Steroidal Saponins from the Roots and Rhizomes of Tupistra chinensis
Abstract
:1. Introduction
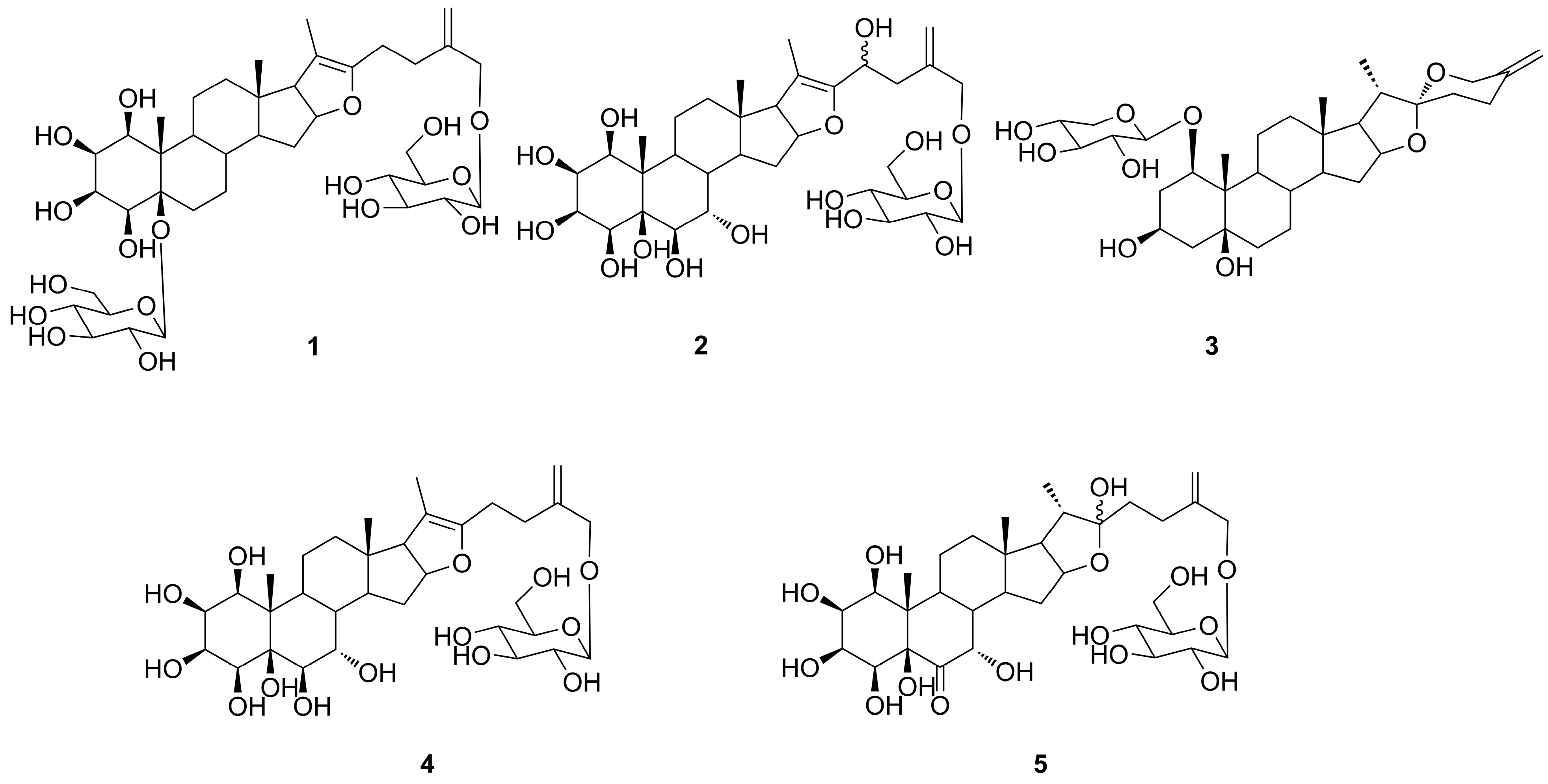
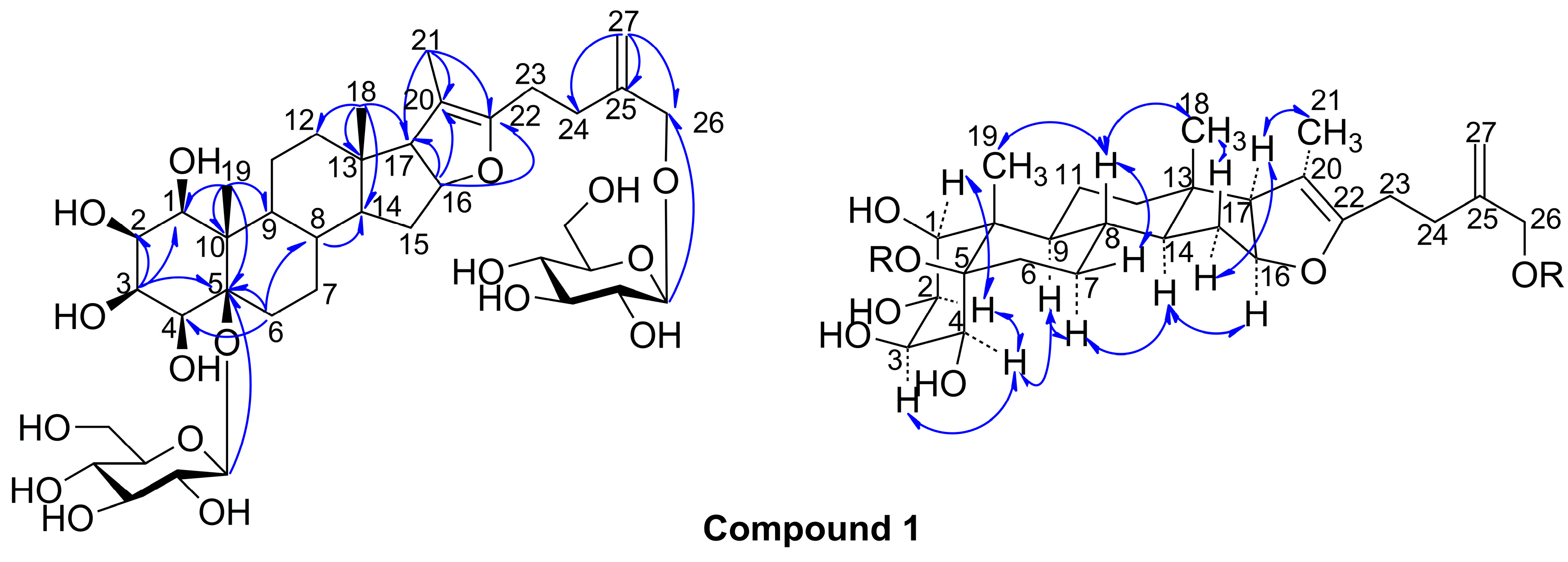
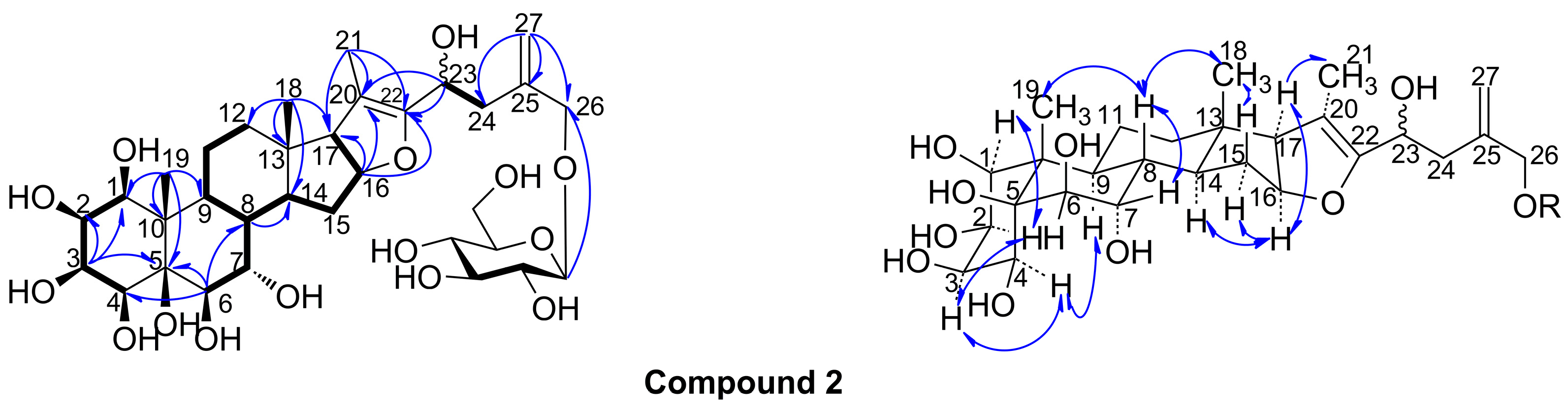
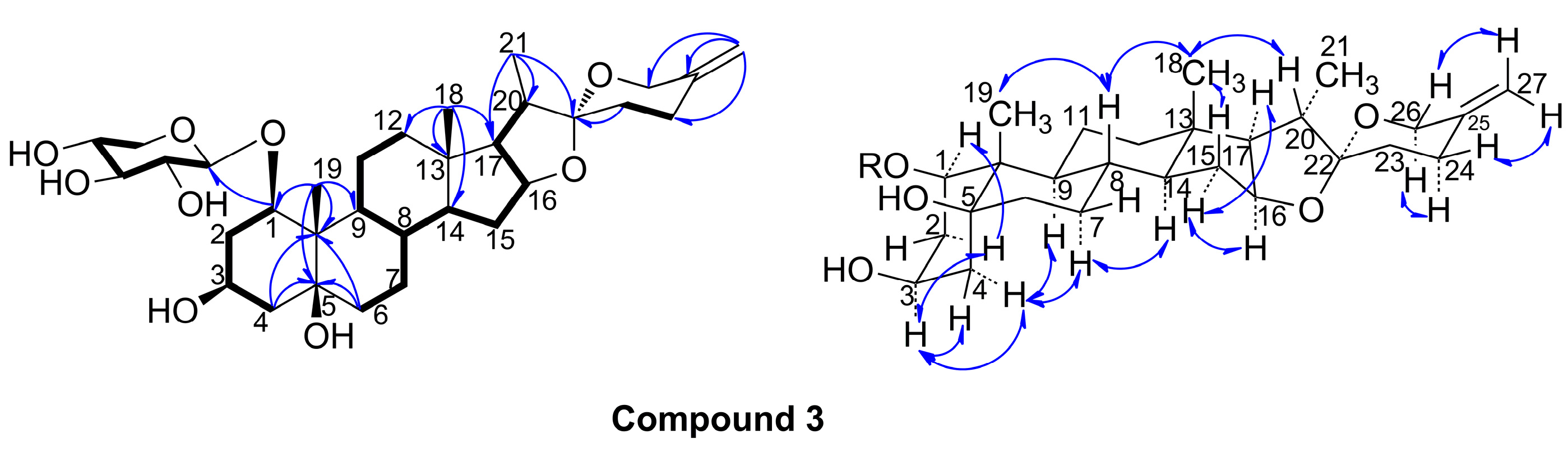
2. Results and Discussion
| Position | 1 | 2 | 3 | |||
|---|---|---|---|---|---|---|
| δc a | δH a (J in Hz) | δc b | δH b (J in Hz) | δc c | δH c (J in Hz) | |
| 1 | 77.8 | 4.25 (brs) | 79.1 | 4.29 (brs) | 82.5 | 4.26 (brs) |
| 2 | 68.1 | 4.38 (brs) | 67.7 | 4.33 (brs) | 30.4 | 2.53 (H-2a, ca.) 1.85 (H-2b, ca.) |
| 3 | 75.2 | 4.70 (brs) | 76.1 | 4.77 (brs) | 67.8 | 4.59 (brs) |
| 4 | 67.6 | 4.08 (brs) | 70.2 | 5.33 (brs) | 40.0 | 2.40 (H-4a, ca.) 2.04 (H-4b, ca.) |
| 5 | 87.4 | - | 78.6 | - | 74.7 | - |
| 6 | 24.9 | 1.93 (ca.), 2.80 (ca.) | 74.0 | 5.03 (brs) | 36.3 | 1.54 (ca.), 1.90 (ca.) |
| 7 | 28.5 | 1.1 (ca.), 1.51 (ca.) | 72.5 | 4.49 (brs) | 29.2 | 0.98 (H-7a, ca.) 1.51 (H-7b, ca.) |
| 8 | 34.4 | 1.59 (ca.) | 34.8 | 2.62 (ca.) | 35.4 | 1.67 (ca.) |
| 9 | 46.6 | 1.19 (ca.) | 37.8 | 2.05 (ca.) | 46.3 | 1.15 (ca.) |
| 10 | 46.2 | - | 46.3 | - | 44.9 | - |
| 11 | 21.9 | 1.41 (ca.), 1.44 (ca.) | 21.9 | 1.61 (ca.), 1.67 (ca.) | 22.3 | 1.14(ca.),1.38 (ca.) |
| 12 | 39.7 | 1.62 (d, 12.0), 1.15 (ca.) | 40.0 | 1.70 (d, 12.0), 1.24 (ca.) | 40.5 | 1.73 (d, 12.5), 1.13 (ca.) |
| 13 | 43.3 | - | 43.8 | - | 41.2 | - |
| 14 | 54.3 | 0.76 (ca.) | 48.9 | 1.96 (ca.) | 56.7 | 1.12 (ca.) |
