A Fungal α-Galactosidase from Tricholoma matsutake with Broad Substrate Specificity and Good Hydrolytic Activity on Raffinose Family Oligosaccharides
Abstract
:1. Introduction
2. Results and Discussion
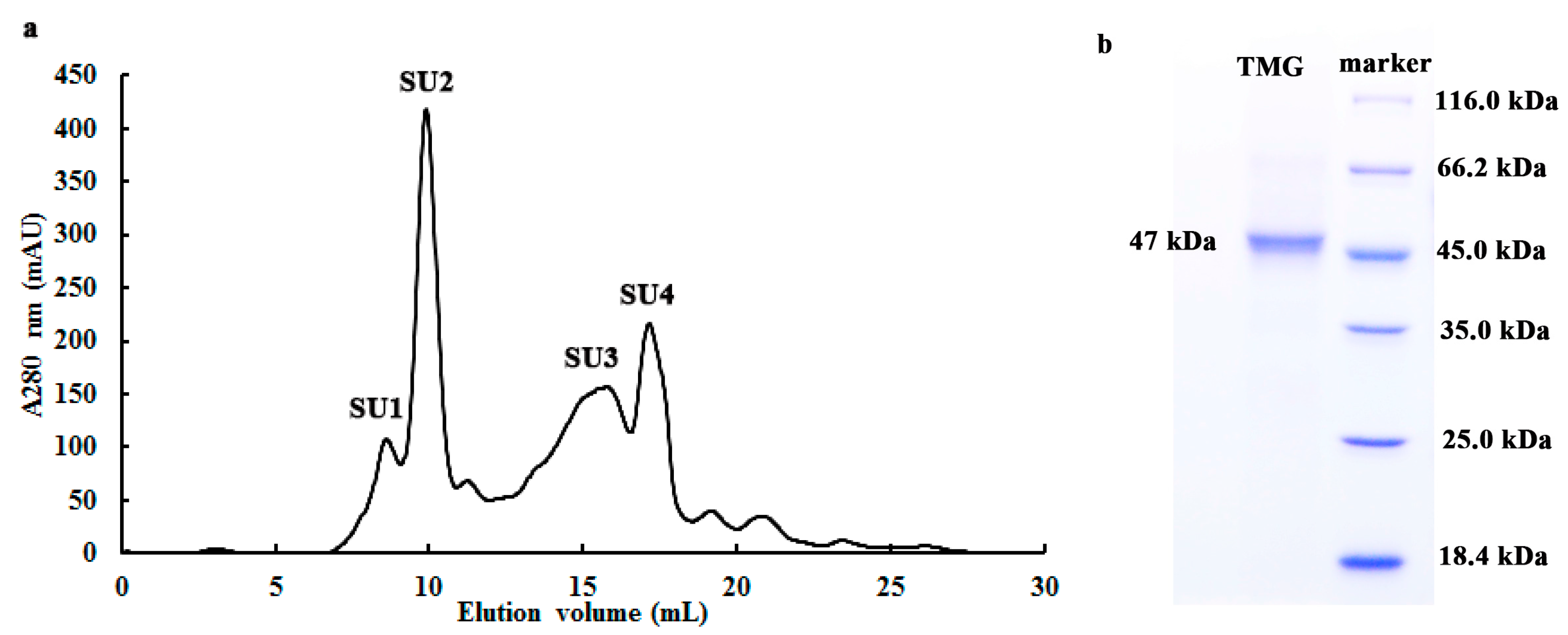
2.1. Purification of TMG
| Chromatographic Fraction | Total Protein (mg/400 g) | Total Activity (U) a | Specific Activity (U/mg) b | Yield (%) | Purification Fold c |
|---|---|---|---|---|---|
| Crude extract | 17,427 | 116,204 | 6.7 | 100 | 1 |
| Q2 | 311.7 | 102,037 | 327.3 | 87.9 | 49.1 |
| MonoQ1 | 95.6 | 34,490 | 360.8 | 29.7 | 54.1 |
| SU2 | 9.6 | 8727 | 909.1 | 7.5 | 136.3 |
2.2. Determination of Molecular Weight and Amino Acid Sequence of TMG
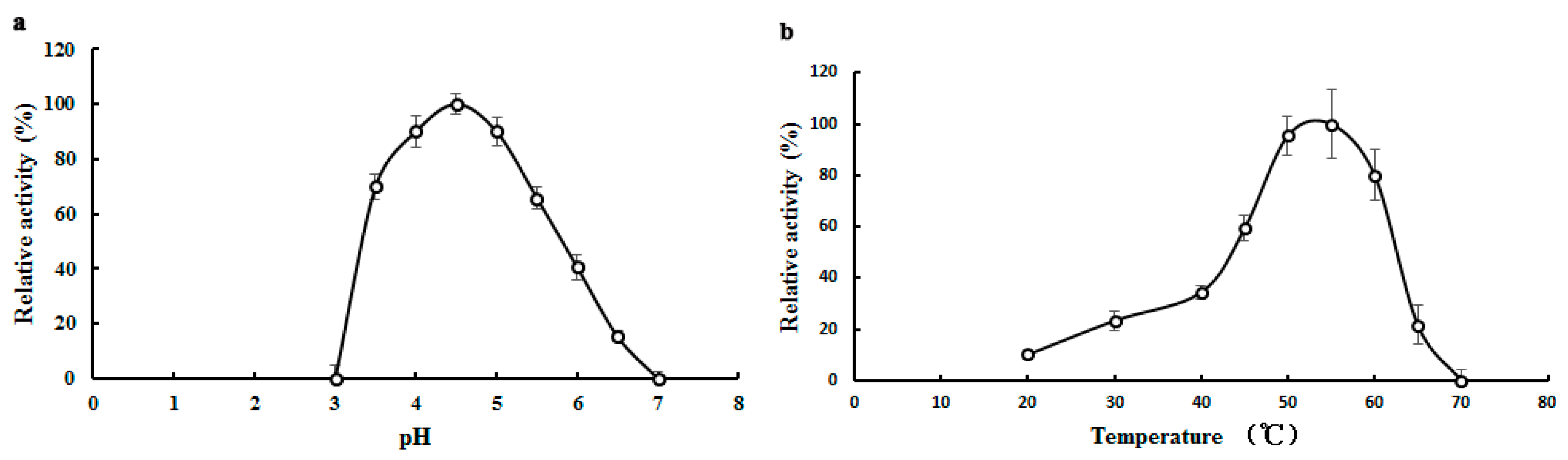
2.3. Biochemical Properties of TMG
| Metal Ion Concentration | Relative—Galactosidase Activity (%) | |||
|---|---|---|---|---|
| 10 mM | 5 mM | 2.5 mM | 1.25 mM | |
| Mg2+ | 30.6 ± 0.03 | 80.90.01 | 82.6 ± 0.02 | 93.0 ± 0.04 |
| Mn2+ | 43.3 ± 0.03 | 69.1 ± 0.07 | 101.6 ± 0.02 | 100.4 ± 0.02 |
| Pb2+ | 9.6 ± 0.02 | 80.0 ± 0.12 | 88.3 ± 0.09 | 95.3 ± 0.05 |
| Al3+ | 72.1 ± 0.01 | 80.7 ± 0.03 | 73.1 ± 0.05 | 82.5 ± 0.02 |
| Cu2+ | ND | ND | 31.8 ± 0.01 | 80.9 ± 0.05 |
| Fe3+ | ND | ND | ND | 31.4 ± 0.02 |
| EDTA | 116.4 ± 0.65 | 96.3 ± 0.84 | 101.3 ± 0.61 | 112.5 ± 0.45 |
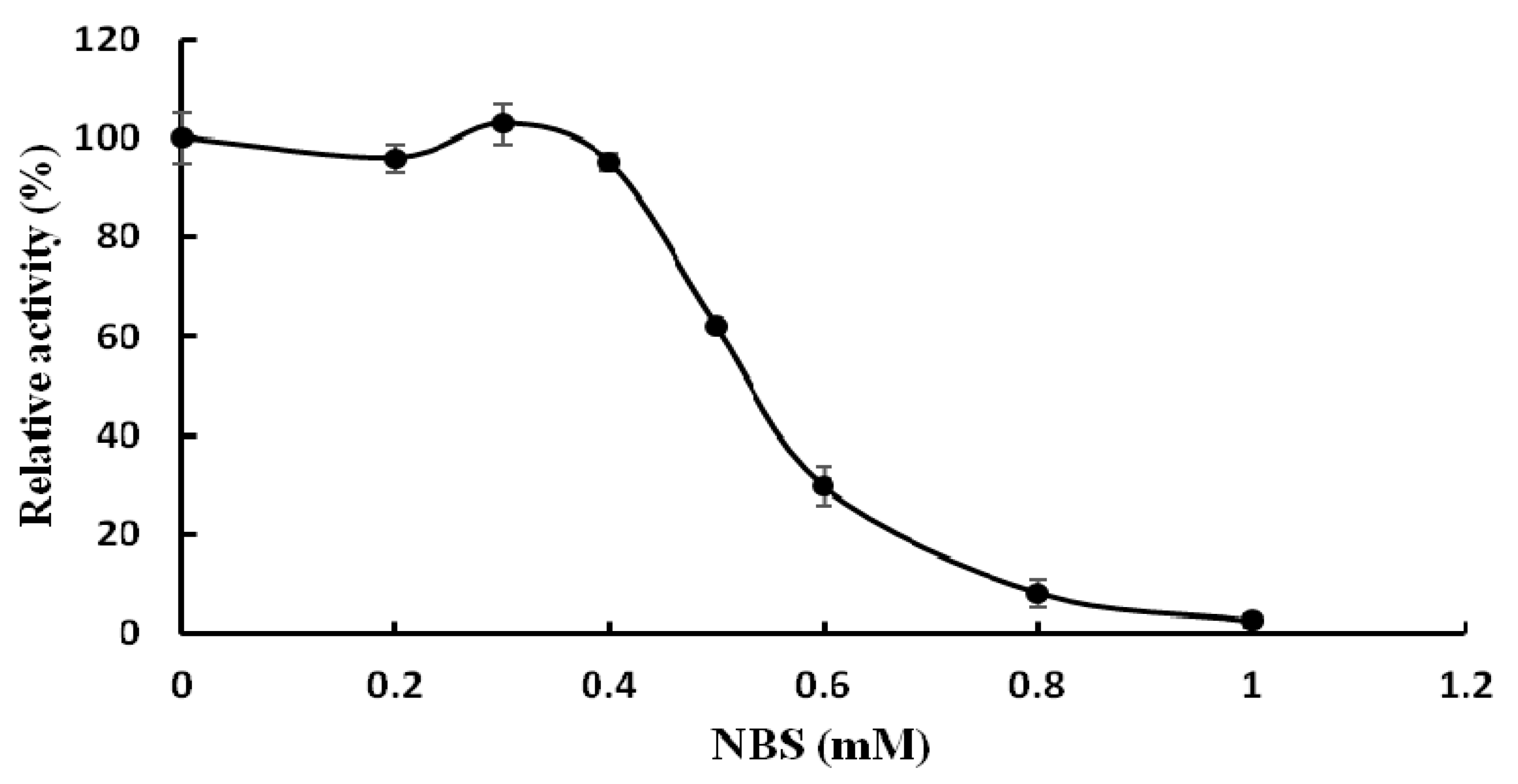
2.4. Substrate Specificity of TMG and Determination of Kinetic Parameters
| Substrate | Concentration (mM) | Relative Activity (%) a |
|---|---|---|
| 4-Nitrophenyl α-d-galactopyranoside (pNPG) | 10 | 100 ± 0.77 |
| 2-Nitrophenyl β-d-galactopyranoside (oNPG) | 10 | 0.9 ± 0.03 |
| 4-Nitrophenyl β- d-glucuronide | 10 | 4 ± 0.01 |
| Stachyose | 50 | 67 ± 0.02 |
| Raffinose | 50 | 84 ± 0.01 |
| Melibiose | 50 | 17 ± 0.11 |
| Locust bean gum | 1% | 12 ± 0.07 |
| Guar gum | 1% | 13 ± 0.05 |
| Substrate | Km (Mm) | Kcat a (s−1) | Kcat/Km (s−1·mM−1) |
|---|---|---|---|
