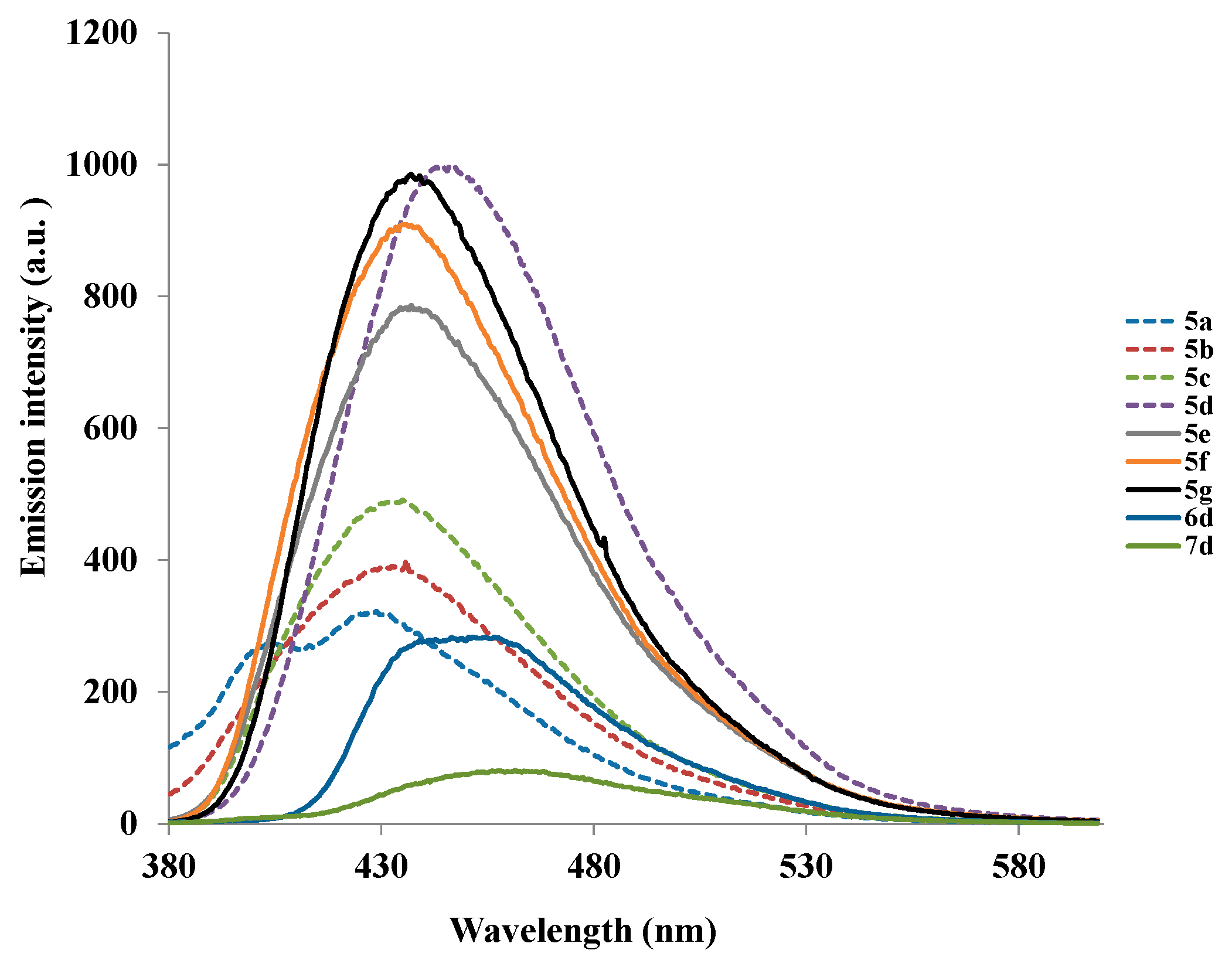
3.3. Typical Procedure for the Synthesis of the 2-Aryl-6-iodoquinazolin-4(3H)-ones 2a–2d
3.3.1. 6-Iodo-2-phenylquinazolin-4(3H)-one (2a)
A stirred mixture of 1 (1.00 g, 3.81 mmol), benzaldehyde (0.48 g, 4.57 mmol) and iodine (1.93 g, 7.62 mmol) in ethanol (100 mL) was heated under reflux for 8 h and then allowed to cool to room temperature. An ice-cold saturated aqueous solution of sodium thiosulphate was added to the mixture and the resultant precipitate was filtered and washed thoroughly with cold water. The product was recrystallized to afford 2a as a white solid (1.11 g, 83%), mp. >345 °C (acetonitrile); νmax (ATR) 542, 624, 698, 777, 834, 1284, 1460, 1562, 1595, 1667, 2853, 2955 cm−1; δH (500 MHz, DMSO-d6) 7.52 (d, J = 8.5 Hz, 1H), 7.54 (t, J = 7.5 Hz, 2H), 7.59 (d, J = Hz, 1H), 8.11 (dd, J = 2.0 and 8.5 Hz, 1H), 8.17 (d, J = 7.0 Hz, 2H), 8.41 (d, J = 2.0 Hz, 1H), 12.69 (brs, 1H); δC (125 MHz, DMSO-d6) 91.3, 123.4, 128.3, 128.9, 130.0, 131.8, 133.8, 134.7, 142.9, 148.8, 154.4, 162.5; m/z 349 (100, MH+); HRMS (ES): MH+, found 348.9833. C14H10IN2O+ requires 348.9760.
3.3.2. 2-(4-Fluorophenyl)-6-iodoquinazolin-4(3H)-one (2b)
A mixture of 1 (1.00 g, 3.81 mmol), 4-fluorobenzaldehyde (0.57 g, 4.57 mmol) and iodine (1.93 g, 7.62 mmol) in ethanol (100 mL) afforded 2b as a white solid (1.30 g, 93%), mp. 337–339 °C (acetonitrile); νmax (ATR) 538, 648, 737, 830, 840, 942, 1154, 1460, 1514, 1572, 1667, 2867, 2926 cm−1; δH (500 MHz, DMSO-d6) 7.39 (t, J = 9.0 Hz, 2H), 7.51 (d, J = 9.0 Hz, 1H), 8.11 (dd, J = 2.0 and 8.5 Hz, 1H), 8.24 (t, J = 9.0 Hz. 2H), 8.40 (d, J = 2.0 Hz, 1H), 12.72 (s, 1H); δC (125 MHz, DMSO-d6) 91.9, 116.1 (d, 2JCF = 21.8 Hz), 123.1, 129.5, 130.0 (d, 2JCF = 3.7 Hz), 130.9 (d, 3JCF = 8.5 Hz) 134.6, 143.3, 148.3, 152.5, 161.4, 164.6 (d, 1JCF = 250.5 Hz); m/z 367 (100, MH+); HRMS (ES): MH+, found 366.9744. C14H9FIN2O+ requires 366.9747.
3.3.3. 2-(4-Chlorophenyl)-6-iodoquinazolin-4(3H)-one (2c)
A mixture of 1 (1.00 g, 3.81 mmol), 4-chlorobenzaldehyde (0.64 g, 4.57 mmol) and iodine (1.93 g, 7.62 mmol) in ethanol (100 mL) afforded 2c as a white solid (1.31 g, 89%), mp. >345 °C (acetonitrile); νmax (ATR) 541, 622, 644, 730, 830, 841, 940, 1091, 1459, 1473, 1556, 1595, 1667, 2922, 3038 cm−1; δH (500 MHz, DMSO-d6) 7.51 (d, J = 8.5 Hz, 1H), 7.61 (d, J = 8.5 Hz, 2H), 8.10 (dd, J = 2.0 and 8.5 Hz, 1H), 8.19 (d, J = 8.5 Hz, 2H), 8.40 (d, J = 2.0 Hz, 1H), 12.74 (br s, 1H); δC (125 MHz, DMSO-d6) 92.0, 123.3, 129.1, 130.0, 130.2, 132.1, 134.7, 136.8, 143.3, 148.4, 152.8, 161.0; m/z 383 (100, MH+); HRMS (ES): MH+, found 382.9448. C14H935ClIN2O + requires 382.9370.
3.3.4. 6-Iodo-2-(4-methoxyphenyl)quinazolin-4(3H)-one (2d)
A mixture of 1 (1.00 g, 3.81 mmol), 4-methoxybenzaldehyde (0.62 g, 4.57 mmol) and iodine (1.93 g, 7.62 mmol) in ethanol (20 mL) afforded 2d as a white solid (1.42 g, 98%), mp. 321–323 °C (acetonitrile); νmax (ATR) 541, 649, 742, 831, 841, 939, 1027, 1250, 1459, 1517, 1594, 1662, 2852, 2922 cm−1; δH (500 MHz, DMSO-d6) 3.84 (s, 3H), 7.07 (d, J = 9.0 Hz, 2H), 7.47 (d, J = 9.0 Hz, 1H), 8.06 (dd, J = 2.0 and 8.5 Hz, 1H), 8.17 (d, J = 8.5 Hz, 2H), 8.37 (d, J = 2.0 Hz, 1H), 12.50 (br s, 1H); δC (125 MHz, DMSO-d6) 55.9, 91.1, 114.5, 123.0, 125.3, 129.8, 130.0, 134.6, 143.1, 148.7, 153.3, 161.8, 162.4; m/z 379 (100, MH+); HRMS (ES): MH+, found 378.9943. C15H12IN2O2+ requires 378.9865.