| 15 | 31.0 | 2.48 (H-15a, ca.) 2.38 (H-15b, ca.) | 34.7 | 2.58 (H-15a, ca.) 1.65 (H-15b, ca.) | 32.7 | 2.07 (H-15a, ca.) 1.48 (H-15b, ca.) |
| 16 | 84.4 | 4.77 (q, 7.5) | 85.1 | 4.87 (ca.) | 81.9 | 4.62 (q, 7.2) |
| 17 | 64.5 | 2.42 (ca.) | 65.3 | 2.57 (ca.) | 63.5 | 1.88 (ca.) |
| 18 | 14.3 | 0.67 (s) | 14.6 | 0.81 (s) | 17.0 | 0.87 (s) |
| 19 | 13.7 | 1.70 (s) | 16.0 | 1.99 (s) | 14.4 | 1.59 (s) |
| 20 | 103.9 | - | 105.9 | - | 42.4 | 2.00(ca.) |
| 21 | 11.7 | 1.58 (s) | 12.1 | 1.74 (s) | 15.5 | 1.10 (d, 8.0) |
| 22 | 151.8 | - | 154.2 | - | 109.9 | - |
| 23 | 34.3 | 1.45 (ca.), 2.04 (ca.) | 64.8 | 5.13 (dd, 6.0, 8.0) | 33.7 | 1.81 (ca.) |
| 24 | 24.6 | 2.37 (ca.), 2.47 (ca.) | 40.3 | 2.88 (H-24a, dd, 6.0, 14.3), 3.10 (H-24b, dd, 8.0, 14.3) | 29.4 | 2.26 (ca.) 2.74 (ca.) |
| 25 | 146.2 | - | 144.4 | - | 144.9 | - |
| 26 | 71.7 | 4.58 (d, 13.0) 4.34 (d, 13.0) | 72.7 | 4.75 (d, 13.0) 4.61 (d, 13.0) | 65.5 | 4.50 (d, 12.1) 4.07 (d, 12.1) |
| 27 | 111.6 | 5.35 (H-27a, s) 5.04 (H-27b, s) | 114.6 | 5.47 (H-27a, s) 5.28 (H-27b, s) | 109.2 | 4.81(H-27a, s) 4.84 (H-27b, s) |
| 1' | 97.4 | 5.28 (d, 7.8) | 104.2 | 5.0 (d, 7.8) | 104.1 | 4.81 (d, 7.2) |
| 2' | 76.2 | 3.95 (ca.) | 75.6 | 4.12 (ca.) | 75.8 | 3.99 (ca.) |
| 3' | 78.6 | 4.01 (ca.) | 80.0 | 4.36 (ca.) | 78.9 | 4.21 (ca.) |
| 4' | 71.9 | 4.02 (ca.) | 72.1 | 4.27 (ca.) | 71.5 | 4.23 (ca.) |
| 5' | 78.8 | 4.22 (ca.) | 79.0 | 3.96 (ca.) | 68.1 | 3.78 (t, 10.5), 4.42 (dd, 4.5, 11.5) |
