Separation of Polyphenols and Caffeine from the Acetone Extract of Fermented Tea Leaves (Camellia sinensis) Using High-Performance Countercurrent Chromatography
Abstract
:1. Introduction
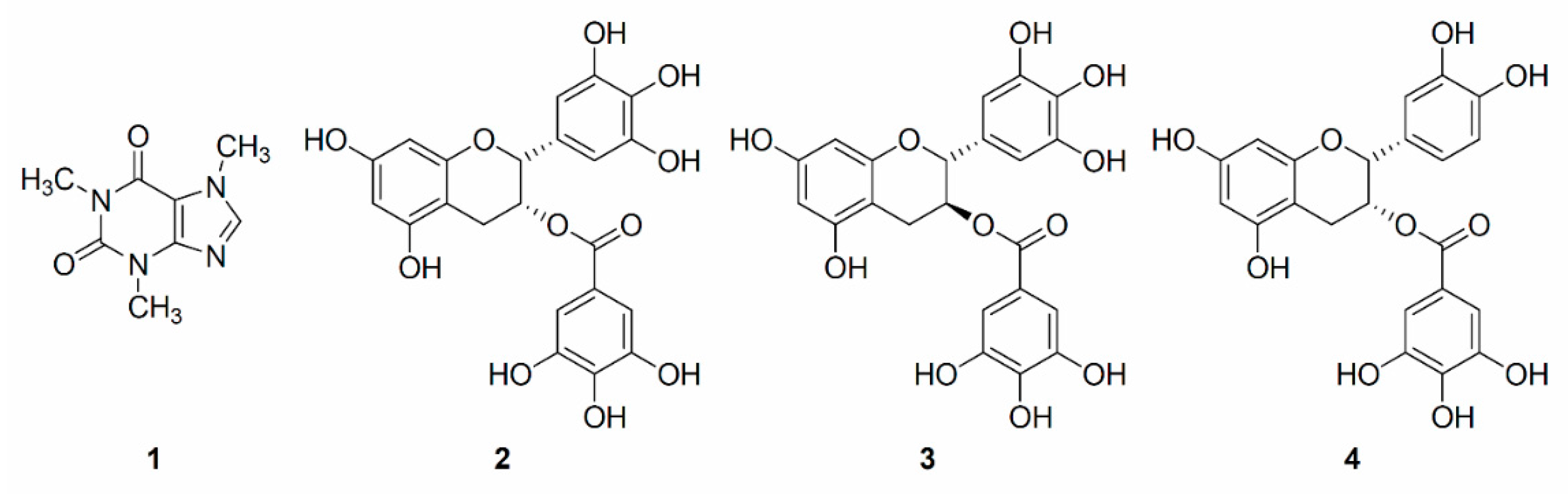
2. Results and Discussion
2.1. HPLC-PDA Analysis of Sample Extract

2.2. Evaluation of Partition Coefficient (K) Value
| HEMWat System (v/v) | Partition Coefficient (K) Value | ||||||
|---|---|---|---|---|---|---|---|
| 1 (K1) | α1 | 2 (K2) | α2 | 3 (K3) | α3 | 4 (K4) | |
| 3:7:3:7 | 0.27 | - | 0.03 | 2.33 | 0.07 | 2.14 | 0.15 |
| 2:8:2:8 | 0.39 | - | 0.23 | 2.04 | 0.47 | 1.74 | 0.82 |
| 1:9:1:9 | 0.60 | 2.28 | 1.37 | 1.78 | 2.45 | 1.52 | 3.74 |
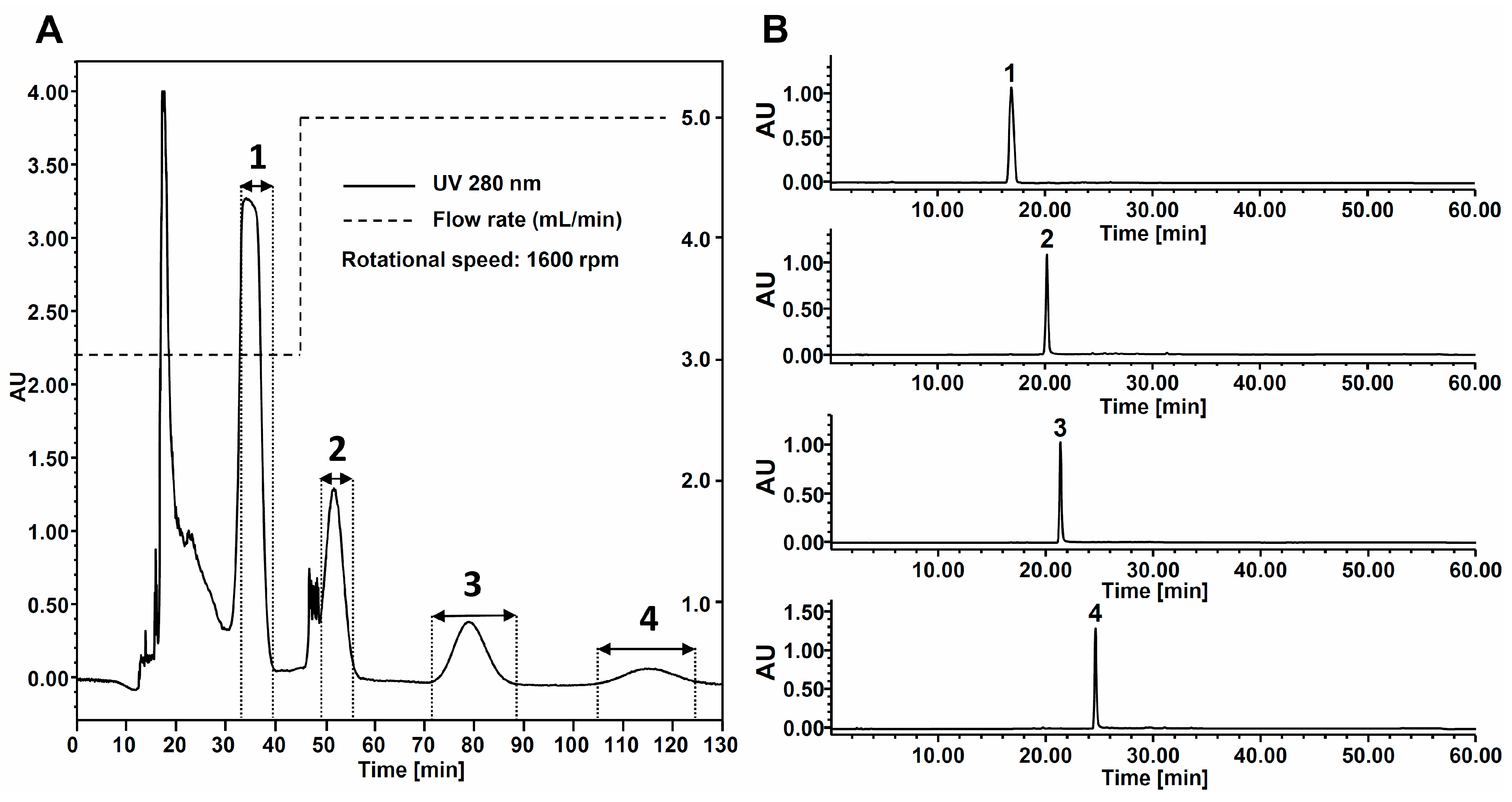
2.3. HPCCC Separation of Sample Extract

3. Experimental Section
3.1. General Experimental Procedures
3.2. Preparation of Sample Extract
3.3. HPLC-PDA Analysis
3.4. Evaluation of Partition Coefficient Value
3.5. HPCCC Procedure
3.6. Identification of HPCCC Peak Fractions
Acknowledgements
Author Contributions
Conflicts of Interest
References
- Preedy, V.R. Tea in Health and Disease Prevention, 1st ed.; Elsevier: New York, NY, USA, 2013; pp. 3–115. [Google Scholar]
- Benzie, I.F.F.; Szeto, Y.T. Total antioxidant capacity of teas by the ferric reducing/antioxidant power assay. J. Agric. Food. Chem. 1999, 47, 633–636. [Google Scholar] [CrossRef] [PubMed]
- Yen, G.C.; Chen, H.Y. Antioxidant activity of various tea extracts in ralation to their antimutagenecity. J. Agric. Food. Chem. 1995, 43, 27–32. [Google Scholar] [CrossRef]
- Lin, Y.L.; Tsai, S.H.; Lin-Shiau, S.Y.; Ho, C.T.; Lin, J.K. Theaflavin-3,3′-digallate from black tea blocks the nitric oxide synthase by down-regulating the activation of NF-kappaB in macrophages. Eur. J. Pharmacol. 1999, 367, 379–388. [Google Scholar] [CrossRef]
- De Mejia, E.C.; Ramirez-Mares, M.V.; Puangpraphant, S. Bioactive components of tea; cnacer, inflammation and behavior. Brain Behav. Immun. 2009, 6, 721–731. [Google Scholar] [CrossRef] [PubMed]