| pPNGal | 0.99 | 9.17 | 9.3 |
| Raffinose | 3.7 | 6.4 | 1.73 |
| Stachyose | 3.5 | 6.7 | 1.9 |
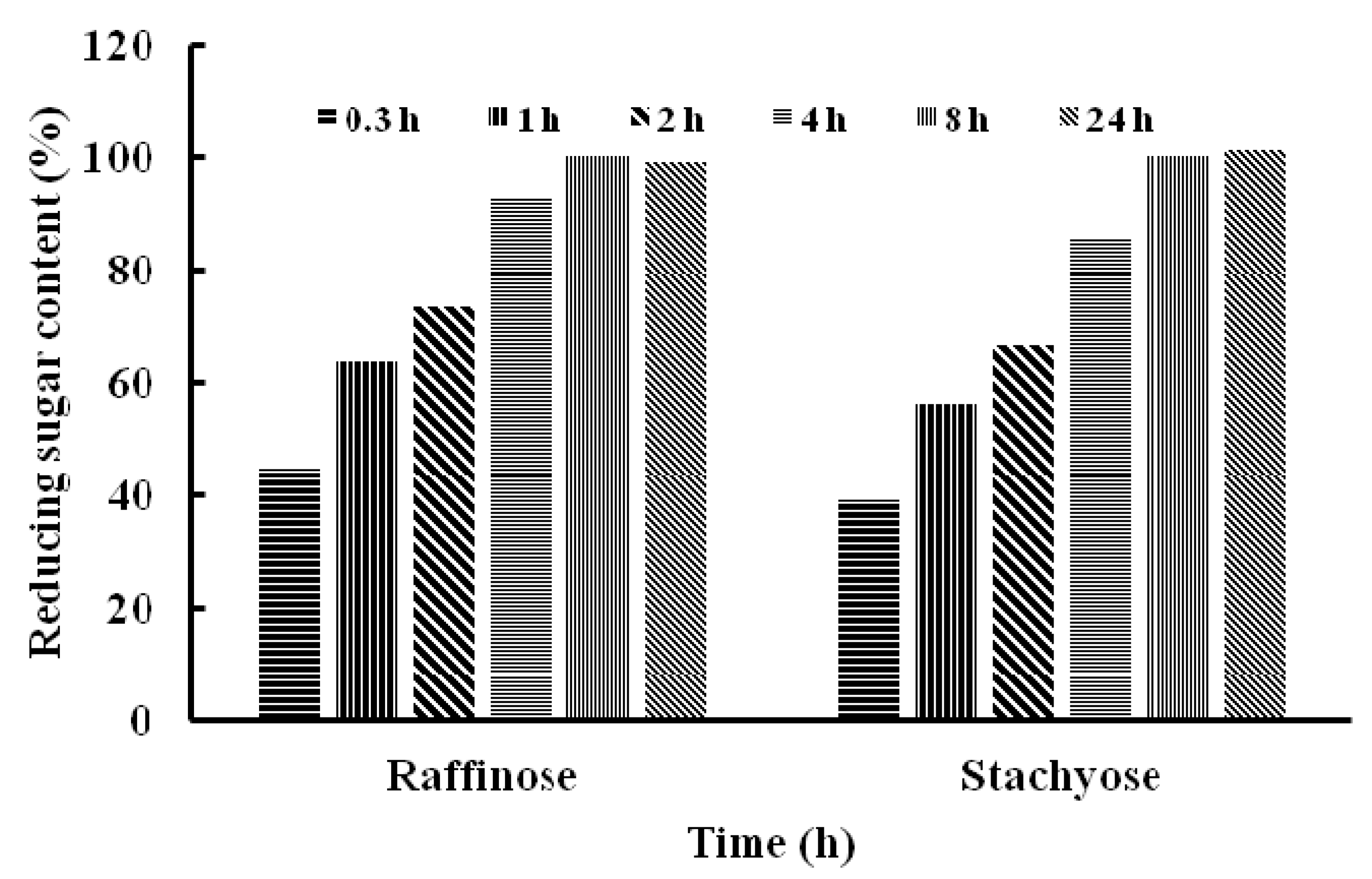
3. Experimental Section
3.1. Materials
3.2. Enzyme Activity Assay
3.3. Purification of α-Galactosidase from Tricholoma Matsutake
3.4. Determination of Molecular Weight and Amino Acid Sequence
3.5. Biochemical Properties of the Enzyme
3.6. Substrate Specificity and Kinetic Parameters
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Katrolia, P.; Jia, H.; Yan, Q.; Song, S.; Jiang, Z.; Xu, H. Characterization of a protease-resistant alpha-galactosidase from the thermophilic fungus Rhizomucor miehei and its application in removal of raffinose family oligosaccharides. Bioresour. Technol. 2012, 110, 578–586. [Google Scholar] [CrossRef] [PubMed]
- Steggerda, F.R. Gastrointestinal gas following food consumption. Ann. N. Y. Acad. Sci. 1968, 150, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Kayastha, A.M. A novel application of Cicer alpha-galactosidase in reduction of raffinose family oligosaccharides in soybean folur. J. Plant Biochem. Biotechnol. 2013, 22, 353–356. [Google Scholar] [CrossRef]
- Ghazi, S.; Rooke, J.A.; Galbraith, H. Improvement of the nutritive value of soybean meal by protease and alpha-galactosidase treatment in broiler cockerels and broiler chicks. Br. Poult. Sci. 2003, 44, 410–418. [Google Scholar] [CrossRef] [PubMed]
- Kotiguda, G.; Kapnoor, S.S.; Kulkarni, D.; Mulimani, V.H. Degradation of raffinose oligosaccharides in soymilk by immobilized alpha-galactosidase of Aspergillus oryzae. J. Microbiol. Biotechnol. 2007, 17, 1430–1436. [Google Scholar] [PubMed]
- Kobayashi, H.; Suzuki, H. Studies on the decomposition of raffinose by α-galactosidase of mold. J. Fermet. Technol. 1972, 50, 625–632. [Google Scholar]
- Mittal, Y.; Sharma, C.B. Development of α-galactosidase isoenzymes in chickpea seeds. Plant Sci. 1991, 77, 185–190. [Google Scholar] [CrossRef]
