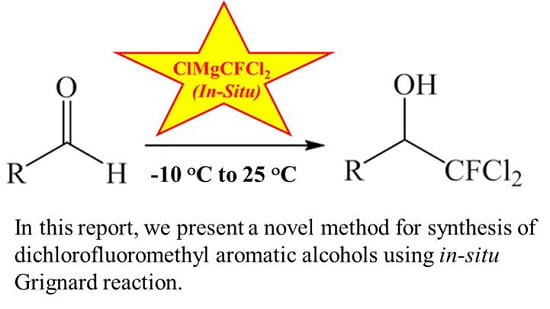
Addition of CFCl3 to Aromatic Aldehydes via in Situ Grignard Reaction
Abstract
:1. Introduction
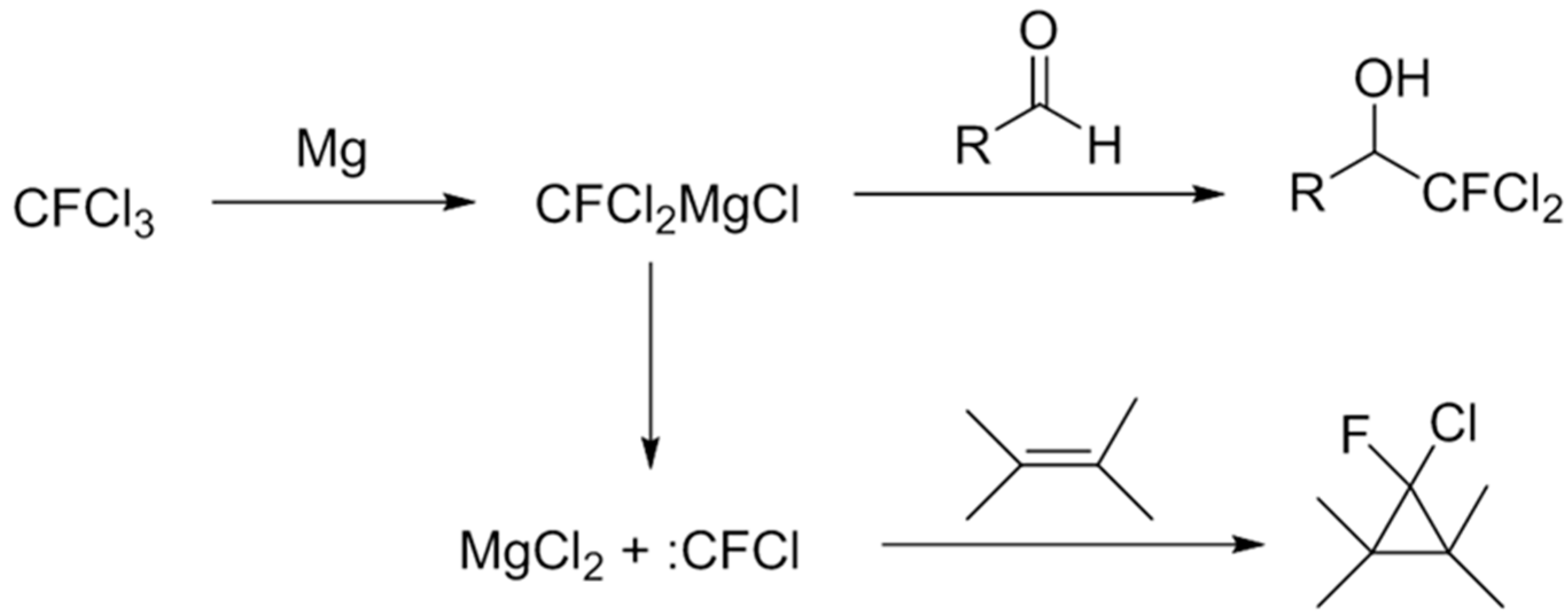
2. Results and Discussion
| Entry | Substrate 1 | Time (h) | Product 2 | Yield (%) |
|---|---|---|---|---|
| 1 |  1a | 4 |  2a | 55 b |
| 2 |  1b | 4 |  2b | 50 b |
| 3 |  1c | 3 |  2c | 20 b |
| 4 |  1d | 3 |  2d | 14 b |
| 5 |  1e | 4 |  2e | 47 b |
| 6 |  1f | 4 |  2f | 65 b |
| 7 |  1g | 4 |  2g | 60 b |
| 8 |  1h | 4 | unknown mixture c | |
| 9 |  1i | 4 | unknown mixture c |

3. Experimental Section
3.1. General
3.2. Materials
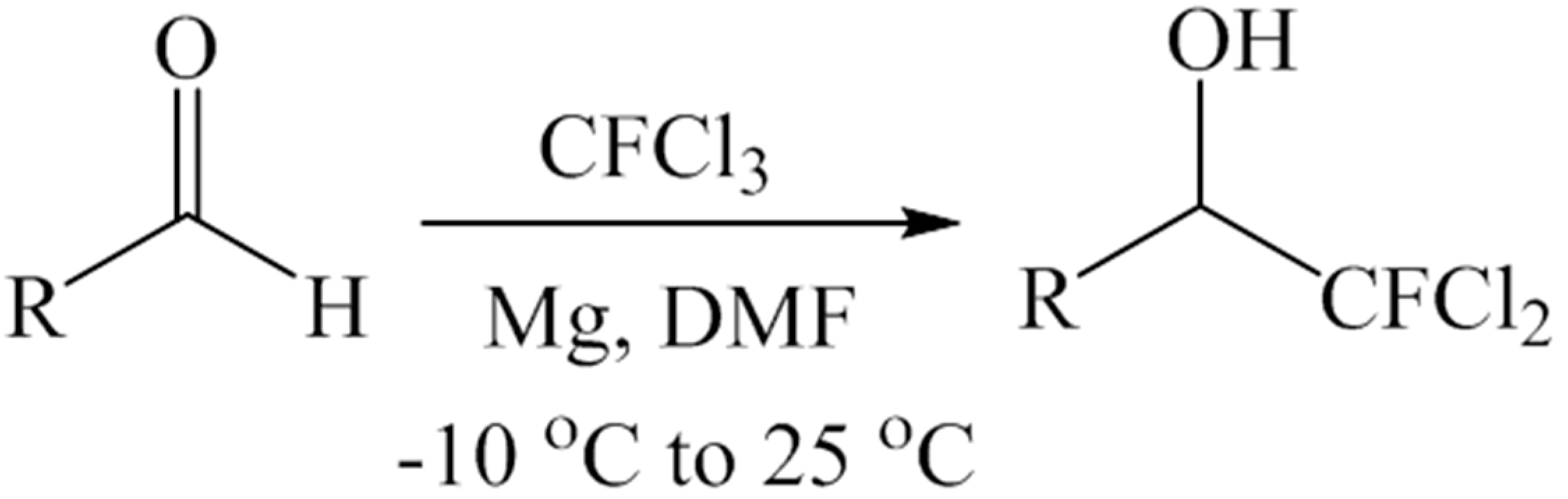
3.3. Method: Activation of CFCl3 by Magnesium Powder and Its Addition to Aromatic Aldehydes-Typical Procedure
3.4. Characterization of the Products