3.4. Typical Procedure for the Synthesis of the 2-Aryl-4-chloro-6-iodoquinazolines 3a–d
3.4.1. 4-Chloro-6-iodo-2-phenylquinazoline (3a)
A stirred mixture of 2a (1.00 g, 2.87 mmol) and phosphoryl chloride (10 mL) was treated dropwise with triethylamine (4 mL) at room temperature. The mixture was then heated under reflux for 6 h and allowed to cool to room temperature. An ice-cold water was added to the mixture and the aqueous layer was extracted with chloroform. The combined organic layers were washed with water and then dried over anhydrous MgSO4, filtered and evaporated under reduced pressure to afford 3a as a brown solid (0.91 g, 86%), mp. 148–150 °C (ethanol); νmax (ATR) 532, 663, 685, 703, 830, 866, 985, 1295, 1410, 1469, 1535, 1555 cm−1; δH (500 MHz, CDCl3) 7.52–7.55 (m, 3H), 7.81 (d, J = 8.5 Hz, 1H), 8.17 (dd, J = 1.5 and 8.5 Hz, 1H), 8.57–8.59 (m, 2H), 8.63 (d, J = 1.5 Hz, 1H); δC (125 MHz, CDCl3) 93.3, 123.8, 128.7, 128.8, 130.3, 131.4, 134.6, 136.2, 143.6, 150.8, 160.4, 160.9; m/z 367 (100, MH+); HRMS (ES): MH+, found 366.9498. C14H935ClIN2+ requires 366.9499.
3.4.2. 4-Chloro-2-(4-fluorophenyl)-6-iodoquinazoline (3b)
A mixture of 2b (1.00 g, 2.60 mmol), phosphoryl chloride (10 mL) and NEt3 (4 mL) afforded 3b as a white solid (1.00 g, 78%), mp. 192–193 °C (ethanol); νmax (ATR) 548, 648, 687, 736, 826, 845, 986, 1156, 1214, 1298, 1343, 1410, 1553, 1595 cm−1; δH (500 MHz, CDCl3) 7.21 (t, J = 9.0 Hz, 2H), 7.83 (d, J = 9.0 Hz, 1H), 8.17 (dd, J = 2.0 and 8.5 Hz, 1H), 8.59 (t, J = 9.0 Hz, 2H), 8.63 (d, J = 2.0 Hz, 1H); δC (125 MHz, CDCl3) 93.3, 115.7 (d, 2JCF = 21.8 Hz), 123.7, 130.2, 131.0 (d, 3JCF = 8.5 Hz), 132.5 (d, 4JCF = 3.7 Hz), 134.6, 143.7, 150.8, 159.4, 160.9, 165.1 (d, 1JCF = 250.2 Hz); m/z 385 (100, MH+); HRMS (ES): MH+, found 384.9398. C14H835ClFIN2+ requires 384.9405.
3.4.3. 4-Chloro-2-(4-chlorophenyl)-6-iodoquinazoline (3c)
A mixture of 2c (1.00 g, 2.61 mmol), phosphoryl chloride (10 mL) and NEt3 (4 mL) afforded 3c as a white solid (0.78 g, 74.5%), mp. 207–209 °C (ethanol); νmax (ATR) 533, 732, 829, 878, 1088, 1295, 1343, 1411, 1534, 1552, 1592 cm−1; δH (500 MHz, CDCl3) 7.50 (d, J = 8.5 Hz, 2H), 7.80 (d, J = 9.0 Hz, 1H), 8.18 (dd, J = 2.0 and 8.5 Hz, 1H), 8.53 (d, J = 9.0 Hz, 2H), 8.63 (d, J = 2.0 Hz, 1H); δC (125 MHz, CDCl3) 93.6, 123.8, 128.9, 130.1, 130.3, 134.6, 134.7, 137.7, 143.8, 150.8, 159.4, 161.0; m/z 401 (100, MH+); HRMS (ES): MH+, found 400.9106. C14H835Cl2IN2+ requires 400.9109.
3.4.4. 4-Chloro-6-iodo-2-(4-methoxyphenyl)quinazoline (3d)
A mixture of 2d (1.00 g, 2.64 mmol), phosphoryl chloride (10 mL) and NEt3 (4 mL) afforded 3d as a yellow solid (0.87 g, 82%), mp. 165–167 °C (ethanol); νmax (ATR) 523, 562, 647, 736, 783, 828, 844, 870, 984, 1026, 1166, 1295, 1411, 1466, 1553, 1595 cm−1; δH (500 MHz, CDCl3) 3.91 (s, 3H), 7.03 (d, J = 9.0 Hz, 2H), 7.76 (d, J = 8.5 Hz, 1H), 8.13 (dd, J = 2.0 and 8.5 Hz, 1H), 8.53 (d, J = 9.0 Hz, 2H), 8.59 (d, J = 2.0 Hz, 1H); δC (125 MHz, CDCl3) 55.4, 92.5, 114.0, 123.4, 128.9, 130.1, 130.5, 134.5, 143.4, 151.0, 160.2, 160.7, 162.4; m/z 397 (100, MH+); HRMS (ES): MH+, found 396.9691. C15H1135ClIN2O+ requires 396.9605.
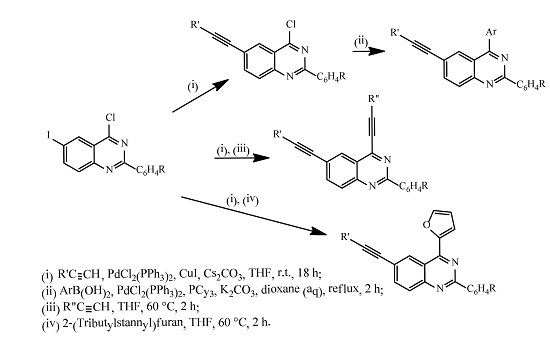
3.5. Typical Procedure for Site-Selective Sonogashira Cross-Coupling of 3a–d with Terminal Alkynes
3.5.1. 4-Chloro-2-phenyl-6-(phenylethynyl)quinazoline (4a)
A stirred mixture of 3a (0.30 g, 0.82 mmol), PdCl2(PPh3)2 (0.057 g, 0.082 mmol), CuI (0.008 g, 0.041 mmol) and Cs2CO3 (0.40 g, 1.23 mmol) in THF (15 mL) was purged with argon gas for 30 min. Phenylacetylene (0.10 g, 0.98 mmol) was added to the mixture using a syringe. The reaction mixture was stirred at room temperature for 24 h and then quenched with an ice-cold water. The product was extracted into chloroform and the combined organic layers were washed with water, dried over Na2SO4, filtered and evaporated under reduced pressure. The residue was recrystallized to afford 4a as a yellow solid (0.26 g, 93%), Rf (2:1 hexane/toluene) 0.36, mp. 164–165 °C; νmax (ATR) 526, 649, 686, 706, 754, 848, 1327, 1361, 1413, 1556, 2208 cm−1; δH (500 MHz, CDCl3) 7.40–7.41 (m, 3H), 7.53–7.54 (m, 3H), 7.60–7.65 (m, 2H), 8.01 (dd, J = 1.5 and 9.0 Hz, 1H), 8.06 (d, J = 9.0 Hz, 1H), 8.41 (d, J = 1.5 Hz, 1H), 8.59–7.70 (m, 2H); δC (125 MHz, CDCl3) 88.2, 92.3, 122.4, 122.5, 123.5, 128.5, 128.6, 128.7, 128.8, 128.9, 129.0, 131.4, 131.8, 136.5, 137.4, 151.2, 160.5, 162.0; m/z 341 (100, MH+); HRMS (ES): MH+, found 341.0838. C22H1435ClN2+ requires 341.0846.
3.5.2. 4-Chloro-2-(4-fluorophenyl)-6-(phenylethynyl)quinazoline (4b)
A mixture of 3b (0.30 g, 0.78 mmol), PdCl2(PPh3)2(0.054 g, 0.078 mmol), CuI (0.007 g, 0.039 mmol), Cs2CO3 (0.38 g, 1.17 mmol) and phenylacetylene (0.096 g, 0.94 mmol) in THF (15 mL) afforded 4b as a yellow solid (0.25 g, 89%), Rf (2:1 hexane/toluene) 0.47, mp. 189–191 °C; νmax (ATR) 524, 600, 682, 751, 841, 995, 1160, 1222, 1366, 1414, 1564, 2214 cm−1; δH (500 MHz, CDCl3) 7.21 (t, J = 8.5 Hz, 2H), 7.39–7.41 (m, 3H), 7.60–7.62 (m, 2H), 8.01 (dd, J = 1.5 and 8.5 Hz, 1H), 8.03 (d, J = 8.5 Hz, 1H), 8.40 (d, J = 1.5 Hz, 1H), 8.61 (t, J = 8.5 Hz, 2H); δC (125 MHz, CDCl3) 88.2, 92.4, 115.7 (d, 2JCF = 21.7 Hz), 122.3, 122.4, 123.5, 128.5, 128.8, 128.9, 129.0, 131.0 (d, 3JCF = 8.5 Hz), 131.8, 132.6 (d, 4JCF = 3.8 Hz), 137.6, 151.2, 159.5, 162.1, 165.1 (d, 1JCF = 250.2 Hz); m/z 359 (100, MH+); HRMS (ES): MH+, found 359.0751. C22H1335ClFN2+ requires 359.0751.