- Gao, Z.; Schaffer, A.A. A novel alkaline alpha-galactosidase from melon fruit with a substrate preference for raffinose. Plant Physiol. 1999, 119, 979–988. [Google Scholar] [CrossRef] [PubMed]
- Smart, E.L.; Pharr, D.M. Characterization of alpha-Galactosidase from Cucumber Leaves. Plant Physiol. 1980, 66, 731–734. [Google Scholar] [CrossRef] [PubMed]
- Du, F.; Zhu, M.; Wang, H.; Ng, T. Purification and characterization of an alpha-galactosidase from Phaseolus coccineus seeds showing degrading capability on raffinose family oligosaccharides. Plant Physiol. Biochem. 2013, 69, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Shi, P.; Huang, H.; Cao, Y.; Meng, K.; Yang, P.; Zhang, R.; Chen, X.; Yao, B. A new alpha-galactosidase from symbiotic Flavobacterium sp. TN17 reveals four residues essential for alpha-galactosidase activity of gastrointestinal bacteria. Appl. Microbiol. Biotechnol. 2010, 88, 1297–1309. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, J.G.; Reis, A.P.; Guimaraes, V.M.; Falkoski, D.L.; da Silva Fialho, L.; de Rezende, S.T. Purification and characterization of Aspergillus terreus alpha-galactosidases and their use for hydrolysis of soymilk oligosaccharides. Appl. Biochem. Biotechnol. 2011, 164, 1111–1125. [Google Scholar] [CrossRef] [PubMed]
- Sripuan, T.; Aoki, K.; Yamamoto, K.; Tongkao, D.; Kumagai, H. Purification and characterization of thermostable alpha-galactosidase from Ganoderma lucidum. Biosci. Biotechnol. Biochem. 2003, 67, 1485–1491. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Shi, P.; Luo, H.; Huang, H.; Yang, P.; Yao, B. A thermophilic alpha-galactosidase from Neosartorya fischeri P1 with high specific activity, broad substrate specificity and significant hydrolysis ability of soymilk. Bioresour. Technol. 2014, 153, 361–364. [Google Scholar] [CrossRef] [PubMed]
