Eco-Friendly Synthesis of a New Class of Pyridinium-Based Ionic Liquids with Attractive Antimicrobial Activity
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry
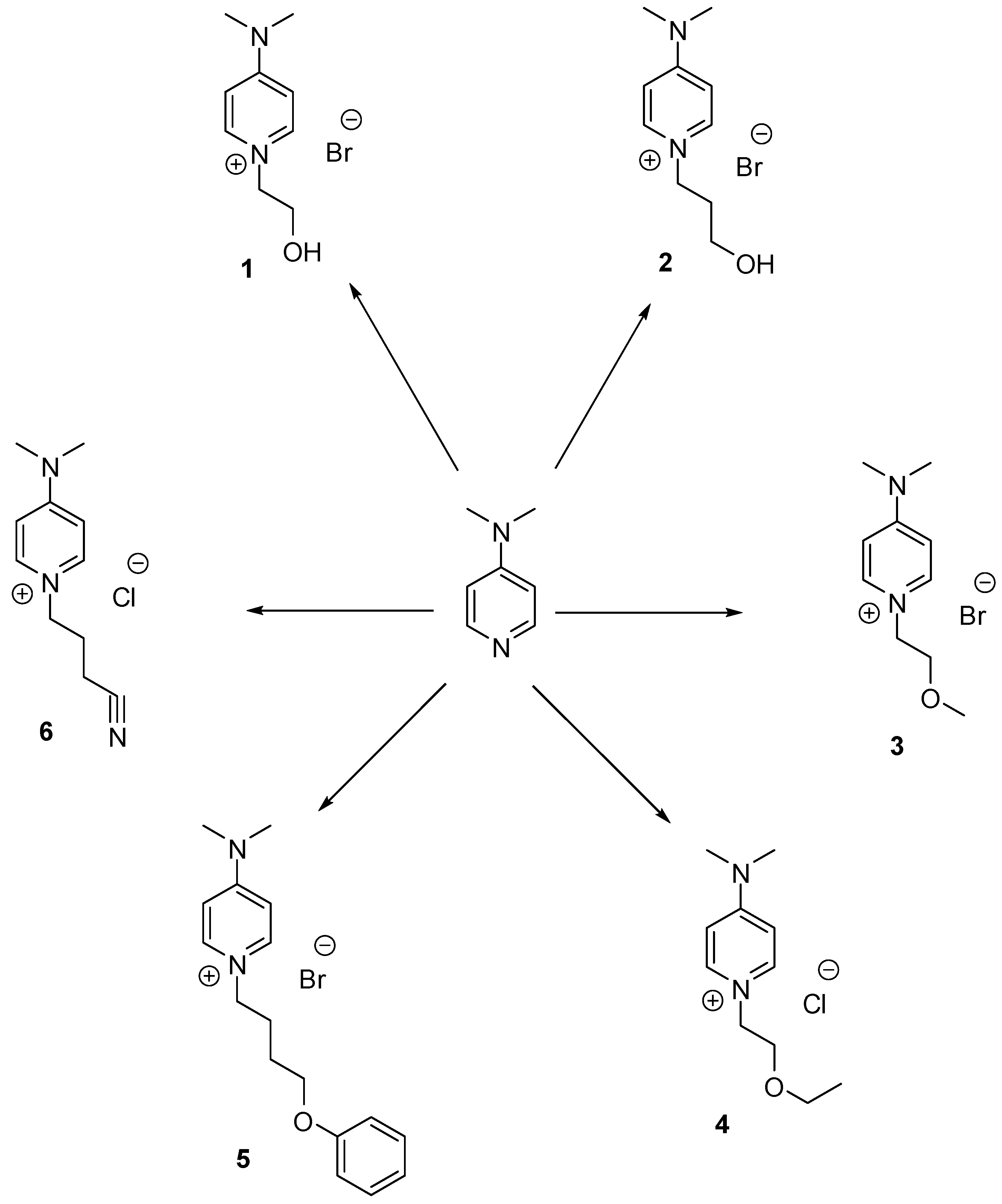

| Ionic Liquid | RX | Yield (%) for the N-Alkylation a | MY | Yield (%) for the Anion Metathesis b |
|---|---|---|---|---|
| 1 | HO(CH2)2Br | 82 | ||
| 7 | NaBF4 | 97 | ||
| 8 | KPF6 | 98 | ||
| 9 | NaOOCCF3 | 96 | ||
| 2 | HO(CH2)3Br | 85 | ||
| 10 | NaBF4 | 97 | ||
| 11 | KPF6 | 99 | ||
| 12 | NaOOCCF3 | 97 | ||
| 3 | CH3O(CH2)2Br | 81 | ||
| 13 | NaBF4 | 94 | ||
| 14 | KPF6 | 92 | ||
| 15 | NaOOCCF3 | 94 | ||
| 4 | CH3CH2O(CH2)2Cl | 79 | 94 | |
| 16 | NaBF4 | 93 | ||
| 17 | KPF6 | 95 | ||
| 18 | NaOOCCF3 | 92 | ||
| 5 | PhO(CH2)4Br | 83 | 93 | |
| 19 | NaBF4 | 94 | ||
| 20 | KPF6 | 94 | ||
| 21 | NaOOCCF3 | 93 | ||
| 6 | NC(CH2)3Cl | 78 | ||
| 22 | NaBF4 | 92 | ||
| 23 | KPF6 | 93 | ||
| 24 | NaOOCCF3 | 92 |
2.2. Antimicrobial Activity
| Compd | Antifungal Activity | Antibacterial Activity | ||||||
|---|---|---|---|---|---|---|---|---|
| A. fumigatus | S. racemosum | G. candidum | C. albicans | S. pneumoniae | B. subtilis | P. aeruginosa | E. coli | |
| 1 | 11.9 | 13.2 | 14.4 | 11.6 | 11.2 | 13.6 | 15.8 | 16.2 |
| 2 | 19.3 | 20.4 | 17.7 | 15.6 | 16.5 | 18.7 | 15.2 | 18.4 |
| 3 | 12.2 | 13.1 | 13.9 | 10.8 | 11.6 | 12.3 | 10.1 | 10.9 |
| 4 | NA | NA | NA | NA | NA | NA | NA | NA |
| 5 | 23.4 | 22.3 | 28.1 | 26.3 | 23.2 | 27.4 | 22.3 | 23.2 |
| 6 | 11.1 | 12.1 | 12.9 | 10.3 | 12.4 | 12.9 | 10.2 | 11.9 |
| 7 | NA | NA | NA | NA | NA | NA | NA | NA |
| 8 | NA | NA | NA | NA | NA | NA | NA | NA |
| 9 | NA | NA | NA | NA | NA | NA | NA | NA |
| 10 | NA | NA | NA | NA | NA | NA | NA | NA |
| 11 | 14.3 | 15.9 | 17.3 | 13.1 | 18.3 | 20.1 | 14.6 | 16.2 |
| 12 | 21.3 | 20.2 | 22.1 | 19.6 | 20.4 | 21.3 | 17.3 | 20.6 |
| 13 | 18.3 | 19.6 | 18.2 | 17.3 | 18.1 | 19.6 | 15.2 | 17.3 |
| 14 | 16.3 | 17.8 | 19.2 | 16.7 | 17.4 | 18.3 | 17.2 | 20.9 |
| 15 | NA | NA | NA | NA | NA | NA | NA | NA |
| 16 | 18.3 | 19.3 | 21.2 | 15.7 | 18.4 | 19.2 | 16.7 | 17.3 |
| 17 | 16.3 | 18.2 | 19.4 | 14.6 | 17.3 | 18.2 | 15.3 | 14.6 |
| 18 | 18.2 | 19.6 | 15.6 | 13.9 | 14.5 | 16.2 | 13.3 | 16.5 |
