New Fluorescence Probes for Biomolecules
Abstract
:1. Introduction
2. Results and Discussion
2.1. The Study of Squarine with BSA Association Process
| Dye | Solvent | Absorption (nm) | ε1 | ε2 | ε3 | ||
|---|---|---|---|---|---|---|---|
| λ1 | λ2 | λ3 | (×105 mol−1·cm−1·L) | (×105 mol−1·cm−1·L) | (×105 mol−1·cm−1·L) | ||
| SQ6 | Dichloromethane | 283 | 316 | 0.61 | 0.46 | ||
| Methanol | 278 | 330 | 0.507 | 0.48 | |||
| DMSO | 284 | 343 | 0.39 | 0.437 | |||
| Water | 275 | 316 | 0.777 | 0.595 | |||
| SQ7 | Tetrahydrofurane | 625 | 1.25 | ||||
| Methanol | 629 | 1.135 | |||||
| Nitromethane | 631 | 1.25 | |||||
| Acetonitrile | 635 | 1.19 | |||||
| DMSO | 639 | 2.45 | |||||
| SQ8 | Dichloromethane | 561 | 614 | 795 | 0.02 | 0.023 | 0.014 |
| Acetone | 554 | 603 | 788 | 0.024 | 0.03 | 0.014 | |
| Methanol | 554 | 609 | 780 | 0.045 | 0.049 | 0.025 | |
| Nitromethane | 555 | 607 | 787 | 0.046 | 0.049 | 0.029 | |
| Acetonitrile | 552 | 602 | 785 | 0.027 | 0.029 | 0.013 | |
| DMSO | 568 | 611 | 0.049 | 0.055 | |||
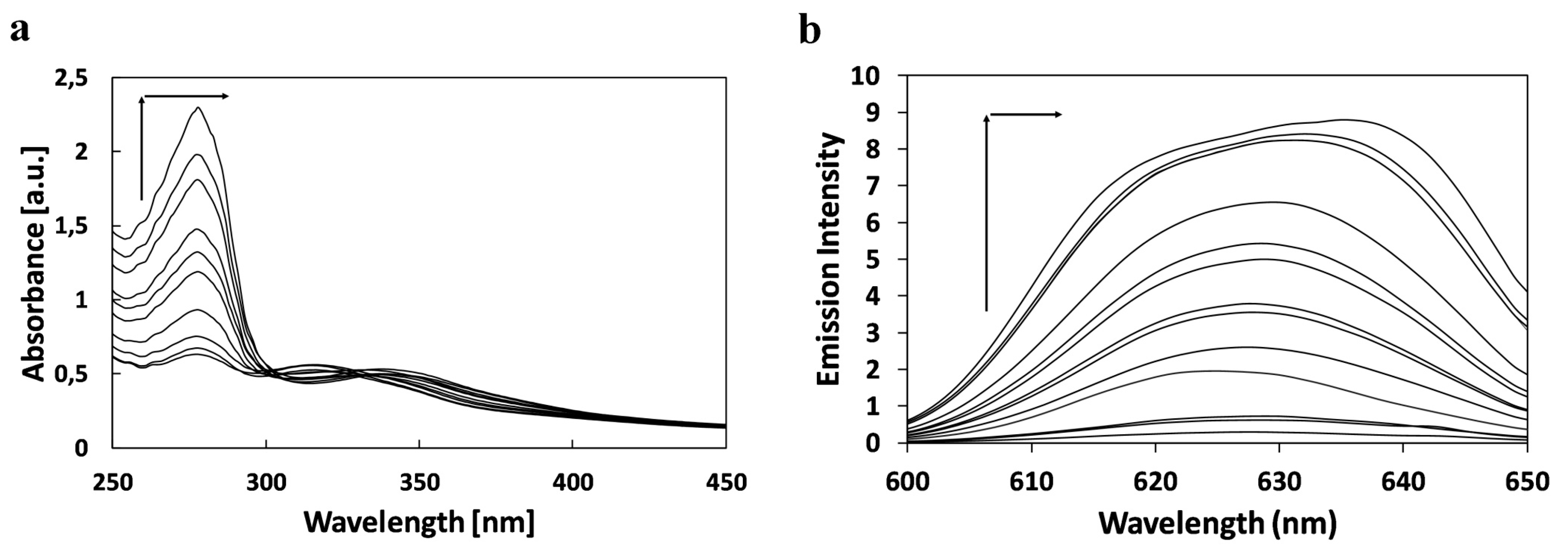
2.2. Study of Quenching Process of BSA by Squarine Dyes
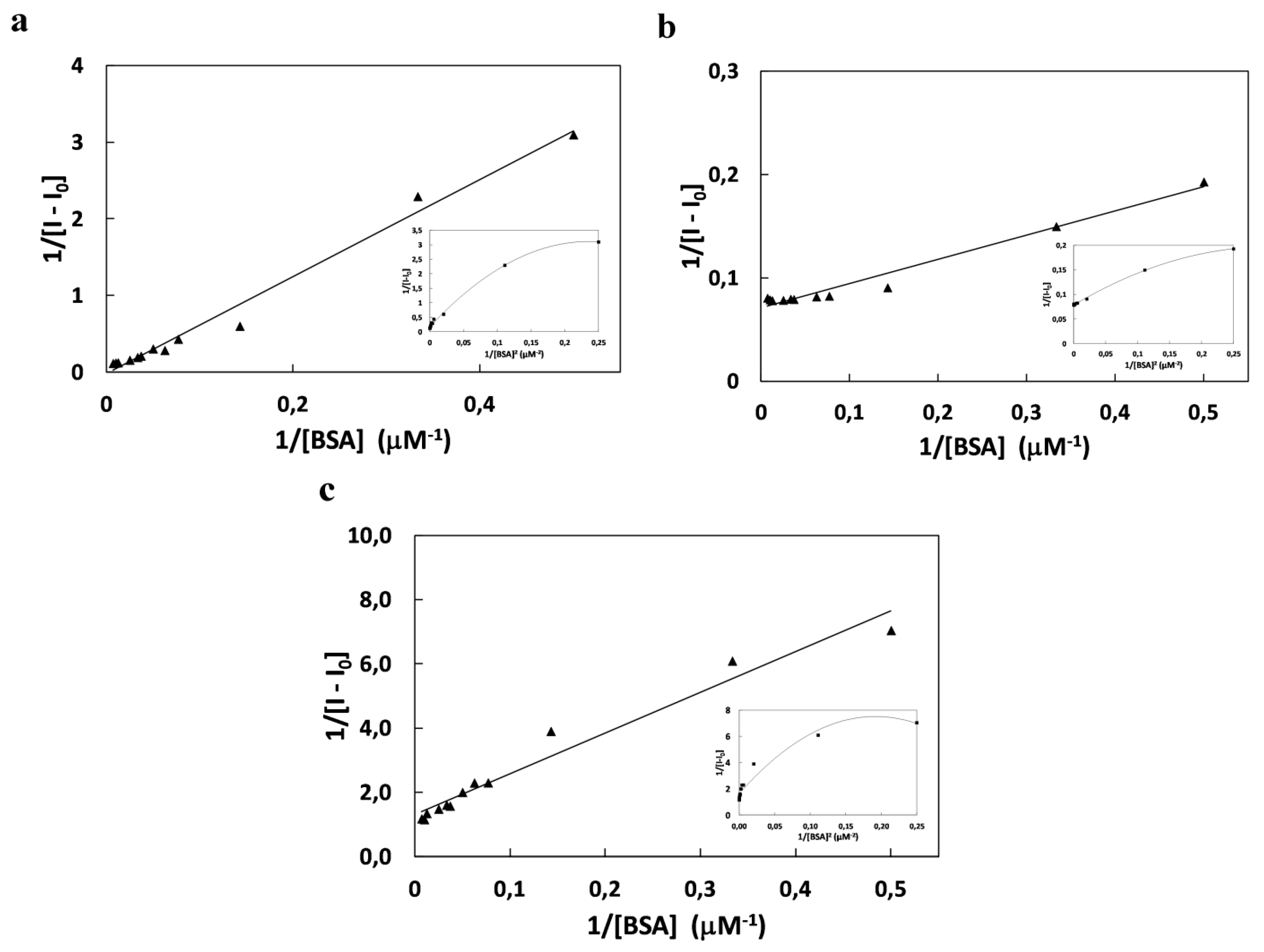
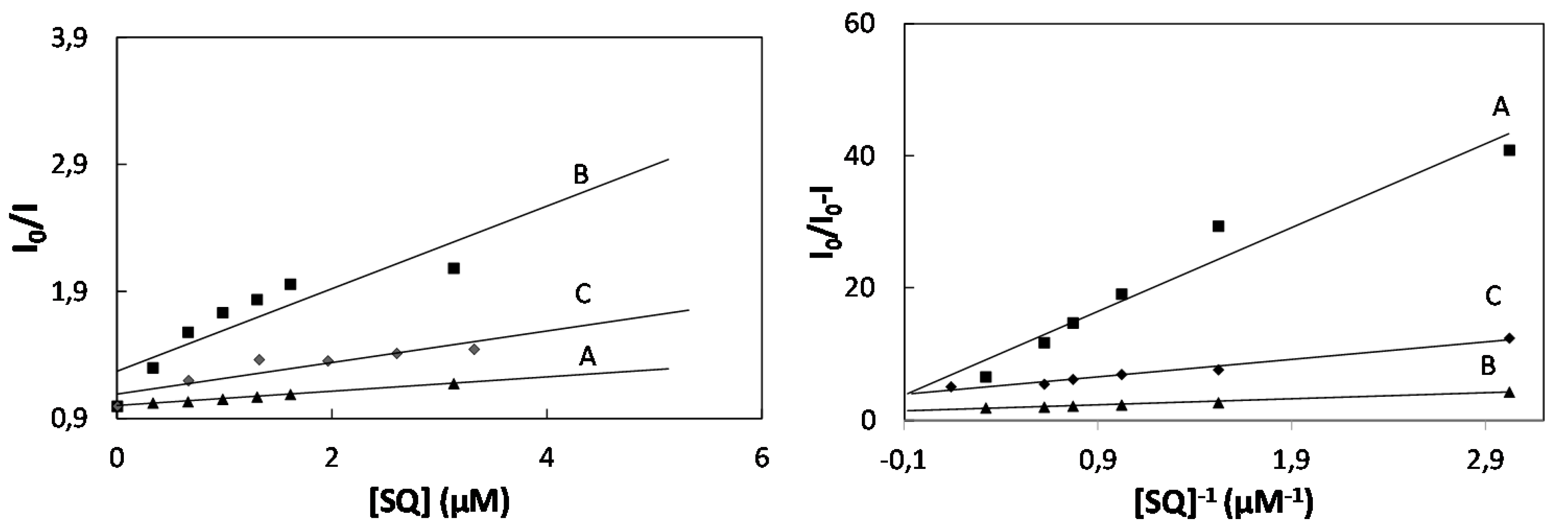
| K (×104 M−2) | Ka (×104 M−1) | KSV (×104 M−1) | Kb (×104 M−1) | n | ΔG (kJmol−1) | |
|---|---|---|---|---|---|---|
| SQ6 | 2.58 | 40.4 | 5.65 | 1.49 | 0.90 | −25.17 |
| SQ7 | 303 | 18,7 | 32.5 | 0.18 | 0.57 | −36.98 |
| SQ8 | 102 | 160 | 12.8 | 0.04 | 0.56 | −34.29 |

3. Experimental Section
3.1. Synthesis
3.2. Materials
3.3. Preparation of Stock Solutions of Dyes and Proteins
3.4. Preparation of Working Solutions