- Sampietro, D.; Quiroga, E.; Sgariglia, M.; Soberon, J.; Vattuone, M.A. A thermostable alpha-galactosidase from Lenzites elegans (Spreng.) ex Pat. MB445947: Purification and properties. Antonie Leeuwenhoek 2012, 102, 257–267. [Google Scholar] [CrossRef] [PubMed]
- Saad, R.R.; Fawzi, E.M. Purification and characterization of a thermostable alpha-galactosidase from Thielavia terrestris NRRL 8126 in solid state fermentation. Acta Biol. Hung. 2012, 63, 138–150. [Google Scholar] [CrossRef] [PubMed]
- Simila, J.; Gernig, A.; Murray, P.; Fernandes, S.; Tuohy, M.G. Cloning and expression of a thermostable alpha-galactosidase from the thermophilic fungus Talaromyces emersonii in the methylotrophic yeast Pichia pastoris. J. Microbiol. Biotechnol. 2010, 20, 1653–1663. [Google Scholar] [PubMed]
- Hoshi, H.; Yagi, Y.; Iijima, H.; Matsunaga, K.; Ishihara, Y.; Yasuhara, T. Isolation and characterization of a novel immunomodulatory alpha-glucan-protein complex from the mycelium of Tricholoma matsutake in basidiomycetes. J. Agric. Food Chem. 2005, 53, 8948–8956. [Google Scholar] [CrossRef] [PubMed]
- Ikekawa, T.; Uehara, N.; Maeda, Y.; Nakanishi, M.; Fukuoka, F. Antitumor activity of aqueous extracts of edible mushrooms. Cancer Res. 1969, 29, 734–735. [Google Scholar] [PubMed]
- Mau, J.L.; Lin, H.C.; Song, S.F. Antioxidant properties of several specialty mushrooms. Food Res. Int. 2002, 35, 519–526. [Google Scholar] [CrossRef]
- Xu, L.; Zhu, M.; Chen, X.; Wang, H.; Zhang, G. A novel laccase from fresh fruiting bodies of the wild medicinal mushroom Tricholoma matsutake. Acta Biochim. Pol. 2015, 62, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Hatakeyama, T.; Mizuno, K.; Kurosawa, S. Purification and properties of a nuclease from the fruit body of Tricholoma matsutake. Biosci. Biotechnol. Biochem. 2010, 74, 206–208. [Google Scholar] [CrossRef] [PubMed]
- Tong, H.; Liu, X.; Tian, D.; Sun, X. Purification, chemical characterization and radical scavenging activities of alkali-extracted polysaccharide fractions isolated from the fruit bodies of Tricholoma matsutake. World J. Microbiol. Biotechnol. 2013, 29, 775–780. [Google Scholar] [CrossRef] [PubMed]