- 2,2-Dichloro-2-fluoro-1-phenylethanol (2a). 1H-NMR (CDCl3): δ = 7.53 (m, 2H), 7.41 (m, 3H), 5.16 (d, J = 8.4 Hz, 1H), 2.97 (br s, 1H, OH).
- 2,2-Dichloro-2-fluoro-1-(2-methylphenyl)ethanol (2b). 1H-NMR (CDCl3): δ = 7.66 (d, J = 6.9 Hz, 1H), 7.25 (m, 3H), 5.43 (d, J = 9.3 Hz, 1H), 2.60 (s, 3H), 3.05 (br s, 1H, OH).
- 2,2-Dichloro-2-fluoro-1-(2-chlorophenyl)ethanol (2c). 1H-NMR (CDCl3): δ = 7.75 (m, 1H), 7.36 (m, 3H), 5.77 (d, J = 9.3 Hz, 1H), 3.17 (br s, 1H, OH).
- 2,2-Dichloro-2-fluoro-1-(4-bromophenyl)ethanol (2d). 1H-NMR (CDCl3): δ = 7.53 (m, 2H), 7.40 (d, J = 8.1 Hz, 2H), 5.11 (d, J = 7.5 Hz, 1H), 3.24 (br s, 1H, OH).
- 2,2-Dichloro-2-fluoro-1-(4-methoxyphenyl)ethanol (2e). 1H-NMR (CDCl3): δ = 7.45 (d, J = 8.4 Hz, 2H), 6.92 (d, J = 8.4 Hz, 2H), 5.10 (d, J = 8.7 Hz, 1H), 3.82 (s, 3H), 2.97 (br s, 1H, OH).