3.5. Spectroscopic Measurements
4. Conclusions
Acknowledgements
Author Contributions
Conflicts of Interest
References
- Carrie, L.; Amiot, S.X.; Song, L.; Lingyun, P.; Julia, X.Z. Near-Infrared Fluorescent Materials for Sensing of Biological Targets. Sensors 2008, 8, 3082–3105. [Google Scholar]
- Demchenko, A.P. Introduction to Fluorescence Sensing; Springer: Amsterdam, The Netherland, 2009. [Google Scholar]
- Changxia, S.; Jinghe, Y.; Lei, L.; Xia, W.; Yang, L.; Shufang, L. Advances in the Study of Luminescence Probes for Proteins. J. Chromatogr. B 2004, 803, 173–190. [Google Scholar]
- Soo-Youl, P.; Kun, J.; Sea-Whe, O. The Novel Functional Chromophores Based on Squarylium Dyes. Korean Chem. Soc. 2005, 26, 428–432. [Google Scholar]
- Kiyose, K.; Aizawa, S.; Sasaki, E.; Kojima, H.; Hanaoka, K.; Terai, T.; Urano, Y.; Nagano, T. Molecular Design Strategies for Near-Infrared Ratiometric Fluorescent Probes Based on the Unique Spectral Properties of Aminocyanines. Chem. Eur. 2009, 15, 9191–9200. [Google Scholar] [CrossRef] [PubMed]
- Loudet, A.; Burgess, K. BODIPY Dyes and their Derivatives: Syntheses and Spectroscopic Properties. Chem. Rev. 2007, 107, 4891–4932. [Google Scholar] [CrossRef] [PubMed]
- Pham, W.; Cassell, L.; Gillman, A.; Koktysh, D.; Gore, J.C. A Near-Infrared Dye for Multichannel Imaging. Chem. Commun. 2008, 16, 1895–1897. [Google Scholar] [CrossRef] [PubMed]
- Lakowicz, J.R. Topics in Fluorescence Spectroscopy: Probe Design and Chemical Sensing; Plenum Press: New York, NY, USA, 1994; Volume 4. [Google Scholar]