- Du, F.; Liu, Q.; Wang, H.; Ng, T. Purification an alpha-galactosidase from Coriolus versicolor with acid-resistant and good degradation ability on raffinose family oligosaccharides. World J. Microbiol. Biotechnol 2014, 30, 1261–1267. [Google Scholar] [CrossRef] [PubMed]
- Ramalingam; Saraswathy, N.; Sadasivam, S.; Subha, K.; Poorani, N. Purification and properties of alpha-galactosidase from white-rot fungus Pleurotus florida. Indian J. Biochem. Biophys. 2007, 44, 76–81. [Google Scholar] [PubMed]
- Singh, N.; Kayastha, A.M. Purification and characterization of alpha-galactosidase from white chickpea (Cicer arietinum). J. Agric. Food chem. 2012, 60, 3253–3259. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Luo, H.; Li, J.; Bai, Y.; Huang, H.; Shi, P.; Fan, Y.; Yao, B. An alpha-galactosidase from an acidophilic Bispora sp. MEY-1 strain acts synergistically with beta-mannanase. Bioresour. Technol. 2010, 101, 8376–8382. [Google Scholar] [CrossRef] [PubMed]
- Patil, A.G.G. α-Galactosidase from bacillus megaterium VHM1 and its application in removal of flatulence-causing factors from soymilk. J. Microbiol. Biotechnol. 2010, 20, 1546–1554. [Google Scholar] [CrossRef] [PubMed]
- Da Silva Fialho, L; Guimaraes, V.M.; Callegari, C.M.; Reis, A.P.; Barbosa, D.S.; de Lima Borgesb, E.E.; Moreira, M.A.; de Rezende, S.T. Characterization and biotechnological application of an acid alpha-galactosidase from Tachigali multijuga Benth. seeds. Phytochemistry 2008, 69, 2579–2585. [Google Scholar]
- Dey, P.M.; Pridham, J.B. Biochemistry of α-galactosidases. Adv. Enzymol. Relat. Areas Mol. Biol. 1972, 36, 114–118. [Google Scholar]
- Gote, M.M.; Khan, M.I.; Khire, J.M. Active site directed chemical modification of alpha-galactosidase from Bacillus stearothermophilus (NCIM 5146): Involvement of lysine, tryptophan and carboxylate residues in catalytic site. Enzyme Microb. Technol. 2007, 40, 1312–1320. [Google Scholar] [CrossRef]