- 2,2-Dichloro-2-fluoro-1-(4-trifluoromethylphenyl)ethanol (2f). 1H-NMR (CDCl3): δ = 7.51 (m, 4H), 5.3 (d, J = 8.1 Hz, 1H), 3.1 (br s, 1H, OH).
- 2,2-Dichloro-2-fluoro-1-(4-fluorophenyl)ethanol (2g). 1H-NMR (CDCl3): δ = 7.75 (m, 2H), 7.36 (m, 2H), 5.8 (d, J = 9.1 Hz, 1H), 3.3 (br s, 1H, OH).
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Welch, C. The Ozone Hole. Available online: http://www.theozonehole.com/cfc.htm (accessed on 6 June 2015).
- Chlorofluorocarbons (CFCs). Available online: http://www.esrl.noaa.gov/gmd/hats/publictn/elkins/cfcs.html (accessed on 6 June 2015).
- United Nations Environmental Program (UNEP). Montreal Protocol on Substances that Deplete the Ozone Layer. Available online: http://ozone.unep.org/pdfs/Montreal-Protocol2000.pdf (accessed on 17 August 2015).
- Deam, R.T.; Dayal, A.R.; McAllister, T.; Mundy, A.E.; Western, R.J.; Besley, L.M.; Farmer, A.J.D.; Horrigan, C.; Murphy, A.B. Interconversion of chlorofluorocarbons in plasmas. J. Chem. Soc. Chem. Commun. 1995, 347–348. [Google Scholar] [CrossRef]
- Shimakoshi, H.; Maeyema, Y.; Kaieda, T.; Matsuo, T.; Matsui, E.; Naruta, Y.; Hisaeda, Y. Hydrophobic vitamin B12. Part 20: Supernucleophilicity of Co(I) heptamethyl cobyrinate toward various organic halides. Bull. Chem. Soc. Jpn. 2005, 78, 859–863. [Google Scholar] [CrossRef]
- Krahl, T.; Kemintz, E. Amorphous aluminum bromide fluoride (ABF). Angew. Chem. Int. Ed. 2004, 43, 6653–6656. [Google Scholar] [CrossRef] [PubMed]
- Nenajdenko, V.G.; Shastin, A.V.; Korotchenko, V.N.; Varseev, G.N.; Balenkova, E.S. A novel approach to 2-chloro-2-fluorostyrenes. Eur. J. Org. Chem. 2003, 2, 302–308. [Google Scholar] [CrossRef]
- Saikia, A.K.; Tsuboi, S. Chemistry of trichlorofluoromethane: Synthesis of chlorofluoromethyl phenyl sulfone and fluoromethyl phenyl sulfone and some of their reactions. J. Org. Chem. 2001, 66, 643–647. [Google Scholar] [CrossRef] [PubMed]
- Zeifman, Y.V.; Postovoi, S.A. Reductive alkylation of perfluorocarboxylic acid esters with CCl3F or CCl4 and synthesis of higher linear perfluoroketones. J. Fluor. Chem. 2004, 125, 1815–1819. [Google Scholar] [CrossRef]
- Seiler, N.; Jung, M.J.; Koch-Weser, J. Enzyme-Activated Irreversible Inhibitors; Elsevier/North-Holland Biomedical Press: Amsterdam, The Netherlands, 1978. [Google Scholar]
- Rando, R.R. Mechanism-based enzyme inhibitors. Pharmacol. Rev. 1984, 36, 111–142. [Google Scholar] [PubMed]
- Banks, R.E. Organofluorine. Chemicals and Their Industrial Applications; Royal Society of Chemistry: London, UK, 1979; Volume 123, pp. 154, 169. [Google Scholar]
- Fuller, R.; Kobayashi, Y. Biomedical Aspects of Fluorine Chemistry; Kodansha/Elsevier: New York, NY, USA, 1982. [Google Scholar]
- Ozone Depletion Potential. Available online: http://en.wikipedia.org/wiki/Ozone_depletion_potential (accessed on 6 June 2015).
- Barkakaty, B.; Takaguchi, Y.; Tsuboi, S. Addition of CFCl3 to aromatic aldehydes under ultrasonic irradiation. Synthesis 2006, 6, 959–962. [Google Scholar] [CrossRef]
- Barkakaty, B.; Takaguchi, Y.; Tsuboi, S. New synthetic routes towards various α-fluorinated aryl ketones and their enantioselective reduction using baker’s yeast. Tetrahedron 2007, 63, 970–976. [Google Scholar] [CrossRef]
- Postovoi, S.A.; Kagramanova, E.M.; Mysov, E.I.; Zeifman, Y.V. Reductive addition of polychlorofluoroalkanes to fluorocarbonyl compounds. Russ. Chem. Bull. (Engl. Transl.) 1996, 45, 1425–1427. [Google Scholar] [CrossRef]
- Material Safety Data Sheet Aluminum MSDS. Available online: http://www.sciencelab.com/msds.php?msdsId=9922844 (accessed on 6 June 2015).