3.5.3. 4-Chloro-2-(4-chlorophenyl)-6-(phenylethynyl)quinazoline (4c)
A mixture of 3c (0.30 g, 0.75 mmol), PdCl2(PPh3)2(0.052 g, 0.074 mmol), CuI (0.007 g, 0.037 mmol), Cs2CO3 (0.36 g, 1.12 mmol) and phenylacetylene (0.091 g, 0.90 mmol) in THF (15 mL) afforded 4c as a yellow solid (0.22 g, 78%), Rf (2:1 hexane/toluene) 0.56, mp. 214–215 °C; νmax (ATR) 523, 680, 747, 836, 1088, 1364, 1404, 1417, 1538, 1552, 2211; cm−1; δH (500 MHz, CDCl3) 7.41–7.42 (m, 3H), 7.51 (d, J = 9.0 Hz, 2H), 7.60–7.62 (m, 2H), 8.03 (dd, J = 1.5 and 8.5 Hz, 1H), 8.07 (d, J = 8.5 Hz, 1H), 8.42 (d, J = 1.5 Hz, 1H), 8.56 (d, J = 9.0 Hz, 2H); δC (125 MHz, CDCl3) 88.1, 92.5, 122.4, 122.4, 123.7, 128.5, 128.7, 128.9, 129.0, 129.1, 130.1, 131.8, 134.9, 137.6, 137.7, 151.1, 159.4, 162.1; m/z 375 (100, MH+); HRMS (ES): MH+, found 375.0468. C22H1335Cl2N2+ requires 375.0456.
3.5.4. 4-Chloro-2-(4-methoxyphenyl)-6-(phenylethynyl)quinazoline (4d)
A mixture of 3d (0.30 g, 0.75 mmol), PdCl2(PPh3)2 (0.053 g, 0.075 mmol), CuI (0.007 g, 0.037 mmol), Cs2CO3 (0.36 g, 1.12 mmol) and phenylacetylene (0.091 g, 0.90 mmol) in THF (15 mL) afforded 4d as a yellow solid (0.21 g, 75%), Rf (1:1 hexane/toluene) 0.50, mp. 170–172 °C; νmax (ATR) 526, 564, 606, 747, 789, 831, 849, 995, 1028, 1173, 1246, 1364, 1413, 1553, 2209 cm−1; δH (500 MHz, CDCl3) 3.90 (s, 3H), 7.03 (d, J = 8.5 Hz, 2H), 7.40–7.41 (m, 3H), 7.60 (d, J = 3.5 Hz, 2H), 7.96 (dd, J = 1.5 and 8.5 Hz, 1H), 7.99 (d, J = 8.5 Hz, 1H), 8.36 (d, J = 1.5 Hz, 1H), 8.54 (d, J = 8.5 Hz, 2H); δC (125 MHz, CDCl3) 55.4, 88.3, 92.0, 114.0, 122.0, 122.6, 122.8, 128.5, 128.7, 128.8, 128.9, 129.1, 130.6, 131.8, 137.3, 151.4, 160.3, 161.8, 162.4; m/z 371 (100, MH+); HRMS (ES): MH+, found 371.0943. C23H1635ClN2O+ requires 341.0951.
3.5.5. 4-(4-Chloro-2-phenylquinazolin-6-yl)but-3-yn-1-ol (4e)
A mixture of 3a (0.30 g, 0.82 mmol), PdCl2(PPh3)2 (0.057g, 0.082 mmol), CuI (0.008 g, 0.041 mmol), Cs2CO3 (0.40 g, 1.23 mmol) and 3-butyn-1-ol (0.068 g, 0.98 mmol) in THF (20 mL) afforded 4e as a yellow solid (0.23 g, 90%), Rf (2:1 hexane/ethyl acetate) 0.37, mp. 139–141 °C; νmax (ATR) 554, 687, 705, 767, 836, 991, 1062, 1211, 1306, 1361, 1417, 1557, 2235, 3218 cm−1; δH (500 MHz, CDCl3) 1.91 (s, 1H), 2.78 (t, J = 6.5 Hz, 2H), 3.90 (dt, J = 6.5 and 8.5 Hz, 2H), 7.52–7.54 (m, 3H), 7.88 (dd, J = 1.5 and 8.5 Hz, 1H), 8.00 (d, J = 8.5 Hz, 1H), 8.28 (d, J = 1.5 Hz, 1H), 8.57–8.59 (m, 2H); δC (125 MHz, CDCl3) 23.9, 61.0, 81.3, 89.9, 122.3, 123.6, 128.6, 128.7, 128.8, 128.9, 131.3, 136.5, 137.6, 151.1, 160.4, 161.9; m/z 309 (100, MH+); HRMS (ES): MH+, found 309.0789. C18H1435ClN2O+ requires 309.0795.
3.5.6. 4-(4-Chloro-2-(4-fluorophenyl)quinazolin-6-yl)but-3-yn-1-ol (4f)
A mixture of 3b (0.30 g, 0.78 mmol), PdCl2(PPh3)2(0.054 g, 0.078 mmol), CuI (0.007 g, 0.039 mmol), Cs2CO3 (0.38 g, 1.17 mmol) and 3-butyn-1-ol (0.066 g, 0.94 mmol) in THF (15 mL) afforded 4f as a yellow solid (0.23 g, 90%), Rf (2:1 hexane/ethyl acetate) 0.39, mp. 180–181 °C; νmax (ATR) 557, 647, 740, 748, 840, 986, 1064, 1150, 1221, 1307, 1361, 1410, 1557, 2235, 3196 cm−1; δH (500 MHz, CDCl3) 1.84 (br s, 1H), 2.79 (t, J = 6.5 Hz, 2H), 3.90 (t, J = 6.5 Hz, 2H), 7.20 (t, J = 9.0 Hz, 2H), 7.90 (dd, J = 1.5 and 8.5 Hz, 1H), 7.98 (J = 8.5 Hz, 1H), 8.29 (d, J = 1.5 Hz, 1H), 8.60 (t, J = 9.0 Hz, 2H); δC (125 MHz, CDCl3) 23.9, 61.0, 81.2, 89.9, 115.7 (d, 2JCF = 20.8 Hz), 122.1, 123.5, 128.8, 128.9, 130.9 (d, 3JCF = 8.5 Hz), 132.6 (d, 4JCF = 3.7 Hz), 137.7, 151.0, 159.3, 161.9, 165.0 (d, 1JCF = 250.3 Hz); m/z 327 (100, MH+); HRMS (ES): MH+, found 327.0694. C18H1335ClFN2O+ requires 327.0700.
3.5.7. 4-(4-Chloro-2-(4-chlorophenyl)quinazolin-6-yl)but-3-yn-1-ol (4g)
A mixture of 3c (0.30 g, 0.75 mmol), PdCl2(PPh3)2(0.052 g, 0.075 mmol), CuI (0.007 g, 0.037 mmol), Cs2CO3 (0.36 g, 1.12 mmol) and 3-butyn-1-ol (0.062 g, 0.89 mmol) in THF (15 mL) afforded 4g as a yellow solid (0.22 g, 85%), Rf (2:1 hexane/ethyl acetate) 0.40, mp. 199–201 °C; νmax (ATR) 556, 647, 701, 735, 840, 991, 1011, 1064, 1091, 1360, 1403, 1418, 1539, 2233, 3213 cm−1; δH (500 MHz, CDCl3) 1.84 (t, J = 6.0 Hz, 1H), 2.79 (t, J = 6.5 Hz, 2H), 3.90 (q, J = 6.0 Hz, 2H), 7.50 (d, J = 8.0 Hz, 2H), 7.90 (dd, J = 1.5 and 8.5 Hz, 1H), 7.99 (J = 8.5 Hz, 1H), 8.30 (d, J = 1.5 Hz, 1H), 8.53 (d, J = 8.5 Hz, 2H); δC (125 MHz, CDCl3) 23.9, 61.0, 81.3, 90.0, 115.6, 115.8, 122.2, 123.6, 128.8, 130.9, 131.0, 132.6, 137.7, 151.0, 159.4, 162.0; m/z 343 (100, MH+); HRMS (ES): MH+, found 343.0402. C18H1335Cl2N2O+ requires 343.0405.
3.5.8. 4-(4-Chloro-2-(4-methoxyphenyl)quinazolin-6-yl)but-3-yn-1-ol (4h)
A mixture of 3d (0.30 g, 0.75 mmol), PdCl2(PPh3)2(0.053 g, 0.075 mmol), CuI (0.007 g, 0.037 mmol), Cs2CO3 (0.36 g, 1.12 mmol) and 3-butyn-1-ol (0.063 g, 0.90 mmol) in THF (15 mL) afforded 4h as a yellow solid (0.21 g, 82%), Rf (2:1 hexane/ethyl acetate) 0.37, mp. 159–160 °C; νmax (ATR) 596, 744, 789, 836, 850, 988, 1033, 1061, 1163, 1173, 1247, 1311, 1361, 1416, 1551, 2230, 3285 cm−1; δH (500 MHz, CDCl3) 1.85 (t, J = 6.0 Hz, 1H), 2.78 (t, J = 6.5, 2H), 3.89 (t, J = 6.5 Hz, 2H), 3.91 (s, 3H), 7.03 (d, J = 9.5 Hz, 2H), 7.86 (dd, J = 1.5 and 8.5 Hz, 1H), 7.95 (d, J = 8.5 Hz, 1H), 8.27 (d, J = 1.5 Hz, 1H), 8.54 (d, J = 9.0 Hz, 2H); δC (125 MHz, CDCl3) 23.9, 55.4, 61.1, 81.4, 89.5, 114.1, 121.9, 122.9, 128.7, 128.8, 129.1, 130.5, 137.6, 151.2, 160.2, 161.8, 162.4; m/z 339 (100, MH+); HRMS (ES): MH+, found 339.0894. C19H1635ClN2O2+ requires 339.0900.