| 6' | 62.8 | 4.52 (ca.), 4.21 (ca.) | 63.2 | 4.58 (dd, 2.0, 11.8), 4.41 (dd, 5.5, 11.8) | - | - |
| 1'' | 103.8 | 4.89 (d, 7.7) | - | - | - | - |
| 2'' | 75.8 | 4.03 (ca.) | - | - | - | - |
| 3'' | 78.5 | 4.22 (ca.) | - | - | - | - |
| 4'' | 71.7 | 4.19 (ca.) | - | - | - | - |
| 5'' | 78.6 | 3.92 (ca.) | - | - | - | - |
| 6'' | 62.6 | 4.52 (ca.), 4.35 (ca.) | - | - | - | - |
| Comp. | 1 μM | 3 μM | 10 μM | 30 μM | 100 μM | IC50 μM |
|---|---|---|---|---|---|---|
| 1 | 1.93 ± 0.95 ** | 13.50 ± 1.81 ** | 14.69 ± 1.41 ** | 16.53 ± 1.26 ** | 16.90 ± 0.69 ** | >100 |
| 2 | 3.95 ± 2.09 ** | 5.75 ± 1.48 ** | 11.50 ± 3.22 ** | 16.17 ± 1.50 ** | 20.04 ± 1.36 ** | >100 |
| 3 | 4.55 ± 1.10 ** | 8.04 ± 1.94 ** | 13.47 ± 0.61 ** | 17.39 ± 0.73 ** | 55.74 ± 0.87 ** | 88.21 ± 1.34 |
| 4 | 4.01 ± 0.86 ** | 9.26 ± 0.44 ** | 11.46 ± 2.91 ** | 13.47 ± 1.49 ** | 26.07 ± 0.99 ** | >100 |
| 5-FU | 3.07 ± 0.52 | 5.21 ± 0.28 | 17.39 ± 1.11 | 47.88 ± 1.38 | 71.96 ± 2.49 | 38.65 ± 1.59 |
| Comp. | 1 μM | 3 μM | 10 μM | 30 μM | 100 μM | IC50 μM |
|---|---|---|---|---|---|---|
| 1 | 3.75 ± 1.24 ** | 11.62 ± 1.88 ** | 12.83 ± 2.02 ** | 14.35 ± 0.77 ** | 20.19 ± 3.63 ** | >100 |
| 2 | 4.17 ± 1.30 ** | 7.68 ± 1.27 ** | 11.07 ± 1.57 ** | 13.80 ± 2.05 ** | 23.11 ± 0.74 ** | >100 |
| 3 | 3.95 ± 0.95 ** | 7.90 ± 1.67 ** | 13.05 ± 1.75 ** | 20.60 ± 2.40 ** | 56.17 ± 1.98 ** | 86.63 ± 2.33 |
