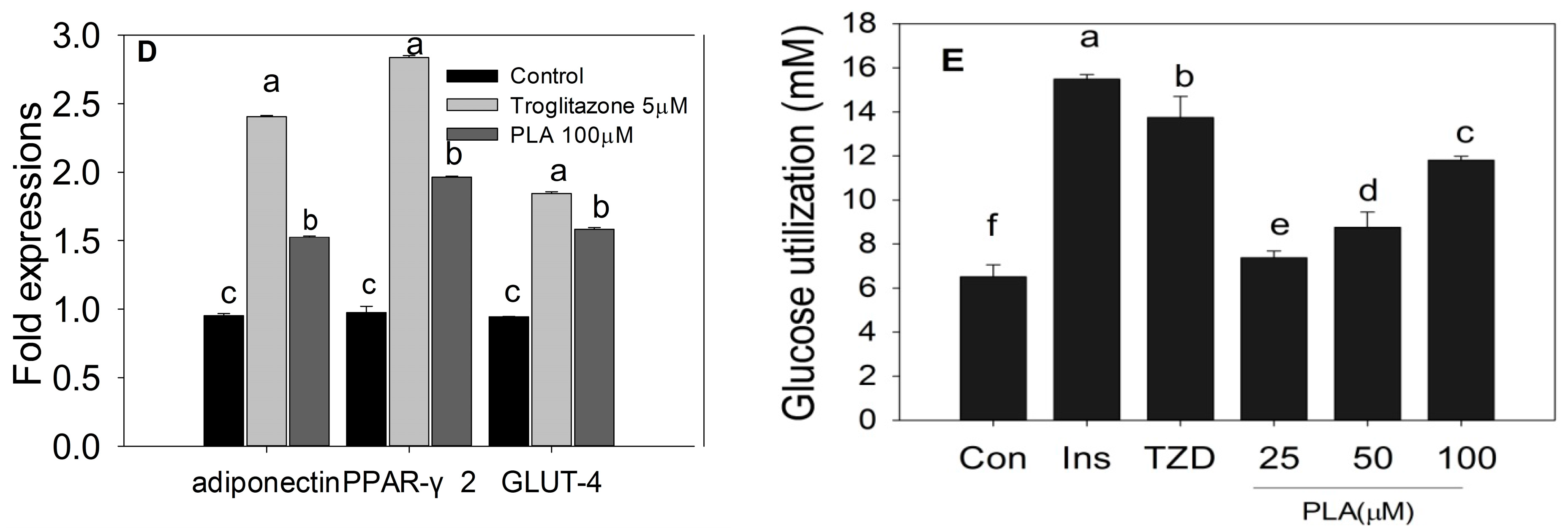
2.4. Discussion
3. Experimental Section
3.1. Chemicals
3.2. Isolation and Characterization of PLA from Lactobacillus spp. KCC-10
3.3. Cell Proliferation
3.4. Differentiation
3.5. Oil Red O Staining
3.6. Measurement of Glycerol Accumulation
3.7. Glucose Utilization Test
3.8. Quantification of PPRA-γ, C/EBP-α, Adiponectin, SREBP-1, FAS, and GLUT-4 mRNA Expression
3.9. Western Blot
3.10. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- White, U.A.; Stephens, J.M. Transcriptional factors that promote formation of white adipose tissue. Mol. Cell. Endocrinol. 2009, 318, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Dubois, S.G.; Heilbronn, L.K.; Smith, S.R.; Albu, J.B.; Kelley, D.E.; Ravussin, E. Decreased expression of adipogenic genes in obese subjects with type 2 diabetes. Obesity 2006, 14, 1543–1552. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.W.; Desantis, O.; Croniger, C.M. Role of Triglyceride/Fatty Acid cycle in development of type-2 diabetes. In Endocrinology and Metabolism; Croniger, C., Ed.; Case Western Reserve University: Cleveland, OH, USA, 2011. [Google Scholar]
- Sun, K.; Kusminski, C.M.; Scherer, P.E. Adipose tissue remodeling and obesity. J. Clin. Investig. 2011, 12, 2094–2101. [Google Scholar] [CrossRef] [PubMed]
- Bays, H.E.; Gonzalez-Campoy, J.M.; Bray, G.A.; Kitabchi, A.E.; Bergman, D.A.; Schorr, A.B.; Rodbard, H.W.; Henry, R.R. Pathogenic potential of adipose tissue and metabolic consequences of adipocyte hypertrophy and increased visceral adiposity. Exp. Rev. Cardiovasc. Ther. 2008, 6, 343–368. [Google Scholar] [CrossRef] [PubMed]
- Karbowska, J.; Kochan, Z. Role of adiponectin in the regulation of carbohydrate and lipid metabolism role of adiponectin in the regulation of carbohydrate and lipid metabolism. J. Physiol. Pharmacol. 2006, 57, 103–113. [Google Scholar] [PubMed]
- Thierry, A.; Maillard, M.B. Production of cheese flavor compounds derived from amino acid catabolism by Propionibacterium freudenreichii. Lait 2002, 82, 17–32. [Google Scholar] [CrossRef]
- Rejiniemon, T.S.; Raishy, R.; Hussain, R.; Rajamani, B. In-vitro functional properties of Lactobacillus plantarum isolated from fermented ragi malt. South Ind. J. Biol. Sci. 2015, 1, 15–23. [Google Scholar]
- Lavermicocca, P.; Valerio, F.V.; Visconti, A. Antifungal activity of phenyllactic acid against molds isolated from bakery products. Appl. Environ. Microbiol. 2003, 69, 634–640. [Google Scholar] [CrossRef] [PubMed]
- Valan Arasu, M.; Jung, M.W.; Ilavenil, S.; Jane, M.; Kim, D.H.; Lee, K.D.; Park, H.S.; Hur, T.Y.; Choi, G.J.; Lim, Y.C.; et al. Isolation and characterization of antifungal compound from Lactobacillus plantarum KCC-10 from forage silage with potential beneficial properties. J. Appl. Microbiol. 2013, 115, 1172–1185. [Google Scholar] [CrossRef] [PubMed]
- Farmer, S.R. Transcriptional control of adipocyte formation. Cell Metab. 2006, 4, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Naowaboot, J.; Chung, C.H.; Pannangpetch, P.; Choi, R.; Kim, B.H.; Lee, M.Y.; Kukongviriyapan, U. Mulberry leaves extract increases adiponectin in murine 3T3-L1 adipocytes. Nutr. Res. 2012, 32, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Luo, N.; Kline, R.L.; Garvey, W.T. Adiponectin promotes adipocyte differentiation, insulin sensitivity and lipid accumulations. J. Lipid Res. 2005, 46, 1369–1379. [Google Scholar]