3.6. Typical Procedure for the Suzuki-Miyaura-Miyaura Cross-Coupling of 4a–h
3.6.1. 4-(4-Fluorophenyl)-2-phenyl-6-(phenylethynyl)quinazoline (5a)
A stirred mixture of 4a (0.10 g, 0.29 mmol), PdCl2(PPh3)2 (0.009 g, 0.014 mmol), PCy3 (0.008 g, 0.029 mmol) and K2CO3 (0.060 g, 0.43 mmol) in 3:1 dioxane/water (v/v; 10 mL) was purged with argon gas for 30 min. 4-Fluorophenylboronic acid (0.048 g, 0.35 mmol) was added to the mixture using a syringe. The reaction mixture was heated at 100 °C for 2 h and then quenched with an ice-cold water. The product was extracted into chloroform and the combined organic layers were washed with water, dried over Na2SO4, filtered and evaporated under reduced pressure. The residue was purified by column chromatography on silica gel to afford 5a as a yellow solid (0.089 g, 76%), Rf (2:1 hexane/toluene) 0.40, mp. 177–180 °C; νmax (ATR) cm−1 520, 570, 595, 685, 708, 752, 846, 1157, 1230, 1400, 1538, 1607, 2204; δH (500 MHz, DMSO-d6) 7.33 (t, J = 8.5 Hz, 2H), 7.38–7.39 (m, 3H), 7.52–7.57 (m, 5H), 7.93 (dd, J = 6.5 and 8.5 Hz, 2H), 7.98 (d, J = 8.5 Hz, 1H), 8.12 (d, J = 8.5 Hz, 1H), 8.24 (d, J = 1.5 Hz, 1H), 8.68 (d, J = 6.5 Hz, 2H); δC (125 MHz, DMSO-d6) 88.7, 91.4, 115.8 (d, 2JCF= 21.7 Hz), 121.4, 122.2, 122.5, 128.4, 128.6, 128.7, 128.8, 129.4, 129.7, 130.8, 131.7, 132.2 (d, 3JCF= 8.6 Hz), 133.3 (d, 4JCF= 2.7 Hz), 136.3, 137.8, 151.5, 160.5, 164.0 (d, 1JCF= 249.3 Hz), 166.8; m/z 401 (100, MH+); HRMS (ES): MH+, found 401.1448. C28H18FN2+ requires 401.1454.
3.6.2. 2,4-Bis(4-fluorophenyl)-6-(phenylethynyl)quinazoline (5b)
A mixture of 4b (0.10 g, 0.28 mmol), PdCl2(PPh3)2 (0.009 g, 0.014 mmol), PCy3 (0.008 g, 0.028 mmol), 4-fluorophenylboronic acid (0.046 g, 0.33 mmol) and K2CO3 (0.060 g, 0.43 mmol) in aqueous dioxane (10 mL) afforded 5b as a yellow solid (0.10 g, 85%), Rf (2:1 hexane/toluene) 0.50, mp. 220–222 °C; νmax (ATR) 523, 560, 588, 685, 750, 802, 840, 1027, 1156, 1220, 1244, 1300, 1400, 1509, 1536, 1600, 2211 cm−1; δH (500 MHz, CDCl3) 7.21 (t, J = 8.5 Hz, 2H), 7.34 (t, J = 8.5 Hz, 2H), 7.37–7.39 (m, 3H), 7.56–7.58 (m, 2H), 7.92 (dd, J = 5.5 and 8.5 Hz, 2H), 7.99 (dd, J = 2.0 and 8.5 Hz, 1H), 8.10 (d, J = 8.5 Hz, 1H), 8.24 (d, J = 2.0 Hz, 1H), 8.71 (t, J = 8.5 Hz, 2H); δC (125 MHz, CDCl3) 88.6, 91.5, 115.5 (d, 2JCF = 21.8 Hz), 115.9 (d, 2JCF = 21.8 Hz), 121.3, 122.3, 122.5, 128.4, 128.8, 129.3, 129.7, 130.8 (d, 3JCF = 8.6 Hz), 131.7, 132.2 (d, 3JCF = 8.6 Hz), 133.3 (d, 4JCF = 3.7 Hz), 134.0 (d, 4JCF = 3.1 Hz), 136.4, 151.4, 159.6, 164.1 (d, 1JCF = 249.3 Hz), 164.8 (d, 1JCF = 249.3 Hz), 166.8; m/z 419 (100, MH+); HRMS (ES): MH+, found 419.1362. C28H17F2N2+ requires 419.1360.
3.6.3. 4-(4-Fluorophenyl)-2-(4-chlorophenyl)-6-(phenylethynyl)quinazoline (5c)
A mixture of 4c (0.10 g, 0.26 mmol), PdCl2(PPh3)2 (0.009 g, 0.013 mmol), PCy3 (0.007 g, 0.026 mmol), 4-fluorophenylboronic acid (0.044 g, 0.32 mmol) and K2CO3 (0.053 g, 0.39 mmol) in aqueous dioxane (10 mL) afforded 5c as a yellow solid (0.094 g, 83%), Rf (2:1 hexane/toluene) 0.50, mp. 206–207 °C; νmax (ATR) 499, 525, 571, 588, 689, 750, 802, 836, 1013, 1089, 1160, 1228, 1314, 1416, 1509, 1536, 1602, 2207 cm−1; δH (500 MHz, CDCl3) 7.34 (t, J = 9.0 Hz, 2H), 7.38–7.39 (m, 3H), 7.50 (d, J = 8.5 Hz, 2H), 7.56–7.57 (m, 2H), 7.91 (t, J = 8.5 Hz, 2H), 7.98 (dd, J = 1.5 and 8.5 Hz, 1H), 8.09 (d, J = 8.5 Hz, 1H), 8.23 (d, J = 1.5 Hz, 1H), 8.63 (d, J = 8.5 Hz, 2H); δC (125 MHz, CDCl3) 88.7, 91.7, 115.9 (d, 2JCF = 21.7 Hz), 121.4, 122.5, 122.6, 128.5, 128.7, 128.8, 129.4, 129.7, 130.0, 131.7, 132.2 (d, 3JCF = 8.5 Hz), 133.3 (d, 4JCF = 3.7 Hz), 136.3, 136.4, 137.1, 151.4, 159.5, 164.1 (d, 1JCF = 249.4 Hz), 166.8; m/z 435 (100, MH+); HRMS (ES): MH+, found 435.1057. C28H17N2F35Cl+ requires 435.1064.
3.6.4. 4-(4-Fluorophenyl)-2-(4-methoxyphenyl)-6-(phenylethynyl)quinazoline (5d)
A mixture of 4d (0.10 g, 0.27 mmol), PdCl2(PPh3)2 (0.009 g, 0.013 mmol), PCy3 (0.007 g, 0.027 mmol), 4-fluorophenylboronic acid (0.044 g, 0.32 mmol) and K2CO3 (0.056 g, 0.40 mmol) in aqueous dioxane (10 mL) afforded 5d as a yellow solid (0.091 g, 78%), Rf (2:1 hexane/toluene) 0.25, mp. 198–200 °C; νmax (ATR) 525, 571, 588, 685, 750, 802, 842, 1027, 1161, 1245, 1300, 1401, 1510, 1536, 1601, 2211 cm−1; δH (500 MHz, CDCl3) 3.91 (s, 3H), 7.05 (d, J = 8.5 Hz, 2H), 7.33 (t, J = 9.0 Hz, 2H), 7.37–7.39 (m, 3H), 7.56 (dd, J = 2.5 and 6.0 Hz, 2H), 7.92 (dd, J = 5.5 and 8.5 Hz, 2H), 7.96 (dd, J = 1.5 and 9.0 Hz, 1H), 8.08 (d, J = 8.5 Hz, 1H), 8.22 (d, J = 1.5 Hz, 1H), 8.65 (d, J = 9.0 Hz, 2H); δC (125 MHz, CDCl3) 55.4, 88.8, 91.2, 113.9, 115.8 (d, 2JCF = 21.8 Hz), 121.1, 121.7, 122.6, 128.4, 128.6, 128.7, 129.1, 129.8, 130.3, 131.7, 132.1 (d, 3JCF = 8.5 Hz), 133.5 (d, 4JCF = 3.7 Hz), 136.2, 151.6, 160.3, 162.0, 164.0 (d, 1JCF = 249.4 Hz), 166.6; m/z 431 (100, MH+); HRMS (ES): MH+, found 431.1556. C29H20FN2O+ requires 431.1560.
3.6.5. 2-(4-Fluorophenyl)-4-(4-methoxyphenyl)-6-(phenylethynyl)quinazoline (5e)
A mixture of 4b (0.10 g, 0.28 mmol), PdCl2(PPh3)2 (0.009 g, 0.014 mmol), PCy3 (0.008 g, 0.028 mmol), 4-methoxyphenylboronic acid (0.050 g, 0.33 mmol) and K2CO3 (0.058 g, 0.42 mmol) in aqueous dioxane (10 mL) afforded 5e as a yellow solid (0.096 g, 79%), Rf (2:1 hexane/toluene) 0.30, mp. 197–200 °C; νmax (ATR) 591, 687, 754, 841, 1145, 1174, 1223, 1255, 1397, 1508, 1534, 1610, 2196 cm−1; δH (500 MHz, CDCl3) 3.96 (s, 3H), 7.16 (d, J = 9.0 Hz, 2H), 7.21 (t, J = 8.5 Hz, 2H), 7.37–7.39 (m, 3H), 7.56–7.58 (m, 2H), 7.91 (d, J = 9.0 Hz, 2H), 7.98 (dd, J = 2.0 and 9.0 Hz, 1H), 8.08 (d, J = 8.5 Hz, 1H), 8.33 (d, J = 2.0 Hz, 1H), 8.71 (dt, J = 3.5 and 8.5 Hz, 2H); δC (125 MHz, CDCl3) 55.5, 88.9, 91.2, 114.2, 115.5 (d, 2JCF = 21.8 Hz), 121.4, 121.9, 122.7, 128.5, 128.7, 129.2, 129.8, 130.3, 130.8 (d, 3JCF = 8.6 Hz), 131.7, 131.9, 134.2 (d, 4JCF = 2.8 Hz), 136.1, 151.5, 159.6, 161.5, 164. (d, 1JCF = 248.3 Hz), 167.5; m/z 431 (100, MH+); HRMS (ES): MH+, found 431.1554. C29H20FN2O+ requires 431.1560.