| 4 | 2.93 ± 1.18 ** | 6.65 ± 0.94 ** | 7.01 ± 2.47 ** | 13.21 ± 1.40 ** | 24.75 ± 1.62 ** | >100 |
| 5-FU | 6.97 ± 0.82 | 9.03 ± 1.21 | 23.76 ± 1.22 | 42.18 ± 1.22 | 69.24 ± 2.05 | 42.78 ± 1.63 |
3. Experimental Section
3.1. General Information
3.2. Plant Material
3.3. Extraction and Isolation
3.4. 1β,2β,3β,4β,5β,26-Hexahydroxyfurost-20(22),25(27)-dien-5,26-O-β-d-glucopyranoside (1)
3.5. 1β,2β,3β,4β,5β,6β,7α,23ξ,26-Nonahydroxyfurost-20(22),25(27)-dien-26-O-β-d-glucopyranoside (2)
3.6. (20S,22R)-Spirost-25(27)-en-1β,3β,5β-trihydroxy-1-O-β-d-xyloside (3)
3.7. Acid Hydrolysis of Compounds 1, 2, 3 and Absolute Sugar Configuration Determination
3.8. Cytotoxicity Assay
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Song, X.; Liu, H. Research and Application of “Qi-Medicines” in Taibai Mountains; People’s Medical Publishing House: Beijing, China, 2011. [Google Scholar]
- Wu, X.; Fan, J.; Ouyang, Z.; Ning, R.; Guo, W.; Shen, Y.; Sun, Y.; Xu, Q. Tupistra chinensis extract attenuates murine fulminant hepatitis with multiple targets against activated T lymphocytes. J. Pharm. Pharmacol. 2014, 66, 453–465. [Google Scholar]
- Pan, Z.; Li, Y.; Liu, J.; Ning, D.; Li, D.; Wu, X.; Wen, Y. A cytotoxic cardenolide and a saponin from the rhizomes of Tupistra chinensis. Fitoterapia 2012, 83, 1489–1493. [Google Scholar]
- Cai, J.; Zhu, Z.; Yu, C.; Lei, L.; Wu, S. Saponin from Tupistra chinensis Baker inhibits mouse sarcoma S-180 cell proliferation in vitro and implanted solid tumor growth in mice. J. South. Med. Univ. 2007, 27, 188–190. [Google Scholar]