- Kumaran, V.S.; Arulmathi, K.; Sundarapandiyan, R.; Kalaiselvi, P. Attenuation of the inflammatory changes and lipid anomalies by epigallocatechin-3-O-gallate in hypercholesterolemic diet fed aged rats. Exp. Gerontol. 2009, 12, 745–751. [Google Scholar] [CrossRef] [PubMed]
- Nakai, M.; Fukui, Y.; Asami, S.; Toyoda-Ono, Y.; Iwashita, T.; Shibata, H.; Mitsunaga, T.; Hashimoto, F.; Kiso, Y. Inhibitory effects of oolong tea polyphenol on pancreatic lipase in vitro. J. Agric. Food. Chem. 2005, 53, 4593–4598. [Google Scholar] [CrossRef] [PubMed]
- Yeh, C.W.; Chen, W.J.; Chiang, G.T.; Lin-Shiau, S.Y.; Lin, J.K. Suppression of fatty acid synthase in MCF-7 breast cancer cells by tea and tea polyphenols: A possible mechanism for their hypolipidemic effects. Pharmacogenomics J. 2003, 3, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Yanagida, A.; Shoji, A.; Shibusawa, Y.; Shindo, H.; Tagashira, M.; Ikeda, M. Analytical separation of tea catechins and food-related polyphenols by high-speed counter-current chromatography. J. Chromatogr. A 2006, 1112, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Li, D.; Wan, X. Combination of HSCCC and Sephadex LH-20 methods an approach to isolation and purification of the main individual theaflavins from black tea. J. Chromatogr. B 2008, 861, 140–144. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Liu, Z.; Huang, J.A.; Dong, X.; Song, L.; Pan, Y.; Liu, F. Preparative isolation and purification of theaflavins and catechins by high-speed countercurrent chromatography. J. Chromatogr. B 2008, 867, 282–286. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.S.; Wijekoon, W.M.B.; Kumar, V.; Punyasiri, P.N.; Abeysinghe, I.S.B. Separation of proanthocyanidins isolated from tea leaves using high-speed counter-current chromatography. J. Chromatogr. A 2009, 1216, 4295–4302. [Google Scholar] [CrossRef] [PubMed]