- Drexhage, K.H. Structure and Properties of Laser Dyes in Dye Lasers; Springer: New York, NY, USA, 1990; pp. 155–200. [Google Scholar]
- Masotti, A.; Vicennati, P.; Boschi, F.; Calderan, L.; Sbarbati, A.; Ortaggi, G. A Novel near Infrared Indocyanine Dye–Polyethylenimine Conjugate Allows DNA delivery imaging in vivo. Bioconjugate Chem. 2008, 19, 983–987. [Google Scholar] [CrossRef] [PubMed]
- Kasten, F.H. Introduction to Fluorescent Probes: Properties, History and Applications. In Fluorescent and Luminescent Probes for Biological Activity; Mason, W.T., Ed.; Academic Press: Cambridge, UK, 1993; pp. 12–33. [Google Scholar]
- Frangioni, J.V. In vivo Near-Infrared Fluorescence Imaging. Curr. Opin. Chem. Biol. 2003, 7, 626–634. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Wu, Z.; Xiong, Z.; Gambhir, S.S.; Chen, X. Near-Infrared Fluorescent RGD Peptides for Optical Imaging of Integrin avb3 Expression in Living Mice. Bioconjug. Chem. 2005, 16, 1433–1441. [Google Scholar] [CrossRef] [PubMed]
- Coskun, A.; Yilmaz, M.D.; Akkaya, E.U. Bis(2-pyridyl)-Substituted Boratriazaindacene as an NIR-Emitting Chemosensor for Hg(II). Org. Lett. 2007, 9, 607–609. [Google Scholar] [CrossRef] [PubMed]
- Ayyappanpillai, A. Chemistry of squarine-derived materials: near-IR dyes, Low Band Gap Systems, and Cation Sensors. Acc. Chem. Res. 2005, 38, 449–459. [Google Scholar]
- Xu, L.; Xu, Y.; Zhu, W.; Zeng, B.; Chunmei Yang, Ch.; Wu, B.; Qian, X. Versatile Trifunctional Chemosensor of Rhodamine Derivative for Zn2+, Cu2+ and His/Cys in Aqueous Solution and Living Cells. Org. Biomol. Chem. 2011, 9, 8284–8287. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Xu, Y.; Zhu, W.; Sun, X.; Xu, Z.; Qian, X. Modulating the Selectivity by Switching Sensing Media: A Bifunctional Chemosensor Selectivity for Cd2+ and Pb2+ in Different Aqueous Solutions. RSC Adv. 2012, 2, 6323–6328. [Google Scholar] [CrossRef]
- Xu, L.; Xu, Y.; Zhu, W.; Yang, C.; Han, L.; Qian, X. A Highly Selective and Sensitive Fluorescence “Turn-on” Probe for Ag+ in Aqueous Solution and Live Cells. Dalton Trans. 2012, 41, 7212–7217. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.B.; Li, H.R.; Luo, Y.; Xu, L. A Naphthalimide-Based Bifunctional Fluorescent Probe for the Differential Detection of Hg2+ and Cu2+ in Aqueous Solution. Dalton Trans. 2014, 43, 8102–8108. [Google Scholar] [CrossRef] [PubMed]
- Un, H.I.; Huang, C.B.; Huang, C.; Jia, T.; Zhao, X.L.; Wang, C.H.; Xu, L.; Yang, H.-B. A Versatile Fluorescent Dye Based on Naphthalimide: Highly Selective Detection of Hg2+ in Aqueous Solution and Living Cells and its Aggregation-Induced Emission Behavior. Org. Chem. Front. 2014, 1, 1083–1090. [Google Scholar] [CrossRef]
- Un, H.I.; Huang, C.B.; Huang, J.; Huang, C.; Jia, T.; Xu, L. A Naphthalimide-Based Fluorescence “Turn-On” Probe for the Detection of Pb2+ in Aqueous Solution and Living Cells. Chem. Asian J. 2014, 9, 3397–3402. [Google Scholar] [CrossRef] [PubMed]
- Joseph, T.; Douglas, B.S.; Terry, J.A.; Sandra, A.A.; Bruce, P.J. Synthesis and Biosensor Performance of a Near-IR thiol-Reactive Fluorophore based on Benzothiazolium Squaraine. Bioconjugate Chem. 2007, 18, 1841–1846. [Google Scholar]