- Li, Q.; Zou, K.; Wang, Y. Experimental study on vitro anti-tumor action of “Kai-Kou-Jian” extract. Chin. J. Ethnomed. Ethnopharm. 2007, 86, 164–167. [Google Scholar]
- Liu, C.; Guo, Z.; Xue, Y.; Cheng, J.; Huang, N.; Zhou, Y.; Cheng, F.; Zou, K. Five new furostanol saponins from the rhizomes of Tupistra chinensis. Fitoterapia 2012, 83, 323–328. [Google Scholar]
- Huang, W.; Zhang, H.; Zou, K.; Chen, J.; Li, X.; Liu, C.; Huang, N. Total saponins of Tupistra chinensis induces apoptosis in A549 cells. Neoplasma 2012, 59, 613–621. [Google Scholar]
- Song, X.; Li, Y.; Zhang, D.; Jiang, Y.; Wang, W.; Song, B.; Tang, Z.; Cui, J.; Yue, Z. Two new spirostanol saponins from the the roots and rhizomes of Tupistra chinensis. Phytochem. Lett. 2015, 13, 6–10. [Google Scholar]
- Chai, J.; Song, X.; Wang, X.; Mei, Q.; Li, Z.; Cui, J.; Tang, Z.; Yue, Z. Two new compounds from the roots and rhizomes of Trillium tschonoskii. Phytochem. Lett. 2014, 10, 113–117. [Google Scholar]
- Li, H.; Pan, X.; Mei, Q.; Song, X.; Pei, Y.; Yue, Z. Isolation and identification of chemical constituents from root and rhizoma of Trillium tschonoskii Maxim. J. Shenyang Pharm. Univ. 2013, 30, 509–516. [Google Scholar]
- Yue, Z.; Qin, H.; Li, Y.; Sun, Y.; Wang, Z.; Yang, T.; Liu, L.; Wang, M.; Feng, F.; Mei, Q. Chemical constituents of the root of Jasminum giraldii. Molecules 2013, 18, 4766–4775. [Google Scholar]