- Degenhardt, A.A.; Engelhardt, U.H.; Lakenbrink, C.; Winterhalte, P. Preparative separation of polyphenols from tea by high-speed countercurrent chromatography. J. Agric. Food. Chem. 2000, 48, 3425–3430. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y. Golden rules and pitfalls in selecting optimum conditions for high-speed counter-current chromatography. J. Chromatogr. A 2005, 1065, 145–168. [Google Scholar] [CrossRef] [PubMed]
- Yoon, K.D.; Chin, Y.W.; Kim, J. Centrifugal partition chromatography: Application to natural products in 1994–2009. J. Liq. Chromatogr. Relat. Technol. 2010, 33, 1208–1254. [Google Scholar] [CrossRef]
- Sutherland, I.A.; Fisher, D. Role of counter-current chromatography in the modernization of Chinese herbal medicines. J. Chromatogr. A 2009, 1216, 740–753. [Google Scholar] [CrossRef] [PubMed]
- Berthod, A.; Ruiz-Ángel, M.J.; Carda-Broch, S. Countercurrent chromatography: People and applications. J. Chromatogr. A 2009, 1216, 4206–4217. [Google Scholar] [CrossRef] [PubMed]
- Wood, P.; Janaway, L.; Hawes, D.; Sutherland, I.A. Stationary Phase Retention in CCC: Modelling the J-Type Centrifuge as a Constant Pressure Drop Pump. J. Liq. Chromatogr. Relat. Technol. 2003, 26, 1373–1396. [Google Scholar] [CrossRef]
- Guzlek, H.; Wood, P.; Janaway, L. Performance comparison using the GUESS mixture to evaluate counter-current chromatography instruments. J. Chromatogr. A 2009, 1216, 4181–4186. [Google Scholar] [CrossRef] [PubMed]
- Marston, A.; Hostettmann, K. Developments in the application of counter-current chromatography to plant analysis. J. Chromatogr. A 2006, 1112, 181–194. [Google Scholar] [CrossRef] [PubMed]
- Du, Q.; Wu, P.; Ito, Y. Low-speed rotary countercurrent chromatography using a convoluted multilayer helical tube for industrial separation. Anal. Chem. 2000, 72, 3363–3365. [Google Scholar] [CrossRef] [PubMed]