- Iwaki, M.; Matsuda, M.; Maeda, N.; Funahashi, T.; Matsuzawa, Y.; Makishima, M.; Shimomura, I. Induction of adiponectin, a fat-derived antidiabetic and antiatherogenic factor, by nuclear receptors. Diabetes 2003, 52, 1655–1663. [Google Scholar] [CrossRef] [PubMed]
- Qiao, L.; Maclean, P.S.; Schaack, J.; Orlicky, D.J.; Darimont, C.; Pagliassotti, M.; Friedman, J.E.; Shao, J. C/EBPa regulates human adiponectin gene transcription through an intronic enhancer. Diabetes 2005, 54, 1744–1754. [Google Scholar] [CrossRef] [PubMed]
- Kadowaki, T.; Yamauchi, T. Adiponectin and Adiponectin Receptors. Endocr. Rev. 2005, 26, 439–451. [Google Scholar] [CrossRef] [PubMed]
- Kadowaki, T.; Yamauchi, T.; Kubota, N.; Hara, K.; Ueki, K.; Tobe, K. Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J. Clin. Investig. 2006, 116, 1784–1792. [Google Scholar] [CrossRef] [PubMed]
- Tontonoz, P.; Kim, J.B.; Graves, R.A.; Spiegelman, B.M. ADD l: A novel helix-loop-helix transcription factor associated with adipocyte determination and differentiation. Mol. Cell. Biol. 1993, 13, 4753–4759. [Google Scholar] [PubMed]
- Kim, T.S.; Leahy, P.; Freake, H.C. Promoter usage determines tissue specific responsiveness of the rat acetyl-CoA carboxylase gene. Biochem. Biophys. Res. Commun. 1996, 225, 647–653. [Google Scholar] [CrossRef] [PubMed]
- Ericsson, J.; Jackson, S.M.; Kim, J.B.; Spiegelman, B.M.; Edwards, P.A. Identification of glycerol-3-phosphate acyltransferase as an adipocytes determination and differentiation factor 1- and sterol regulatory element binding protein-responsive gene. J. Biol. Chem. 1997, 272, 7298–7305. [Google Scholar] [CrossRef] [PubMed]
- Shimano, H.; Horton, J.D.; Hammer, R.E.; Shimomura, I.; Brown, M.S.; Goldstein, J.L. Overproduction of cholesterol and fatty acids cause massive liver enlargement in transgenic mice expressing truncated SREBP-1a. J. Clin. Investig. 1996, 98, 1575–1584. [Google Scholar] [CrossRef] [PubMed]
- Foretz, M.; Guichard, C.; Ferré, P.; Foufelle, F. Sterol regulatory binding element binding protein-1c is a major mediator of insulin action on the hepatic expression of glucokinase and lipogenesis-related genes. Proc. Natl. Acad. Sci. USA 1999, 96, 12737–12742. [Google Scholar] [CrossRef] [PubMed]
- Shimomura, I.; Bashmakov, Y.; Ikemoto, S.; Horton, J.D.; Brown, M.S.; Goldstein, J.L. Insulin selectively increases SREBP-1c mRNA in the livers of rats with streptozotocin-induced diabetes. Proc. Natl. Acad. Sci. USA 1999, 96, 13656–13661. [Google Scholar] [CrossRef] [PubMed]
- Florence, M.; Perla, S.; Josiane, S.; Takahiko, M.; Takuya, K.; Shuh, N.; Philippe, G.; Zoubir, A.; Raymond, N.; Gérard, A. Arachidonic acid and prostacyclin signaling promote adipose tissue development: A human health concern? J. Lipid Res. 2003, 44, 271–279. [Google Scholar]
- Maier, T.; Leibundgut, M.; Ban, N. The crystal structure of mammalian fatty acid synthase. Science 2008, 321, 1315–1322. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.B.; Spiegelman, B.M. ADD1/SREBP1 promotes adipocyte differentiation and gene expression linked to fatty acid metabolism. Genes Dev. 1996, 10, 1096–1107. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.B.; Wright, H.M.; Wright, M.; Spiegelman, B.M. ADD1/SREBP1 activates PPARγ through the production of endogenous ligand. Proc. Natl. Acad. Sci. USA 1998, 95, 4333–4337. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.B.; Sarraf, P.; Wright, M.; Yao, K.M.; Mueller, E.; Solanes, G.; Lowell, B.B.; Spiegrlman, B.M. Nutritional and insulin regulation of fatty acid synthetase and leptin gene expression through ADD-1/SREBP1. J. Clin. Investig. 1995, 101, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Staels, B.; Fruchart, J.C. Therapeutic roles of peroxisome proliferator activated receptor agonists. Diabetes 2005, 54, 2460–2470. [Google Scholar]