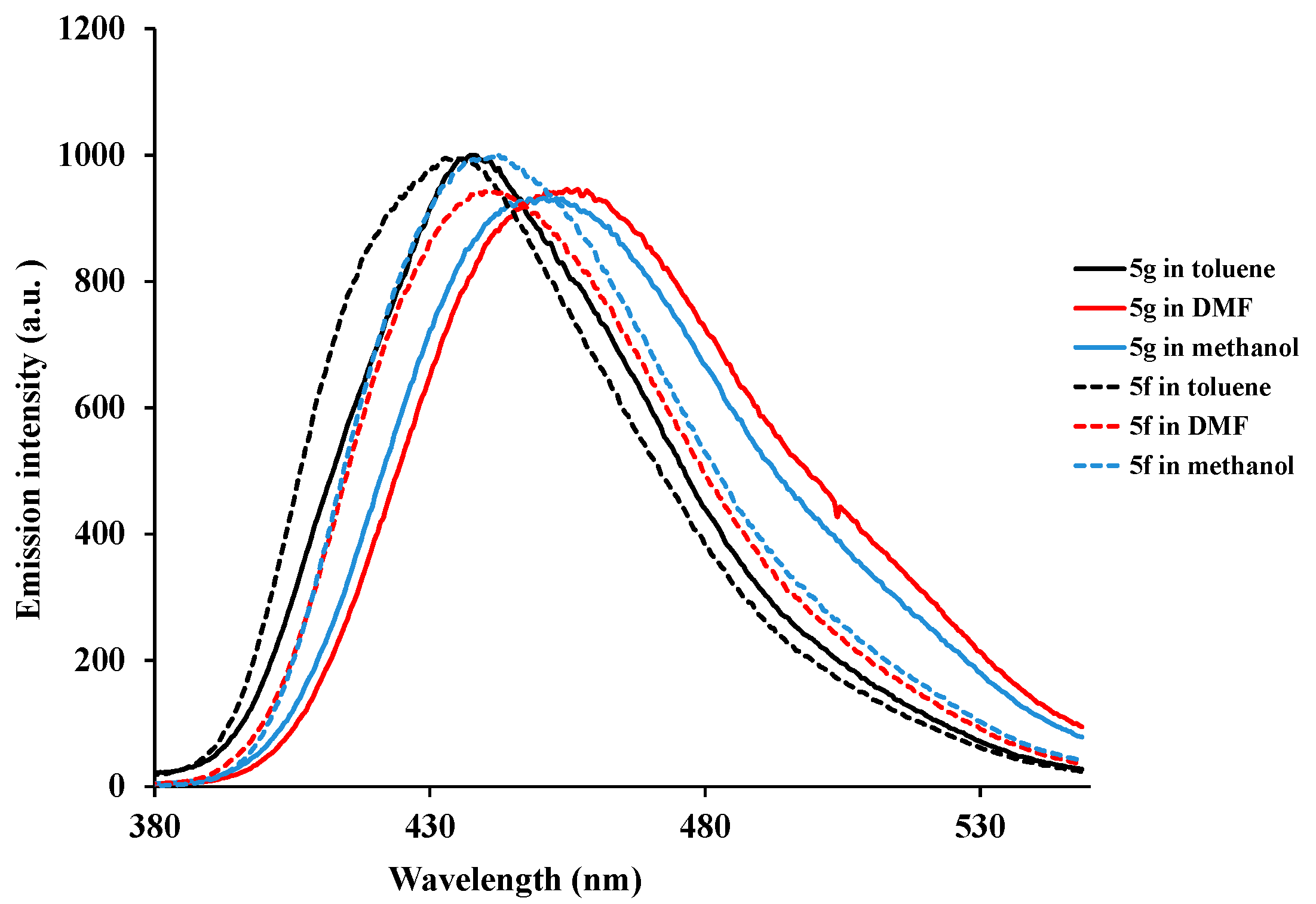
3.6.6. 2-(4-Chlorophenyl)-4-(4-methoxyphenyl)-6-(2-phenylethynyl)quinazoline (5f)
A mixture of 4c (0.10 g, 0.26 mmol), PdCl2(PPh3)2 (0.009 g, 0.013 mmol), PCy3 (0.007 g, 0.026 mmol), 4-methoxyphenylboronic acid (0.048 g, 0.32 mmol) and K2CO3 (0.053 g, 0.39 mmol) in aqueous dioxane (10 mL) afforded 5f as a yellow solid (0.082 g, 70%), Rf (2:1 hexane/toluene) 0.37, mp. 186–187 °C; νmax (ATR) 524, 539, 581, 685, 751, 803, 841, 1012, 1090, 1256, 1395, 1531, 1609, 2204 cm−1; δH (500 MHz, CDCl3) 3.96 (s, 3H), 7.17 (d, J = 9.0 Hz, 2H), 7.38–7.39 (m, 3H), 7.50 (d, J = 8.5 Hz, 2H), 7.56–7.58 (m, 2H), 7.91 (d, J = 8.5 Hz, 2H), 7.97 (d, J = 8.5 Hz, 1H), 8.08 (d, J = 7.0 Hz, 1H), 8.33 (s, 1H), 8.65 (d, J = 8.0 Hz, 2H); δC (125 MHz, CDCl3) 55.5, 88.8, 91.3, 114.2, 121.5, 122.1, 122.6, 128.6, 128.4, 128.7, 129.0, 129.2, 130.0, 130.2, 131.7, 131.8, 136.2, 136.5, 136.8, 151.4, 159.5, 161.4, 167.4; m/z 447 (100, MH+); HRMS (ES): MH+, found 447.1249. C29H2035ClN2O+ requires 447.1264.
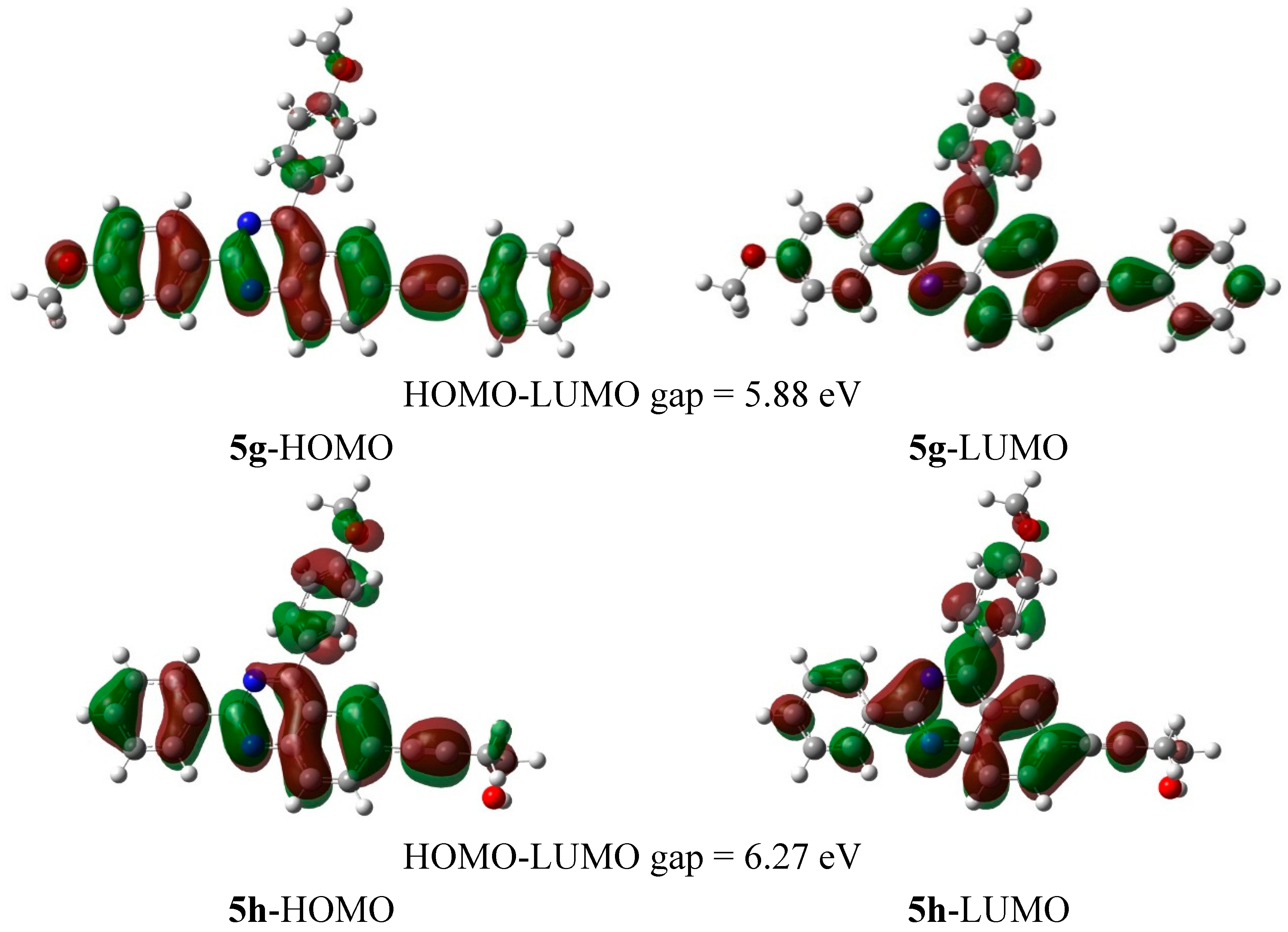
3.6.7. 2,4-Bis(4-methoxyphenyl)-6-(phenylethynyl)quinazoline (5g)
A mixture of 4d (0.10 g, 0.27 mmol), PdCl2(PPh3)2 (0.009 g, 0.013 mmol), PCy3 (0.007 g, 0.027 mmol), 4-methoxyphenylboronic acid (0.049 g, 0.32 mmol) and K2CO3 (0.056 g, 0.40 mmol) in aqueous dioxane (10 mL) afforded 5g as a yellow solid (0.095 g, 79%), Rf (2:1 hexane/toluene) 0.14, mp. 170–172 °C; νmax (ATR) 525, 574, 685, 749, 801, 840, 1024, 1159, 1246, 1299, 1399, 1511, 1532, 1604, 2202 cm−1; δH (500 MHz, CDCl3) 3.91 (d, J = 2.0 Hz, 3H), 3.95 (d, J = 2.5 Hz, 3H), 7.05 (dd, J = 2.5 and 8.5 Hz, 2H), 7.16 (dd, J = 2.5 and 9.0 Hz, 2H), 7.37–7.38 (m, 3H), 7.55–7.57 (m, 2H), 7.91 (dd, J = 2.5 and 8.5 Hz, 2H), 7.94 (dd, J = 2.0 and 8.5 Hz, 1H), 8.05 (dd, J = 2.5 and 9.0 Hz, 1H), 8.31 (s, 1H), 8.66 (dd, J = 2.5 and 8.5 Hz, 2H); δC (125 MHz, CDCl3) 55.4, 55.5, 89.0, 90.9, 13.9, 114.2, 121.2, 121.3, 122.8, 128.4, 128.6, 129.0, 129.9, 130.3, 130.4, 130.7, 131.7, 131.8, 136.0, 151.7, 160.4, 161.4, 161.9, 167.3; m/z 443 (100, MH+); HRMS (ES): MH+, found 443.1750. C30H23N2O2+ requires 443.1760.