- Mathew, C.D.; Balasubramaniam, K. Chemical modification of alpha-galactosidase from coconut. Phytochemistry 1986, 25, 2439–2443. [Google Scholar] [CrossRef]
- Huang, K.; Chen, S.Z.; Yang, K.Y. Crystallization and chemical modification of disulfide bond of calf chymosin. Chin. J. Biotechnol. 1991, 7, 83–92. [Google Scholar] [PubMed]
- Chen, Z.; Yan, Q.; Jiang, Z.; Liu, Y.; Li, Y. High-level expression of a novel α-galactosidase gene from Rhizomucor miehei in Pichia pastoris and characterization of the recombinant enyzme. Protein Expr. Purif. 2015, 110, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Srivastava, G.; Talat, M.; Raghubanshi, H.; Srivastava, O.N.; Kayastha, A.M. Cicer alpha-galactosidase immobilization onto functionalized graphene nanosheets using response surface method and its applications. Food Chem. 2014, 142, 430–438. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Kayastha, A.M. Cicer alpha-galactosidase immobilization onto chitosan and Amberlite MB-150: Optimization, characterization, and its applications. Carbohydr. Res. 2012, 358, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Park, I.; Cho, J. Immobilization of the antarctic Bacillus sp. LX-1 alpha-galactosidase on eudragit L-100 for the production of a functional feed additive. Asian Australas. J. Anim. Sci. 2013, 26, 552–557. [Google Scholar] [CrossRef] [PubMed]
- Bayraktar, H.; Serilmez, M.; Karkas, T.; Celem, E.B.; Onal, S. Immobilization and stabilization of alpha-galactosidase on Sepabeads EC-EA and EC-HA. Intl. J. Biol. Macromol. 2011, 49, 855–860. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, O.; Ortiz, C.; Berenguer-Murcia, A.; Torres, R.; Rodrigues, R.C.; Fernandez-Lafuente, R. Strategies for the one-step immobilization-purification of enzymes as industrial biocatalysts. Biotechnol. Adv. 2015, 33, 435–456. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, R.C.; Ortiz, C.; Berenguer-Murcia, A.; Torres, R.; Fernandez-Lafuente, R. Modifying enzyme activity and selectivity by immobilization. Chem. Soc. Rev. 2013, 42, 6290–6307. [Google Scholar] [CrossRef] [PubMed]
- Husain, Q. Beta galactosidases and their potential applications: A review. Crit. Rev. Biotechnol. 2010, 30, 41–62. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Wang, Y.; Luo, H.; Shi, P.; Meng, K.; Zhou, Z.; Zhang, Z.; Yao, B. Molecular cloning and expression of a novel protease-resistant GH-36 alpha-galactosidase from Rhizopus sp. F78 ACCC 30795. J. Microbiol. Biotechnol. 2009, 19, 1295–1300. [Google Scholar] [PubMed]
- Laemmli, U.K.; Favre, M. Maturation of the head of bacteriophage T4. I. DNA packaging events. J. Mol. Biol. 1973, 80, 575–599. [Google Scholar] [CrossRef]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing suGAR. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Geng, X.; Tian, G.; Zhao, Y.; Zhao, L.; Wang, H.; Ng, T.B. A Fungal α-Galactosidase from Tricholoma matsutake with Broad Substrate Specificity and Good Hydrolytic Activity on Raffinose Family Oligosaccharides. Molecules 2015, 20, 13550-13562. https://doi.org/10.3390/molecules200813550
Geng X, Tian G, Zhao Y, Zhao L, Wang H, Ng TB. A Fungal α-Galactosidase from Tricholoma matsutake with Broad Substrate Specificity and Good Hydrolytic Activity on Raffinose Family Oligosaccharides. Molecules. 2015; 20(8):13550-13562. https://doi.org/10.3390/molecules200813550
Chicago/Turabian StyleGeng, Xueran, Guoting Tian, Yongchang Zhao, Liyan Zhao, Hexiang Wang, and Tzi Bun Ng. 2015. "A Fungal α-Galactosidase from Tricholoma matsutake with Broad Substrate Specificity and Good Hydrolytic Activity on Raffinose Family Oligosaccharides" Molecules 20, no. 8: 13550-13562. https://doi.org/10.3390/molecules200813550