- Singh, R.B.; Mahanta, S.; Bagchi, A.; Guchhait, N. Interaction of Human Serum Albumin with Charge Transfer Probe Ethyl Ester of N,N-dimethylamino Naphtyl Acrylic Acid: an Extrinsic Fluorescence Probe for Studying Protein Environment. Photochem. Photobiol. Sci. 2009, 8, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Guchhait, N. Chemically Induced Unfolding of Bovine Serum Albumin by Urea and Sodium Dodecyl Sufate: A Spectral Study with Polarity-Sensitive Charge-Transfer Fluorescent Probe (E)-3-(4-methylaminophenyl)acrylic acid methyl ester. Chem. Phys. Chem. 2009, 10, 16641–16671. [Google Scholar]
- Lehrer, S.S. Solute Perturbation of Protein Fluorescence. The Quenching of the Tryptophyl Fluorescence of Model Compounds and of Lysozyme by Iodide Ion. Biochemistry 1971, 10, 32543–32263. [Google Scholar]
- Sample Availability: Samples of the compounds are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jurek, K.; Kabatc, J.; Kostrzewska, K.; Grabowska, M. New Fluorescence Probes for Biomolecules. Molecules 2015, 20, 13071-13079. https://doi.org/10.3390/molecules200713071
Jurek K, Kabatc J, Kostrzewska K, Grabowska M. New Fluorescence Probes for Biomolecules. Molecules. 2015; 20(7):13071-13079. https://doi.org/10.3390/molecules200713071
Chicago/Turabian StyleJurek, Katarzyna, Janina Kabatc, Katarzyna Kostrzewska, and Marlena Grabowska. 2015. "New Fluorescence Probes for Biomolecules" Molecules 20, no. 7: 13071-13079. https://doi.org/10.3390/molecules200713071
APA StyleJurek, K., Kabatc, J., Kostrzewska, K., & Grabowska, M. (2015). New Fluorescence Probes for Biomolecules. Molecules, 20(7), 13071-13079. https://doi.org/10.3390/molecules200713071