- Spiegelman, B.M. PPAR-gamma: Adipogenic regulator and thiazolidinedione receptor. Diabetes 1998, 47, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Shintani, M.; Nishimura, H.; Yonemitsu, S.; Ogawa, Y.; Hayashi, T.; Hosoda, K.; Inoue, G.; Nakao, K. Troglitazone Not Only Increases GLUT4 but Also Induces Its Translocation in Rat Adipocytes. Diabetes 2001, 50, 2296–2300. [Google Scholar] [CrossRef] [PubMed]
- Fukumoto, H.; Kayano, T.; Buse, J.B.; Edwards, Y.; Pilch, P.F.; Bell, G.I.; Seino, S. Cloning and characterization of the major insulin-responsive glucose transporter expressed in human skeletal muscle and other insulin-responsive tissues. J. Biol. Chem. 1989, 264, 7776–7779. [Google Scholar] [PubMed]
- Rathi, S.; Grover, J.K.; Vats, V. The effect of Momordica charantia and Mucuna pruriens in experimental diabetes and their effect on key metabolic enzymes involved in carbohydrate metabolism. Phytother. Res. 2002, 16, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Tsao, T.S.; Lodish, H.F.; Fruebis, J. ACRP30, a new hormone controlling fat and glucose metabolism. Eur. J. Pharmacol. 2002, 440, 213–221. [Google Scholar] [CrossRef]
- Choi, K.C.; Roh, S.G.; Hong, Y.H.; Shrestha, Y.B.; Hishikawa, D.; Chen, C.; Kojima, M.; Kangawa, K.; Sasaki, S. The role of ghrelin and growth hormone secretagogues receptor on rat adipogenesis. Endocrinology 2003, 144, 754–759. [Google Scholar] [CrossRef] [PubMed]
- Ilavenil, S.; Valan Arasu, M.; Lee, J.C.; Kim, D.H.; Roh, S.G.; Park, H.S.; Choi, G.J.; Mayakrishnan, V.; Choi, K.C. Trigonelline attenuates the adipocyte differentiation and lipid accumulation in 3T3-L1 cells. Phytomedicine 2014, 21, 758–765. [Google Scholar] [CrossRef] [PubMed]
- Divya, V.; Harini, S.; Manjunath, S.P.; Sowmya, S.S.; Dixit, M.N.; Kakali, D. Novel method to differentiate 3T3-L1 cells in vitro to produce highly sensitive adipocytes for a GLUT4 mediated glucose uptake using fluorescent glucose analog. J. Cell Commun. Signal. 2013, 7, 129–140. [Google Scholar]
- Sample Availability: Samples of the compounds are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ilavenil, S.; Kim, D.H.; Valan Arasu, M.; Srigopalram, S.; Sivanesan, R.; Choi, K.C. Phenyllactic Acid from Lactobacillus plantarum PromotesAdipogenic Activity in 3T3-L1 Adipocyte via Up-Regulationof PPAR-γ2. Molecules 2015, 20, 15359-15373. https://doi.org/10.3390/molecules200815359
Ilavenil S, Kim DH, Valan Arasu M, Srigopalram S, Sivanesan R, Choi KC. Phenyllactic Acid from Lactobacillus plantarum PromotesAdipogenic Activity in 3T3-L1 Adipocyte via Up-Regulationof PPAR-γ2. Molecules. 2015; 20(8):15359-15373. https://doi.org/10.3390/molecules200815359
Chicago/Turabian StyleIlavenil, Soundharrajan, Da Hye Kim, Mariadhas Valan Arasu, Srisesharam Srigopalram, Ravikumar Sivanesan, and Ki Choon Choi. 2015. "Phenyllactic Acid from Lactobacillus plantarum PromotesAdipogenic Activity in 3T3-L1 Adipocyte via Up-Regulationof PPAR-γ2" Molecules 20, no. 8: 15359-15373. https://doi.org/10.3390/molecules200815359