| 19 | 24.2 | 23.3 | 29.2 | 27.3 | 24.6 | 28.3 | 24.2 | 24.9 |
| 20 | 16.3 | 18.2 | 20.1 | 14.3 | 19.3 | 20.4 | 16.2 | 20.3 |
| 21 | 15.3 | 16.9 | 19.2 | 13.4 | 18.2 | 16.8 | 15.9 | 17.5 |
| 22 | NA | NA | NA | NA | NA | NA | NA | NA |
| 23 | NA | NA | NA | NA | NA | NA | NA | NA |
| 24 | NA | NA | NA | NA | NA | NA | NA | NA |
| Amphotericin B | 20.4 | 17.3 | 26.3 | 22.0 | --- | --- | --- | --- |
| Ampicillin | --- | --- | --- | --- | 20.8 | 26.7 | --- | --- |
| Gentamicin | --- | --- | --- | --- | --- | --- | 16.1 | 18.3 |
Minimum Inhibitory Concentration (MIC)
| Compd | Antifungal Activity | Antibacterial Activity | ||||||
|---|---|---|---|---|---|---|---|---|
| A. fumigatus | S. racemosum | G. candidum | C. albicans | S. pneumoniae | B. subtilis | P. aeruginosa | E. coli | |
| 2 | 3.9 | 3.9 | 7.81 | 31.25 | 15.63 | 3.9 | 62.5 | 7.81 |
| 5 | 0.98 | 0.98 | 0.24 | 0.49 | 0.98 | 0.49 | 1.95 | 0.98 |
| 12 | 1.95 | 3.9 | 0.98 | 3.9 | 3.9 | 1.95 | 15.63 | 1.95 |
| 13 | 7.81 | 3.9 | 7.81 | 15.63 | 7.81 | 3.9 | 125 | 15.63 |
| 14 | 31.25 | 7.81 | 3.9 | 15.63 | 15.63 | 7.81 | 15.63 | 1.95 |
| 16 | 7.81 | 3.9 | 1.95 | 62.5 | 7.81 | 0.49 | 31.25 | 7.81 |
| 19 | 0.49 | 0.98 | 0.49 | 0.49 | 0.49 | 0.24 | 0.49 | 0.24 |
| 20 | 31.25 | 7.81 | 3.9 | 125 | 3.9 | 3.9 | 31.25 | 3.9 |
| Amphotericin B | 3.9 | 15.63 | 0.49 | 0.98 | --- | --- | --- | --- |
| Ampicillin | --- | --- | --- | --- | 3.9 | 0.49 | --- | --- |
| Gentamicin | --- | --- | --- | --- | --- | --- | 31.25 | 7.81 |
3. Experimental Section
3.1. Apparatus
3.2. Synthesis
3.3. Characterization
3.4. Determination of Minimum Inhibitory Concentrations
4. Conclusions
Supplementary Materials
Acknowledgement
Conflicts of Interest
References
- Earle, M.J.; Esperanca, J.M.S.; Gilea, M.A.; Lopes, J.N.C.; Rebelo, L.P.N.; Magee, J.W.; Seddon, K.R.; Widegren, J.A. The distillation and volatility of ionic liquids. Nature 2006, 439, 831–834. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.Z.; Gui, J.S.; Lv, X.M.; Zhang, G.Q.; Li, H.W. Study on properties of ionic liquid BMIBF4. Acta Chim. Sin. 2005, 63, 577–580. [Google Scholar]