3.6.8. 4-(4-(4-Methoxyphenyl)-2-phenylquinazolin-6-yl)but-3-yn-1-ol (5h)
A mixture of 4e (0.10 g, 0.32 mmol), PdCl2(PPh3)2 (0.011 g, 0.016 mmol), PCy3 (0.009 g, 0.032 mmol), 4-fluorophenylboronic acid (0.058 g, 0.38 mmol) and K2CO3 (0.066 g, 0.48 mmol) in aqueous dioxane (10 mL) afforded 5h as a yellow solid (0.110 g, 90%), Rf (2:1 hexane/ethyl acetate) 0.26, mp. 181–183 °C; νmax (ATR) 528, 568, 687, 751, 800, 848, 1026, 1167, 1242, 1408, 1511, 1532, 2210, 3032, 3060 cm−1; δH (500 MHz, CDCl3) 1.81 (s, 1H), 2.75 (t, J = 6.5 Hz, 2H), 3.86 (t, J 5.5 Hz, 2H), 3.95 (s, 3H), 7.15 (d, J = 8.0 Hz, 2H), 7.51–7.55 (m, 3H), 7.85 (dd, J = 1.5 and 8.5 Hz, 1H), 7.89 (d, J = 9.0 Hz, 2H), 8.06 (d, J = 9.0 Hz, 1H), 8.22 (s, 1H), 8.69 (dd, J = 1.5 and 8.5 Hz, 2H); δC (125 MHz, CDCl3) 23.9, 55.4, 61.0, 81.9, 88.5, 114.1, 121.3, 121.9, 128.5, 128.6, 129.1, 129.8, 130.2, 130.6, 131.8, 136.2, 138.0, 151.4, 160.4, 161.3, 167.3; m/z 381 (100, MH+); HRMS (ES): MH+, found 381.1597. C25H21N2O2+ requires 381.1603.
3.7. Typical Procedure for the One-Pot Sequential Sonogashira cross-Coupling of 2a–d with Terminal Alkynes
3.7.1. 4-(2-Phenyl-6-(phenylethynyl)quinazolin-4-yl)but-3-yn-1-ol (6a)
A stirred mixture of 3a (0.30 g, 0.82 mmol), PdCl2(PPh3)2 (0.057 g, 0.082 mmol), CuI (0.008 g; 0.041 mmol) and Cs2CO3 (0.40 g, 1.23 mmol) in THF (20 mL) was purged with argon gas for 30 min. Phenylacetylene (0.084 g, 0.82 mmol) was added to the mixture using a syringe. The reaction mixture was stirred at room temperature for 18 h until the starting material was consumed (tlc monitoring). A solution of 3-butyn-1-ol (0.069 g, 0.98 mmol) in THF (5 mL) was added by means of a syringe and the mixture was heated at 80 °C for 2 h and then quenched with an ice-cold water. The product was extracted into chloroform and the combined organic layers were washed with water, dried over Na2SO4, filtered and evaporated under reduced pressure. The residue was purified by column chromatography on silica gel to afford 6a as a yellow solid (0.24 g, 78%), Rf (1:1 hexane/ethyl acetate) 0.65, mp. 163–164 °C; νmax (ATR) 522, 527, 690, 709, 748, 837, 1059, 1402, 1531, 1737, 2229, 3059, 3256 cm−1; δH (500 MHz, CDCl3) 2.74 (br s, 1H), 2.95 (t, J = 6.5 Hz, 2H), 4.02 (t, J = 6.5 Hz, 2H), 7.39–7.42 (m, 3H), 7.51–7.55 (m, 3H), 7.61–7.63 (m, 2H), 7.95 (dd, J = 2.0 and 8.5 Hz, 1H), 8.00 (d, J = 9.0 Hz, 1H), 8.35 (d, J = 2.0 Hz, 1H), 8.56–8.58 (m, 2H); δC (125 MHz, CDCl3) 24.3, 60.6, 78.8, 88.7, 91.9, 97.8, 122.6, 122.7, 123.7, 128.5, 128.6, 128.7, 128.8, 129.0, 129.5, 130.7, 131.8, 136.9, 137.4, 150.3, 152.3, 161.1; m/z 375 (100, MH+); HRMS (ES): MH+, found 375.1487. C26H19N2O+ requires 375.1497.
3.7.2. 4-(2-(4-Fluorophenyl)-6-(phenylethynyl)quinazolin-4-yl)but-3-yn-1-ol (6b)
A mixture of 3b (0.30 g, 0.78 mmol), PdCl2(PPh3)2 (0.054 g, 0.078 mmol), CuI (0.007 g, 0.039 mmol), Cs2CO3 (0.38 g, 1.17 mmol) and phenylacetylene (0.079 g, 0.78 mmol) in THF (20 mL), followed by the addition of 3-butyn-1-ol (0.065 g, 0.93 mmol) in THF (5 mL) was treated as described for 6a to afford 6b as a yellow solid (0.17 g, 55%), Rf (1:1 hexane/ethyl acetate) 0.60, mp. 174–175 °C; νmax (ATR) 528, 570, 691, 744, 758, 839, 1060, 1154, 1224, 1295, 1404, 1532, 2230, 3050, 3271 cm−1; δH (500 MHz, CDCl3) 2.25 (br s, 1H), 2.98 (t, J = 6.0 Hz, 2H), 4.04 (d, J = 6.0 Hz, 2H), 7.20 (t, J = 8.5 Hz, 2H), 7.40–7.41 (m, 3H), 7.61–7.63 (m, 2H), 7.96 (dd, J = 2.0 and 8.5 Hz, 1H), 8.00 (d, J = 8.5 Hz, 1H), 8.39 (d, J = 2.0 Hz, 1H), 8.61 (t, J = 8.5 Hz, 2H); δC (125 MHz, CDCl3) 24.3, 60.6, 78.6, 88.6, 91.9, 98.1, 115.6 (d, 2JCF = 21.8 Hz), 122.6, 122.7, 123.5, 128.5, 128.9, 129.4, 130.8 (d, 3JCF = 8.5 Hz), 131.8, 133.7 (d, 4JCF = 3.0 Hz), 137.0, 146.1, 150.2, 152.3, 160.1, 164.8 (d, 1JCF = 248.3 Hz); m/z 393 (100, MH+); HRMS (ES): MH+, found 393.1412. C26H18FN2O+ requires 393.1403.