- Martins, I.L.; Miranda, J.P.; Oliveira, N.G.; Fernandex, A.S.; Gongalves, S.; Antunes, A.M.M. Synthesis and biological activity of 6-selenocaffeine: potential modulator of chemotherapeutic drugs in breast cancer cells. Molecules 2013, 18, 5251–5264. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.C.; Wang, M.N.; Tseng, T.Y.; Sung, J.S.; Tsai, T.H. Pharmacokinetics of (−)-epigallocatechin-3-gallate in conscious and freely moving rats and its brain regional distribution. J. Agric. Food. Chem. 2007, 55, 1517–1524. [Google Scholar] [CrossRef] [PubMed]
- Sang, S.; Lee, M.J.; Hou, Z.; Ho, C.T.; Yang, C.S. Stability of tea polyphenol (−)-epigallocatechin-3-gallate and formation of dimers and epimers under common experimental conditions. J. Agric. Food. Chem. 2005, 53, 9478–9484. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Lee, J.Y.; Kim, S.M.; Park, D.A.; Jin, C.; Hong, S.P.; Lee, Y.S. A new epicatechin gallate and calpain inhibitory activity from Orostachys japonicus. Fitoterapia 2009, 80, 73–76. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds 1–4 are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, S.J.; Hong, Y.D.; Lee, B.; Park, J.S.; Jeong, H.W.; Kim, W.G.; Shin, S.S.; Yoon, K.D. Separation of Polyphenols and Caffeine from the Acetone Extract of Fermented Tea Leaves (Camellia sinensis) Using High-Performance Countercurrent Chromatography. Molecules 2015, 20, 13216-13225. https://doi.org/10.3390/molecules200713216
Choi SJ, Hong YD, Lee B, Park JS, Jeong HW, Kim WG, Shin SS, Yoon KD. Separation of Polyphenols and Caffeine from the Acetone Extract of Fermented Tea Leaves (Camellia sinensis) Using High-Performance Countercurrent Chromatography. Molecules. 2015; 20(7):13216-13225. https://doi.org/10.3390/molecules200713216
Chicago/Turabian StyleChoi, Soo Jung, Yong Deog Hong, Bumjin Lee, Jun Seong Park, Hyun Woo Jeong, Wan Gi Kim, Song Seok Shin, and Kee Dong Yoon. 2015. "Separation of Polyphenols and Caffeine from the Acetone Extract of Fermented Tea Leaves (Camellia sinensis) Using High-Performance Countercurrent Chromatography" Molecules 20, no. 7: 13216-13225. https://doi.org/10.3390/molecules200713216