- Ui, K.; Yamamoto, K.; Ishikawa, K.; Minami, T.; Takeuchi, K.; Itagaki, M.; Watanabe, K.; Koura, N. Development of non-flammeable lithium secondary battery with room-temperature ionic liquid electrolyte: Performance of electroplated Al film negative electrode. J. Power Sources 2008, 183, 347–350. [Google Scholar] [CrossRef]
- Kubota, K.; Nohira, T.; Goto, T.; Hagiwara, R. Novel inorganic ionic liquids possessing low melting temperatures and wide electro-chemical windows: Binary mixtures of alkali bis(fluorosulfonyl) amides. Electrochem. Commun. 2008, 10, 1886–1888. [Google Scholar] [CrossRef]
- Ahrens, S.; Peritz, A.; Strassner, T. Tunable arylalkyl ionic liquids (TAAILs): The next generation of ionic liquids. Angew. Chem. Int. Ed. Engl. 2009, 48, 7908–7910. [Google Scholar] [CrossRef] [PubMed]
- Marisa, C.B.; Russell, G.E.; Richard, G.C. Non-haloaluminate room-temperature ionic liquids in electrochemistry—A review. Chem. Phys. Chem. 2004, 5, 1106–1120. [Google Scholar]
- Anderson, J.L.; Armstrong, D.W. High-stability ionic liquids, a new class of stationary phases for gas chromatography. Anal. Chem. 2003, 75, 4851–4858. [Google Scholar] [CrossRef] [PubMed]
- Docherty, K.M.; Kulpa, C.F., Jr. Toxicity and antimicrobial activity of imidazolium and pyridinium ionic liquids. Green Chem. 2005, 7, 185–189. [Google Scholar] [CrossRef]
- Procuranti, B.; Myles, L.; Gathergood, N.; Connon, S.J. Pyridinium ion catalysis of carbonyl protection reactions. Synthesis 2009, 23, 4082–4086. [Google Scholar]
- Myles, L.; Gore, R.; Spulak, M.; Gathergood, N.; Connon, S.J. Highly recyclable, imidazolium derived ionic liquids of low antimicrobial and antifungal toxicity: A new strategy for acid catalysis. Green Chem. 2010, 12, 1157–1162. [Google Scholar] [CrossRef]
- Endres, F. Ionic liquids: Solvents for the electrodeposition of metals and semiconductors. Chem. Phys. Chem. 2002, 3, 144–154. [Google Scholar]
- Zhang, Q.H.; Zhang, S.G.; Deng, Y.Q. Recent advances in ionic liquid catalysis. Green Chem. 2011, 13, 2619–2637. [Google Scholar] [CrossRef]
- Ferlin, N.; Courty, M.; Gatard, S.; Spulak, M.; Quilty, B.; Beadham, I.; Ghavre, M.; Haiß, A.; Kümmerer, K.; Gathergood, N.; et al. Biomass derived ionic liquids: Synthesis from natural organic acids, characterization, toxicity, biodegradation and use as solvents for catalytic hydrogenation processes. Tetrahedron 2013, 69, 6150–6161. [Google Scholar] [CrossRef]
