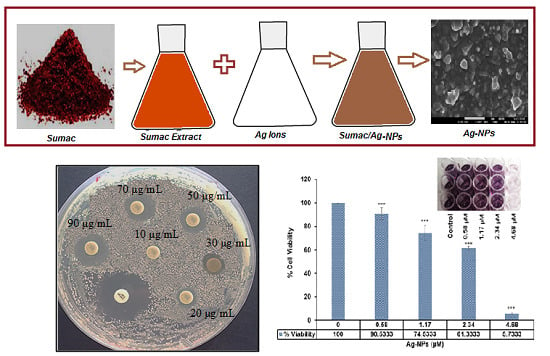
Sumac Silver Novel Biodegradable Nano Composite for Bio-Medical Application: Antibacterial Activity
Abstract
:1. Introduction
2. Results and Discussion






2.1. Antimicrobial Activity
| Concentration (μg/mL) | Inhibition Zone (mm) |
|---|---|
| 10 | - |
| 20 | 8.5 ± 0.53 |
| 30 | 10.2 ± 0.46 |
| 50 | 11.8 ± 0.41 |
| 70 | 13.3 ± 0.12 |
| 90 | 14.3 ± 0.32 |
| Positive control | 17.2 ± 0.38 |

2.2. Cell Cytotoxicity

3. Experimental Section
3.1. Preparation of Sumac Extracts
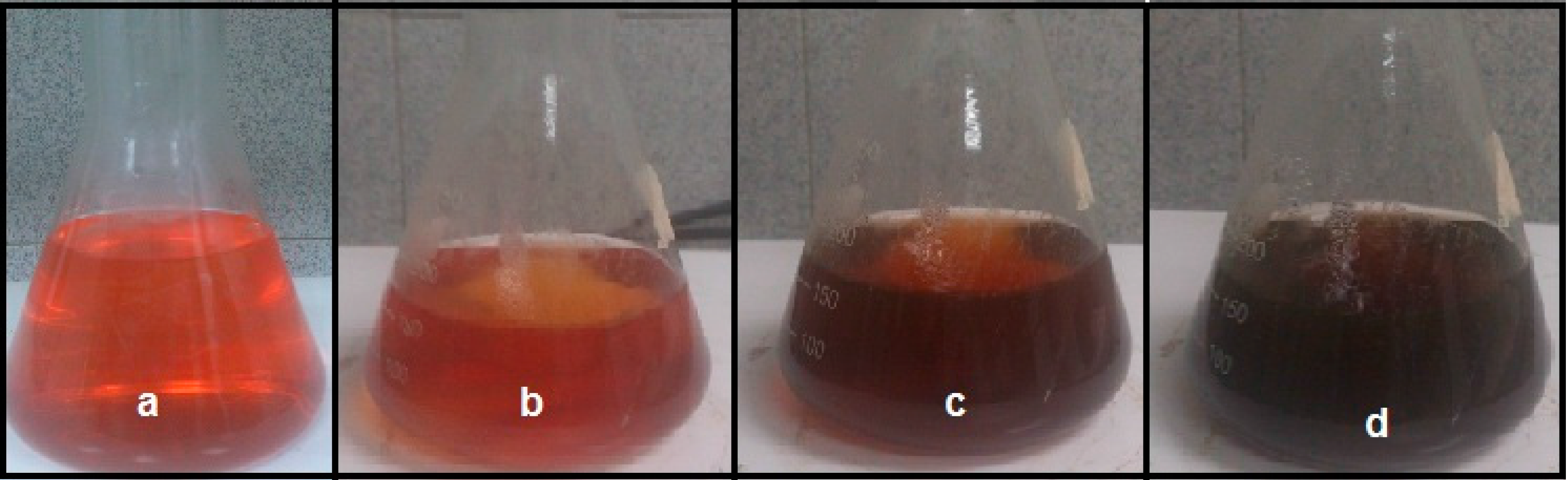
3.2. Biosynthesis of Ag-NPs
3.3. Characterization of Ag-NPs
3.4. Antimicrobial Assays
3.5. Cytotoxicity Test
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References and Notes
- Morones, J.R.; Elechiguerra, J.L.; Camacho, A.; Ramirez, J.T. The bactericidal effect of silver nanoparticles. Nanotechnology 2005, 16, 2346–2353. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, B.; Gholamhoseinpoor, F. A study on the stability and green synthesis of silver nanoparticles using Ziziphora tenuior (Zt) extract at room temperature. Spectrochim. Acta Mol. Biomol. Spectrosc. 2015, 134, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Azizi, S.; Namvar, F.; Mahdavi, M.; Bin Ahmad, M.; Mohamad, R. Biosynthesis of silver nanoparticles using brown marine macroalga, sargassum muticum aqueous extract. Materials 2013, 6, 5942–5950. [Google Scholar] [CrossRef]
- Das, S.K.; Marsili, E. A green chemical approach for the synthesis of gold nanoparticles: Characterization and mechanistic aspect. Rev. Environ. Sci. Biotechnol. 2010, 9, 199–204. [Google Scholar] [CrossRef]
- Liu, L.; Liu, T.; Tade, M.; Wang, S.; Li, X.; Liu, S. Less is more, greener microbial synthesis of silver nanoparticles. Enzyme Microb. Technol. 2014, 67, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Senapati, S.; Khan, M.I.; Kumar, R.; Sastry, M. Extracellular biosynthesis of monodisperse gold nanoparticles by a novel extremophilic actinomycete, thermomonospora sp. Langmuir 2003, 19, 3550–3553. [Google Scholar] [CrossRef]
- Khandelwal, N.; Singh, A.; Jain, D.; Upadhyay, M.K.; Verma, H.N. Green synthesis of silver nanoparticles using Argimone mexicana leaf extract and evaluation of their antimicrobial activities. Dig. J. Nanomater. Biostruct. 2010, 5, 483–489. [Google Scholar]
- Jakobek, L.; Seruga, M.; Novak, I. Flavonols, Phenolic acid and antioxidant activity of some red fruit. J. Food. Technol. 2007, 51, 369–378. [Google Scholar]
- Nasar-Abbas, S.M.; Kadir Halkman, A. Antimicrobial effect of water extract of sumac (Rhuscoriaria L.) on the growth of some food borne bacteria including pathogens. Int. J. Food. Microbiol. 2003, 97, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Elechiguerra, J.L.; Burt, J.L.; Morones, J.R.; Camacho-Bragado, A.; Gao, X.; Lara, H.H.; Yocaman, M. Interaction of silver nanoparticles with HIV-1. J. Nanobiotechnol. 2005. [Google Scholar] [CrossRef]
- Shiraishi, Y.; Toshima, N. Oxidation of ethylene catalyzed by colloidal dispersions of poly (sodium acrylate)-protected silver nanoclusters. Colloids Surf. A 2000, 169, 59–66. [Google Scholar] [CrossRef]