- Hudson, C.; Dale, J. Studies on the forms of D-glucose and their mutarotation. J. Am. Chem. Soc. 1917, 39, 320–328. [Google Scholar]
- Zhang, X.; Chen, C.; Yang, J.; Ni, W.; Liu, H. New minor spirostane glycosides from Ypsilandra thibetica. Helv. Chim. Acta 2012, 95, 1087–1093. [Google Scholar]
- Yan, W.; Ohtani, K.; Kasai, R.; Yamasaki, K. Steroidal saponins from fruits of Tribulus terrestris. Phytochemistry 1996, 42, 1417–1422. [Google Scholar]
- Liu, C.; Guo, Z.; Xue, Y.; Zhang, H.; Zhang, H.; Zou, K.; Huang, N. Tupisteroide A–C, three new polyhydroxylated steroidal constituents from the roots of Tupistra chinensis. Magn. Reson. Chem. 2012, 50, 320–324. [Google Scholar]
- Xu, L.; Zou, K.; Wang, J.; Wu, J.; Zhou, Y.; Dan, F.; Yang, J. New polyhydroxylated furostanol saponins with inhibitory action against NO production from Tupistra chinensis Rhizomes. Molecules 2007, 12, 2029–2037. [Google Scholar]
- Li, Y.; Fan, L.; Sun, Y.; Miao, X.; Zhang, F.; Meng, J.; Han, J.; Zhang, D.; Zhang, R.; Yue, Z.; et al. Paris saponin VII from trillium tschonoskii reverses multidrug resistance of adriamycin-resistant MCF-7/ADR cells via P-glycoprotein inhibition and apoptosis augmentation. J. Ethnopharmcol. 2014, 154, 728–734. [Google Scholar]