- Moniruzzaman, M.; Nakashima, K.; Kamiya, N.; Goto, M. Recent advances of enzymatic reactions in ionic liquids. Biochem. Eng. J. 2010, 48, 295–314. [Google Scholar] [CrossRef]
- Messali, M. A green microwave-assisted synthesis, characterization and comparative study of new pyridazinium-based ionic liquids derivatives towards corrosion of mild steel in acidic environment. J. Mater. Environ. Sci. 2011, 2, 174–185. [Google Scholar]
- Messali, M.; Bousskri, A.; Anejjar, A.; Salghi, R.; Hammouti, B. Electrochemical Studies of New Pyridazinium-Based Ionic Liquid, (1–4-Nitro Phenyl-1-ethanone) Pyridazinium bromide, On Carbon Steel Corrosion in Hydrochloric Acid Medium. Int. J. Electrochem. Sci. 2015, 10, 4532–4551. [Google Scholar]
- Biswas, A.; Shogren, R.L.; Stevenson, D.G.; Willett, J.L.; Bhowmik, P.K. Ionic liquids as solvents for biopolymers: Acylation of starch and zein protein. Carbohydr. Polym. 2006, 66, 546–550. [Google Scholar] [CrossRef]
- Pernak, J.; Sobaszkiewicz, K.; Mirska, I. Anti-microbial activities of ionic liquids. Green Chem 2003, 5, 52–56. [Google Scholar] [CrossRef]
- Carson, L.; Chau, P.K.W.; Earle, M.J.; Gilea, M.A.; Gilmore, B.F.; Gorman, S.P.; McCann, M.T.; Seddon, K.R. Antibiofilm activities of 1-alkyl-3-methylimidazolium chloride ionic liquids. Green Chem. 2009, 11, 492–497. [Google Scholar] [CrossRef]
- Messali, M. An efficient and green sonochemical synthesis of some new eco-friendly functionalized ionic liquids. Arab. J. Chem. 2014, 7, 63–70. [Google Scholar] [CrossRef]
- Messali, M.; Ahmed, S.A. A green microwave-assisted synthesis of new pyridazinium-based ionic liquids as an environmentally friendly alternative. Green Sustain. Chem. 2011, 1, 70–75. [Google Scholar] [CrossRef]
- Messali, M.; Aouad, M.R.; El-Sayed, W.S.; Ali, A.A.; Ben Hadda, T.; Hammouti, B. New eco-friendly 1-alkyl-3-(4-phenoxybutyl) imidazolium-based ionic liquids derivatives: A green ultrasound-assisted Synthesis, characterization, antimicrobial activity and POM analyses. Molecules 2014, 19, 11741–11759. [Google Scholar] [CrossRef] [PubMed]