3.7.3. 4-(2-(4-Chlorophenyl)-6-(phenylethynyl)quinazolin-4-yl)but-3-yn-1-ol (6c)
A mixture of 3c (0.30 g, 0.75 mmol), PdCl2(PPh3)2 (0.052 g, 0.074 mmol), CuI (0.007 g, 0.037 mmol), Cs2CO3 (0.36 g, 1.12 mmol) and phenylacetylene (0.076 g, 0.75 mmol) in THF (20 mL), followed by the addition of 3-butyn-1-ol (0.062 g, 0.89 mmol) in THF (5 mL) was treated as described for 6a to afford 6c as a yellow solid (0.20 g, 65%), Rf (1:1 hexane/ethyl acetate) 0.70, mp. 202–204 °C; νmax (ATR) 525, 567, 684, 737, 749, 837, 1039, 1087, 1296, 1402, 1532, 2233, 3048, 3252 cm−1; δH (500 MHz, CDCl3) 2.40 (br s, 1H), 2.97 (t, J = 6.5 Hz, 2H), 4.03 (t, J = 6.5 Hz, 2H), 7.40–7.41 (m, 3H), 7.48 (d, J = 8.5 Hz, 2H) 7.60–7.62 (m, 2H), 7.94 (d, J = 8.5 Hz, 2H), 8.31 (s, 1H), 8.50 (d, J = 8.5 Hz, 2H); δC (125 MHz, CDCl3) 24.3, 60.6, 78.6, 88.6, 92.1, 98.1 122.6, 122.9, 123.6, 128.5, 128.8, 128.9, 129.0, 129.4, 130.0, 131.8, 135.9, 137.0, 137.1, 150.3, 152.3, 160.0; m/z 409 (100, MH+); HRMS (ES): MH+, found 409.1104. C26H18N2O35Cl+ requires 409.1108.
3.7.4. 4-(2-(4-Methoxyphenyl)-6-(phenylethynyl)quinazolin-4-yl)but-3-yn-1-ol (6d)
A mixture of 3d (0.30 g, 0.75 mmol), PdCl2(PPh3)2 (0.053 g, 0.075 mmol), CuI (0.007 g, 0.037 mmol), Cs2CO3 (0.36 g, 1.12 mmol) and phenylacetylene (0.076 g, 0.75 mmol) in THF (20 mL), followed by the addition of 3-butyn-1-ol (0.063 g, 0.90 mmol) in THF (5 mL) was treated as described for 6a to afford 6d as a yellow solid (0.20 g, 66%), Rf (1:1 hexane/ethyl acetate) 0.50, mp. 141–142 °C; νmax (ATR) 527, 569, 690, 748, 837, 1027, 1059, 1217, 1402, 1531, 1737, 2229, 3040, 3256 cm−1; δH (500 MHz, CDCl3) 2.74 (br s, 1H), 2.95 (t, J = 6.5 Hz, 2H), 3.89 (s, 3H), 4.02 (t, J = 6.5 Hz, 2H), 7.02 (d, J = 9.0 Hz, 2H), 7.39–7.41 (m, 3H), 7.60–7.62 (m, 2H), 7.76 (dd, J = 2.0 and 8.5 Hz, 1H), 7.94 (d, J = 8.5 Hz, 1H), 8.31 (d, J = 2.0 Hz, 1H), 8.52 (d, J = 8.5 Hz, 2H); δC (125 MHz, CDCl3) 24.4, 55.4, 60.6, 78.7, 88.8, 91.6, 97.7, 113.9, 122.1, 122.7, 123.3, 128.5, 128.7, 128.8, 129.5, 130.0, 130.4, 131.8, 136.8, 150.3, 152.2, 160.8, 162.1; m/z 405 (100, MH+); HRMS (ES): MH+, found 405.1600. C27H21N2O2+ requires 405.1603.
3.7.5. 4-(2-Phenyl-4-(phenylethynyl)quinazolin-6-yl)but-3-yn-1-ol (6e)
A mixture of 3a (0.30 g, 0.82 mmol), PdCl2(PPh3)2 (0.057 g, 0.082 mmol), CuI (0.008 g, 0.041 mmol), Cs2CO3 (0.40 g, 1.23 mmol) and 3-butyn-1-ol (0.057 g, 0.82 mmol) in THF (20 mL), followed by the addition of phenylacetylene (0.10 g, 0.98 mmol) in THF (5 mL) was treated as described for 6a to afford 6e as a yellow solid (0.19 g, 61%), Rf (1:1 hexane/ethyl acetate) 0.67, mp. 126–127 °C; νmax (ATR) 546, 687, 708, 755, 838, 1037, 1309, 1409, 1527, 2208, 2922, 3058, 3358 cm−1; δH (500 MHz, CDCl3) 1.89 (s, 1H), 2.80 (t, J = 5.5 Hz, 2H), 3.90 (t, J = 5.5 Hz, 2H), 7.48–7.55 (m, 6H), 7.81 (d, J = 6.5 Hz, 2H), 7.88 (d, J = 8.5 Hz, 1H), 8.02 (d, J = 8.5 Hz, 1H), 8.41 (s, 1H), 8.64 (d, J = 6.5 Hz, 2H); δC (125 MHz, CDCl3) 23.9, 61.0, 81.8, 85.4, 89.4, 982, 121.2, 122.8, 123.6, 128.6, 128.7, 128.8, 129.0, 129.4, 130.2, 130.8, 132.6, 137.1, 137.5, 150.3, 152.3 161.2; m/z 375 (100, MH+); HRMS (ES): MH+, found 375.1495. C26H19N2O+ requires 375.1497.
3.7.6. 4-(2-(4-Fluorophenyl)-4-(phenylethynyl)quinazolin-6-yl)but-3-yn-1-ol (6f)
A mixture of 3b (0.30 g, 0.78 mmol), PdCl2(PPh3)2 (0.054 g, 0.078 mmol), CuI (0.007 g, 0.039 mmol), Cs2CO3 (0.38 g, 1.17 mmol) and 3-butyn-1-ol (0.054 g, 0.78 mmol) in THF (20 mL), followed by the addition of phenylacetylene (0.095 g, 0.93 mmol) in THF (5 mL) was treated as described for 6a to afford 6f as a yellow solid (0.16 g, 52%), Rf (1:1 hexane/ethyl acetate) 0.50, mp. 150–152 °C; νmax (ATR) 566, 690, 742, 760, 835, 1037, 1148, 1221, 1308, 1404, 1509, 1527, 2209, 2922, 3316 cm−1; δH (500 MHz, CDCl3) 2.02 (br s, 1H), 2.79 (t, J = 6.0 Hz, 2H), 3.90 (t, J = 6.0 Hz, 2H), 7.20 (t, J = 9.0 Hz, 2H), 7.46–7.51 (m, 3H), 7.79 (d, J = 7.5 Hz, 2H), 7.85 (dd, J = 2.0 and 8.5 Hz, 1H), 7.97 (d, J = 9.0 Hz, 1H), 8.36 (d, J = 2.0 Hz, 1H), 8.64 (t, J = 7.5 Hz, 2H); δC (125 MHz, CDCl3) 25.0, 61.1, 81.7, 85.3, 89.5, 98.4, 115.6 (d, 2JCF = 21.8 Hz), 121.2, 122.9, 123.5, 128.7, 128.9, 129.4, 130.3, 130.9 (d, 3JCF = 8.6 Hz), 131.7, 133.5 (d, 4JCF = 3.5 Hz), 136.9, 137.2, 150.2, 152.3, 160.2, 164.8 (d, 1JCF = 249.3 Hz); m/z 393 (100, MH+); HRMS (ES): MH+, found 393.1396. C26H18FN2O+ requires 393.1403.
3.7.7. 4-(2-(4-Chlorophenyl)-4-(phenylethynyl)quinazolin-6-yl)but-3-yn-1-ol (6g)
A mixture of 3c (0.30 g, 0.75 mmol), PdCl2(PPh3)2 (0.052 g, 0.074 mmol), CuI (0.007 g, 0.037 mmol), Cs2CO3 (0.36 g, 1.12 mmol) and 3-butyn-1-ol (0.052 g, 0.75 mmol) in THF (20 mL), followed by the addition of phenylacetylene (0.091 g, 0.89 mmol) in THF (5 mL) was treated as described for 6a to afford 6g as a yellow solid (0.23 g, 75%), Rf (1:1 hexane/ethyl acetate) 0.40, mp. 181–182 °C; νmax (ATR) 569, 683, 736, 748, 838, 1012, 1089, 1167, 1308, 1401, 1530, 2213, 2921, 3256 cm−1; δH (500 MHz, CDCl3) 1.86 (br s, 1H), 2.80 (t, J = 6.0 Hz, 2H), 3.91 (t, J = 6.5 Hz, 2H), 7.47–7.51 (m, 5H), 7.80 (d, J = 8.5 Hz, 2H), 7.88 (dd, J = 2.0 and 8.5 Hz, 1H), 8.00 (d, J = 8.5 Hz, 1H), 8.40 (d, J = 2.0 Hz, 1H), 8.60 (d, J = 8.5 Hz, 2H); δC (125 MHz, CDCl3) 24.0, 61.1, 81.7, 85.3, 89.7, 98.5, 121.2, 123.1, 123.6, 128.7, 128.8, 128.9, 129.4, 130.0, 130.3, 132.6, 136.0, 137.1, 137.3, 150.2, 152.3, 160.1; m/z 409 (100, MH+); HRMS (ES): MH+, found 409.1101. C26H18N2O35Cl+ requires 409.1108.