- Li, Y.; Sun, Y.; Fan, L.; Zhang, F.; Meng, J.; Han, J.; Guo, X.; Zhang, D.; Zhang, R.; Yue, Z.; et al. Paris saponin VII inhibits growth of colorectal cancer cells through Ras signaling pathway. Biochem. Pharmacol. 2014, 88, 150–157. [Google Scholar]
- Pan, W.; Chang, F.; Wei, L.; Wu, Y. New flavans, spirostanol sapogenins, and a pregnane genin from Tupistra chinensis and their cytotoxicity. J. Nat. Prod. 2003, 66, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds 3–5 are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Wang, X.; He, H.; Zhang, D.; Jiang, Y.; Yang, X.; Wang, F.; Tang, Z.; Song, X.; Yue, Z. Steroidal Saponins from the Roots and Rhizomes of Tupistra chinensis. Molecules 2015, 20, 13659-13669. https://doi.org/10.3390/molecules200813659
Li Y, Wang X, He H, Zhang D, Jiang Y, Yang X, Wang F, Tang Z, Song X, Yue Z. Steroidal Saponins from the Roots and Rhizomes of Tupistra chinensis. Molecules. 2015; 20(8):13659-13669. https://doi.org/10.3390/molecules200813659
Chicago/Turabian StyleLi, Yuze, Xin Wang, Hao He, Dongdong Zhang, Yi Jiang, Xinjie Yang, Fei Wang, Zhishu Tang, Xiaomei Song, and Zhenggang Yue. 2015. "Steroidal Saponins from the Roots and Rhizomes of Tupistra chinensis" Molecules 20, no. 8: 13659-13669. https://doi.org/10.3390/molecules200813659
APA StyleLi, Y., Wang, X., He, H., Zhang, D., Jiang, Y., Yang, X., Wang, F., Tang, Z., Song, X., & Yue, Z. (2015). Steroidal Saponins from the Roots and Rhizomes of Tupistra chinensis. Molecules, 20(8), 13659-13669. https://doi.org/10.3390/molecules200813659