- Jain, P.; Pradeep, T. Potential of silver nanoparticle-coated polyurethane foam as an antibacterial water filter. Biotechnol. Bioeng. 2005, 90, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Krishnaraj, C.; Jagan, E.G.; Ramachandran, R.; Abirami, S.M.; Mohan, N.; Kalaichelvan, P.T. Effect of biologically synthesized silver nanoparticles on Bacopa monnieri (Linn.) Wettst. plant growth metabolism. Process. Biochem. 2012, 47, 651–658. [Google Scholar] [CrossRef]
- Rai, M.; Deshmukh, S.D.; Ingle, A.P.; Gupta, I.R.; Galdiero, M.; Galdiero, S. Metal nanoparticles: The protective nanoshield against virus infection. Crit. Rev. Microbiol. 2014. [Google Scholar] [CrossRef] [PubMed]
- Franci, G.; Falanga, A.; Galdiero, S.; Palomba, L.; Rai, M.; Morelli, G.; Galdiero, M. Silver nanoparticles as potential antibacterial agents. Molecules 2015, 20, 8856–8874. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, B.; Rostami, A.; Momeni, S.S. Facile green synthesis of silver nanoparticles using seed aqueous extract of Pistacia atlantica and its antibacterial activity. Spectrochim. Acta A 2015, 134, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Sadhasivam, S.; Shanmugam, P.; Yun, K. Biosynthesis of silver nanoparticles by Streptomyces hygroscopicus and antimicrobial activity against medically important pathogenic microorganisms. Colloids Surf. B 2010, 81, 358–362. [Google Scholar] [CrossRef] [PubMed]
- Devina Merin, D.; Prakash, S.; Valentine Bhimba, B. Antibacterial screening of silver nanoparticles synthesized by marine micro algae. Asian Pac. J. Trop. Biomed. 2010, 3, 797–799. [Google Scholar] [CrossRef]
- Vigneshwaran, N.; Nachane, R.P.; Balasubramanya, R.H.; Varadarajan, P.V. A novelone pot “green” synthesis of stable silver nanoparticles using soluble starch. Carbohydr. Res. 2006, 341, 2012–2018. [Google Scholar] [CrossRef] [PubMed]
- Raut Rajesh, W.; Lakkakula Jaya, R.; Kolekar Niranjan, S.; Mendhulkar Vijay, D.; Kashid Sahebrao, B. Phytosynthesis of silver nanoparticle using gliricidia sepium (Jacq.). Curr. Nanosci. 2009, 5, 117–122. [Google Scholar]
- Namvar, F.; Azizi, S.; Ahmad, M.B.; Shameli, K.; Mohamad, R.; Mahdavi, M. Md Tahir P Green synthesis and characterization of gold nanoparticles using the marine macroalgae Sargassum muticum. Res. Chem. Intermed. 2014. [Google Scholar] [CrossRef]
- Nath, S.S.; Chakdar, D.; Gope, G. Synthesis of CdS and ZnS quantum dots and their applications in electronics. NanoTrends 2007, 2, 40–44. [Google Scholar]
- Dubey, S.P.; Lahtinen, M.; Sillanpa, M. Tansy fruit mediated greener synthesis of silver and gold nanoparticles. Process. Biochem. 2010, 45, 1065–1071. [Google Scholar] [CrossRef]
- Singaravelu, G.; Arockiamary, J.; Ganesh, K.; Govindaraju, K. A novel extracellular synthesis of monodisperse gold nanoparticlesusing marine alga, Sargassum wightii Greville. Colloids Surf. B Biointerfaces 2007, 57, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Creighton, J.A.; Eadont, D.G. Ultra violet-visible absorption spectra of the colloidal metallic elements. J. Chem. Soc. Faraday Trans. 1991, 87, 3881–3891. [Google Scholar] [CrossRef]
- Sahayaraj, K.; Roobadevi, M.; Rajesh, S.; Azizi, S. Vernonia cinerea (L.) Less. silver nanocomposite and its antibacterial activity against a cotton pathogen. Res. Chem. Intermed. 2014. [Google Scholar] [CrossRef]