- Messali, M.; Aouad, M.R.; Ali, A.A.; Ben Hadda, T.; Hammouti, B. Synthesis, characterization, and POM analyses of novel bioactive imidazolium-based ionic liquids. Med. Chem. Res. 2015, 24, 1387–1395. [Google Scholar] [CrossRef]
- Katritzky, A.R.; Mokrosz, M.J. The preparation of some 1-vinylpyridinium salts. Heterocycles 1984, 22, 505–512. [Google Scholar] [CrossRef]
- European Committee for Antimicrobial Susceptibility Testing (EUCAST) of the European Society of Clinical Microbiology and Infectious Diseases (ESCMID). Determination of minimum inhibitory concentrations (MICs) of antibacterial agents by agar dilution. Clin. Microbiol. Infect. 2000, 6, 509–515. [Google Scholar]
- National Committee for Clinical Laboratory Standards. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically Approved StandardM7-A5, 5th ed.; NCCLS: Wayne, PA, USA, 2000. [Google Scholar]
- Clinical and Laboratory Standards Institute (CLSI). Document M26-A. In Methods of Determining Bactericidal Activity of Antimicrobial Agents for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically; Approved Guideline; CLSI: Wayne, PA, USA, 1999. [Google Scholar]
- Nomura, H.; Isshiki, Y.; Sakuda, K.; Sakuma, K.; Kondo, S. The Antibacterial Activity of Compounds Isolated from Oakmoss against Legionella pneumophila and Other Legionella spp. Biol. Pharm. Bull. 2012, 35, 1560–1567. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds 1–24 are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Messali, M. Eco-Friendly Synthesis of a New Class of Pyridinium-Based Ionic Liquids with Attractive Antimicrobial Activity. Molecules 2015, 20, 14936-14949. https://doi.org/10.3390/molecules200814936
Messali M. Eco-Friendly Synthesis of a New Class of Pyridinium-Based Ionic Liquids with Attractive Antimicrobial Activity. Molecules. 2015; 20(8):14936-14949. https://doi.org/10.3390/molecules200814936
Chicago/Turabian StyleMessali, Mouslim. 2015. "Eco-Friendly Synthesis of a New Class of Pyridinium-Based Ionic Liquids with Attractive Antimicrobial Activity" Molecules 20, no. 8: 14936-14949. https://doi.org/10.3390/molecules200814936
APA StyleMessali, M. (2015). Eco-Friendly Synthesis of a New Class of Pyridinium-Based Ionic Liquids with Attractive Antimicrobial Activity. Molecules, 20(8), 14936-14949. https://doi.org/10.3390/molecules200814936