- Material Safety Data Sheet Stannous Chloride MSDS. Available online: http://www.sciencelab.com/msds.php?msdsId=9925079 (accessed on 6 June 2015).
- Material Safety Data Sheet Magnesium MSDS. Available online: http://www.sciencelab.com/msds.php?msdsId=9924535 (accessed on 6 June 2015).
- Seelig, M.S. Magnesium requirements in Human Nutrition. Available online: http://www.mgwater.com/require.shtml (accessed on 6 June 2015).
- Hu, C.M.; Tu, M.H. Reductive addition of trichlorofluoromethane to ketones initiated by the Mg/LiCl system. J. Fluor. Chem. 1994, 67, 9–10. [Google Scholar] [CrossRef]
- Barl, N.M.; Werner, V.; Samann, C.; Knochel, P. The halogen/magnesium-exchange using iPrMgCl.LiCl and related exchange reagents. Heterocycles 2014, 88, 827–844. [Google Scholar]
- Krasovskiy, A.; Knochel, P. A LiCl-Mediated Br/Mg exchange reaction for the preparation of functionalized aryl- and heteroarylmagnesium compounds from organic bromides. Angew. Chem. Int. Ed. 2004, 43, 3333–3336. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, R.W.; Leo, P.G.N.; Harms, K. Highly enantiomerically enriched α-haloalkyl grignard reagents. Chem. Eur. J. 2000, 6, 3359–3365. [Google Scholar] [CrossRef]
- Lin, H.; Yang, M.; Huang, P.; Cao, W. A facile procedure for the generation of dichlorocarbene from the reaction of carbon tetrachloride and magnesium using ultrasonic irradiation. Molecules 2003, 8, 608–613. [Google Scholar] [CrossRef]
- Dolbier, W.R., Jr.; Burkholder, C.R. Chlorofluorocarbene from reaction of fluorotrichloromethane with reduced titanium. Synthesis of 1-chloro-1-fluoro cyclopropanes. J. Org. Chem. 1990, 55, 589–594. [Google Scholar] [CrossRef]
- Burton, D.J.; Wiemers, D.M. A remarkable simple preparation of (trifluoromethyl)cadmium and-zinc reagents directly from difluorodihalomethanes. J. Am. Chem. Soc. 1985, 107, 5014–5015. [Google Scholar] [CrossRef]
- Burton, D.J.; Hahnfeld, J.L. Fluorochloro-, fluorobromo-, and monofluorocarbene generation via organo lithium reagents. J. Org. Chem. 1977, 42, 828–831. [Google Scholar] [CrossRef]
- Barhdadi, R.; Simsen, B.; Troupel, M.; Nedelec, J.Y. Direct electroreduction or use of an electrogenerated base: Two ways for the coupling of polyhalogenated compounds with aldehydes or ketones. Tetrahedron 1997, 53, 1721–1728. [Google Scholar] [CrossRef]
- Ando, T.; Namigata, F.; Kataoka, M.; Yachida, K.; Funasaka, W. 1,1 Diaryl- and 1-Aryl-2-chloro-2-fluoroethylenes. Bull. Chem. Soc. Jpn. 1967, 40, 1275–1278. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds might be available from the authors on request.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barkakaty, B.; Talukdar, B.; Lokitz, B.S. Addition of CFCl3 to Aromatic Aldehydes via in Situ Grignard Reaction. Molecules 2015, 20, 15098-15107. https://doi.org/10.3390/molecules200815098
Barkakaty B, Talukdar B, Lokitz BS. Addition of CFCl3 to Aromatic Aldehydes via in Situ Grignard Reaction. Molecules. 2015; 20(8):15098-15107. https://doi.org/10.3390/molecules200815098
Chicago/Turabian StyleBarkakaty, Balaka, Bandana Talukdar, and Bradley S. Lokitz. 2015. "Addition of CFCl3 to Aromatic Aldehydes via in Situ Grignard Reaction" Molecules 20, no. 8: 15098-15107. https://doi.org/10.3390/molecules200815098