- Janardhanan, R.; Karuppaiah, M.; Hebalkar, N.; Rao, T.N. Synthesis and surface chemistry of nano silver particles. Polyhedron 2009, 28, 2522–2530. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Sajadi, M.; Maham, M. Green synthesis of palladium nanoparticles using Hippophae rhamnoides Linn leaf extract and their catalytic activity for the Suzuki-Miyaura coupling in water. J. Mol. Catal. A Chem. 2015, 396, 297–303. [Google Scholar] [CrossRef]
- Falca, L.; Eduarda, M.A.M. Tannins characterization in historic leathers by complementary analytical techniques ATR-FTIR, UV-Vis and chemical tests. J. Cult. Herit. 2012, 14, 499–508. [Google Scholar] [CrossRef]
- Vijayaraghavan, K.; Mahadevan, A.; Sathishkumar, M.; Pavagadhia, S.; Balasubramanian, R. Biosynthesis of Au(0) from Au(III) via biosorption and bioreduction using brown marine alga Turbinaria conoides. Chem. Eng. J. 2011, 167, 223–227. [Google Scholar] [CrossRef]
- Rai, M.; Kon, K.; Ingle, A.; Duran, N.; Galdiero, S.; Galdiero, M. Broad-spectrum bioactivities of silver nanoparticles: The emerging trends and future prospects. Appl. Microbiol. Biotechnol. 2014, 98, 1951–1961. [Google Scholar] [CrossRef] [PubMed]
- Periasamy, S.; Joo, H.S.; Duong, A.C.; Bach, T.H.; Tan, V.Y.; Chatterjee, S.S.; Cheung, G.Y.; Otto, M. How Staphylococcus aureus biofilms develop their characteristic structure. Proc. Natl. Acad. Sci. USA 2012, 109, 1281–1286. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Dai, T.; Huang, L.; Kurup, D.B.; Tegos, G.P.; Jahnke, A.; Wharton, T.; Hamblin, M.R. Photodynamic therapy with a cationic functionalized fullerene rescues mice from fatal wound infections. Nanomedicine 2010, 5, 1525–1533. [Google Scholar] [CrossRef] [PubMed]
- Lazar, V. Quorum sensing in biofilms—How to destroy the bacterial citadels or their cohesion/power. Anaerobe 2011, 17, 280–285. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Sample of the compound as a powder is available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghorbani, P.; Soltani, M.; Homayouni-Tabrizi, M.; Namvar, F.; Azizi, S.; Mohammad, R.; Moghaddam, A.B. Sumac Silver Novel Biodegradable Nano Composite for Bio-Medical Application: Antibacterial Activity. Molecules 2015, 20, 12946-12958. https://doi.org/10.3390/molecules200712946
Ghorbani P, Soltani M, Homayouni-Tabrizi M, Namvar F, Azizi S, Mohammad R, Moghaddam AB. Sumac Silver Novel Biodegradable Nano Composite for Bio-Medical Application: Antibacterial Activity. Molecules. 2015; 20(7):12946-12958. https://doi.org/10.3390/molecules200712946
Chicago/Turabian StyleGhorbani, Parisa, Mozhgan Soltani, Masoud Homayouni-Tabrizi, Farideh Namvar, Susan Azizi, Rosfarizan Mohammad, and Amin Boroumand Moghaddam. 2015. "Sumac Silver Novel Biodegradable Nano Composite for Bio-Medical Application: Antibacterial Activity" Molecules 20, no. 7: 12946-12958. https://doi.org/10.3390/molecules200712946
APA StyleGhorbani, P., Soltani, M., Homayouni-Tabrizi, M., Namvar, F., Azizi, S., Mohammad, R., & Moghaddam, A. B. (2015). Sumac Silver Novel Biodegradable Nano Composite for Bio-Medical Application: Antibacterial Activity. Molecules, 20(7), 12946-12958. https://doi.org/10.3390/molecules200712946