3.7.8. 4-(2-(4-Methoxyphenyl)-4-(phenylethynyl)quinazolin-6-yl)but-3-yn-1-ol (6h)
A mixture of 3d (0.30 g, 0.75 mmol), PdCl2(PPh3)2 (0.053 g, 0.075 mmol), CuI (0.007 g, 0.037 mmol), Cs2CO3 (0.36 g, 1.12 mmol) and 3-butyn-1-ol (0.052 g, 0.75 mmol) in THF (20 mL), followed by the addition of phenylacetylene (0.091 g, 0.90 mmol) in THF (5 mL) was treated as described for 6a to afford 6h as a yellow solid (0.18 g, 59%), Rf (1:1 hexane/ethyl acetate) 0.51, mp. 175–177 °C; νmax (ATR) 560, 687, 746, 756, 836, 1025, 1166, 1246, 1306, 1409, 1514, 1528, 2211, 2926, 3418 cm−1; δH (500 MHz, CDCl3) 1.91 (br s, 1H), 2.79 (t, J = 6.0 Hz, 2H), 3.90 (t, J = 6.0 Hz, 2H), 3.91 (s, 3H), 7.04 (d, J = 9.0 Hz, 2H), 7.46–7.50 (m, 3H), 7.80 (dd, J = 2.0 and 8.0 Hz, 2H), 7.84 (dd, J = 1.5 and 9.0 Hz, 1H), 7.96 (d, J = 9.0 Hz, 1H), 8.37 (d, J = 1.5 Hz, 1H), 8.60 (d, J = 9.0 Hz, 2H); δC (125 MHz, CDCl3) 24.0, 55.4, 61.1, 81.8, 85.5, 89.2, 97.9, 113.9, 121.3, 122.3, 123.3, 128.4, 128.6, 128.8, 129.5, 130.1, 130.2, 130.4, 132.6, 137.1, 150.4, 161.0, 162.1; m/z 405 (100, MH+); HRMS (ES): MH+, found 405.1602. C27H21N2O2+ requires 405.1603.
3.8. Typical Procedure for the One-Pot Sequential Sonogashira and Stille Cross-Coupling of 2a–d
3.8.1. 4-(Furan-2-yl)-2-phenyl-6-(phenylethynyl)quinazoline (7a)
A stirred mixture of 3a (0.10 g, 0.27 mmol), PdCl2(PPh3)2 (0.019 g, 0.027 mmol), CuI (0.002 g, 0.013 mmol) and Cs2CO3 (0.13 g, 0.40 mmol) in THF (10 mL) was purged with argon gas for 30 min. Phenylacetylene (0.027 g, 0.27 mmol) was added to the mixture using a syringe. The reaction mixture was stirred at room temperature for 18 h until the starting material was consumed (tlc monitoring). A solution of 2-(tributylstannyl)furan (0.11 g, 0.32 mmol) in THF (5 mL) was added by means of a syringe and the mixture was heated at 80 °C for 2 h and then quenched with an ice-cold water. The product was extracted into chloroform and the combined organic layers were washed with water, dried over MgSO4, filtered and evaporated under reduced pressure. The residue was purified by column chromatography on silica gel to afford 7a as a yellow solid (0.056 g, 56%), Rf (2:1 hexane/toluene) 0.25, mp. 184–186 °C; νmax (ATR) 525, 592, 686, 703, 752, 918, 996, 1314, 1474, 1532, 2202 cm−1; δH (500 MHz, CDCl3) 6.72 (dd, J = 1.5 and 3.0 Hz, 1H), 7.39–7.40 (m, 3H), 7.51–7.56 (m, 3H) , 7.61–7.63 (m, 2H), 7.75 (d, J = 3.5 Hz, 1H), 7.86 (s, 1H), 7.95 (dd, J = 1.5 and 8.5 Hz, 1H), 8.04 (d, J = 8.5 Hz, 1H), 8.66 (d, J = 7.0 Hz, 2H), 9.10 (d, J = 1.5 Hz, 1H); δC (125 MHz, CDCl3) 89.2, 91.1, 112.4, 116.3, 119.3, 122.2, 122.8, 128.4, 128.5, 128.6, 128.7, 129.3, 130.0, 130.6, 131.7, 136.1, 137.8, 146.1, 152.2, 153.9, 154.7, 160.4; m/z 373 (100, MH+); HRMS (ES): MH+, found 373.1332. requires 373.1341.
3.8.2. 2-(4-Fluorophenyl)-4-(furan-2-yl)-6-(phenylethynyl)quinazoline (7b)
A mixture of 3b (0.10 g, 0.26 mmol), PdCl2(PPh3)2 (0.018 g, 0.026 mmol), CuI (0.002 g, 0.013 mmol), Cs2CO3 (0.13 g, 0.39 mmol) and phenylacetylene (0.026 g, 0.26 mmol) in THF (10 mL), followed by the addition of 2-(tributylstannyl)furan (0.11 g, 0.31 mmol) in THF (5 mL) was treated as for 7a to afford 7b as a yellow solid (0.062 g, 62%), Rf (2:1 hexane/toluene) 0.30, mp. 195–196 °C; νmax (ATR) 524, 574, 684, 742, 840, 1015, 1220, 1398, 1511, 1534, 2203 cm−1; δH (500 MHz, CDCl3) 6.72 (dd, J = 1.5 and 3.5 Hz, 1H), 7.39–7.41 (m, 3H), 7.50 (d, J = 8.0 Hz, 2H), 7.61–7.62 (m, 2H), 7.73 (d, J = 3.5 Hz, 1H), 7.87 (s, 1H), 7.95 (dd, J = 2.0 and 8.5 Hz, 1H), 8.01 (d, J = 9.0 Hz, 1H), 8.66 (d, J = 8.5 Hz, 2H) 9.09 (d, J = 1.5 Hz, 1H); δC (125 MHz, CDCl3) 89.1, 91.2, 112.4, 115.4 (d, 2JCF = 21.7 Hz), 116.3, 119.2, 122.3, 122.8, 128.4, 128.7, 129.1, 130.0, 130.6 (d, 3JCF = 8.5 Hz), 131.7, 134.0 (d, 4JCF = 2.7 Hz), 136.2, 146.2, 152.1, 153.8, 154.7, 159.5, 164.7 (d, 1JCF = 249.3 Hz); m/z 391 (100, MH+); HRMS (ES): MH+, found 391.1238. C26H16N2OF+ requires 391.1247.
3.8.3. 2-(4-Chlorophenyl)-4-(furan-2-yl)-6-(phenylethynyl)quinazoline (7c)
A mixture of 3c (0.10 g, 0.25 mmol), PdCl2(PPh3)2 (0.018 g, 0.025 mmol), CuI (0.002 g, 0.012 mmol), Cs2CO3 (0.12 g, 0.37 mmol) and phenylacetylene (0.026 g, 0.25 mmol) in THF (10 mL), followed by the addition of 2-(tributylstannyl)furan (0.11 g, 0.30 mmol) in THF (5 mL) was treated as for 7a to afford 7c as a yellow solid (0.070 g, 69%), Rf (2:1 hexane/toluene) 0.36, mp. 164–166 °C; νmax (ATR) 524, 539, 682, 736, 745, 849, 1013, 1089, 1400, 1532, 1580, 2207 cm−1; δH (500 MHz, CDCl3) 6.72 (dd, J = 1.5 and 3.5 Hz, 1H), 7.39–7.40 (m, 3H), 7.49 (d, J = 8.5 Hz, 2H), 7.61–7.62 (m, 2H), 7.72 (d, J = 3.5 Hz, 1H), 7.87 (s, 1H), 7.94 (dd, J = 1.5 and 8.5 Hz, 1H), 8.01 (d, J = 8.5 Hz, 1H), 8.60 (t, J = 9.0 Hz, 2H) 9.09 (d, J = 1.5 Hz, 1H); δC (125 MHz, CDCl3) 89.1, 91.3, 112.5, 116.4, 119.3, 122.5, 122.7, 128.4, 128.6, 128.7, 129.2, 129.8, 130.0, 131.7, 132.6, 136.3, 136.8, 146.2, 152.1, 153.8, 154.7, 159.4; m/z 407 (100, MH+); HRMS (ES): MH+, found 407.0939. C26H16N2O35Cl+ requires 407.0951.
3.8.4. 4-(Furan-2-yl)-2-(4-methoxyphenyl)-6-(phenylethynyl)quinazoline (7d)
A mixture of 3d (0.10 g, 0.25 mmol), PdCl2(PPh3)2 (0.018 g, 0.025 mmol), CuI (0.002 g, 0.012 mmol), Cs2CO3 (0.12 g, 0.37 mmol) and phenylacetylene (0.026 g, 0.25 mmol) in THF (10 mL), followed by the addition of 2-(tributylstannyl)furan (0.11 g, 0.30 mmol) in THF (5 mL) was treated as for 7a to afford 7d as a yellow solid (0.059 g, 59%), Rf (toluene) 0.50, mp. 148–150 °C; νmax (ATR) 529, 574, 574, 690, 744, 836, 1010, 1163, 1246, 1407, 1514, 2217 cm−1; δH (500 MHz, CDCl3) 3.91 (s, 3H), 6.71 (dd, J = 1.5 and 3.5 Hz, 1H), 7.05 (d, J = 8.5 Hz, 2H), 7.38–741 (m, 3H), 7.60–7.62 (m, 2H), 7.72 (d, J = 3.5 Hz, 1H), 7.86 (s, 1H), 7.93 (dd, J = 1.5 and 8.5 Hz, 1H), 7.99 (d, J = 8.5 Hz, 1H), 8.62 (d, J = 8.5 Hz, 2H) 9.07 (d, J = 1.5 Hz, 1H); δC (125 MHz, CDCl3) 55.3, 89.3, 90.9, 112.4, 113.8, 116.1, 119.1, 121.7, 122.9, 128.4, 128.6, 129.0, 130.0, 130.2, 130.5, 131.7, 136.0, 146.0, 152.2, 153.9, 154.6, 160.3, 161.9; m/z 403 (100, MH+); HRMS (ES): MH+, found 403.1439. C27H19N2O2+ requires 403.1447.