Synthesis of Oxygen Heterocycles via Aromatic C-O Bond Formation Using Arynes
Abstract
:1. Introduction
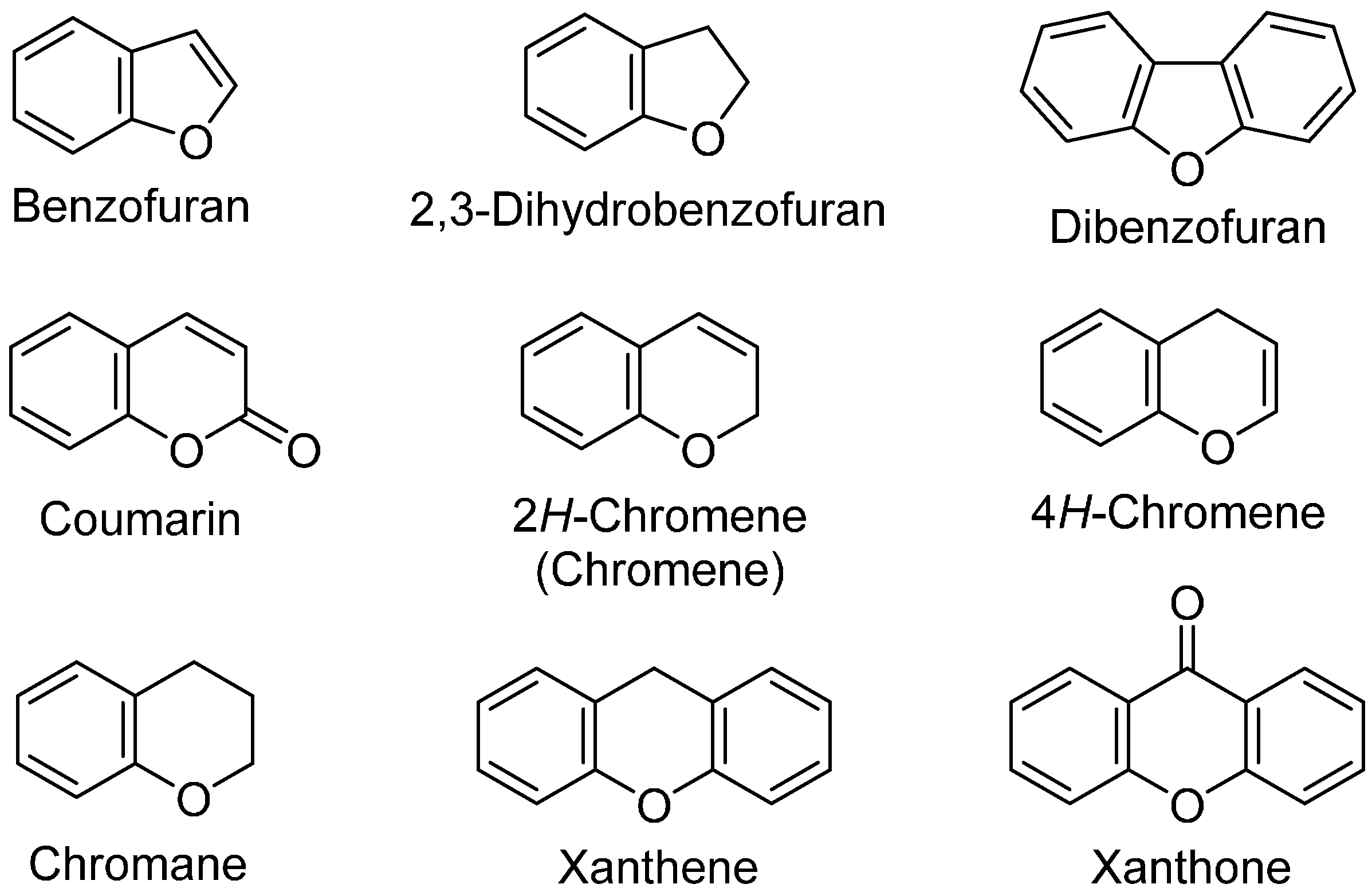

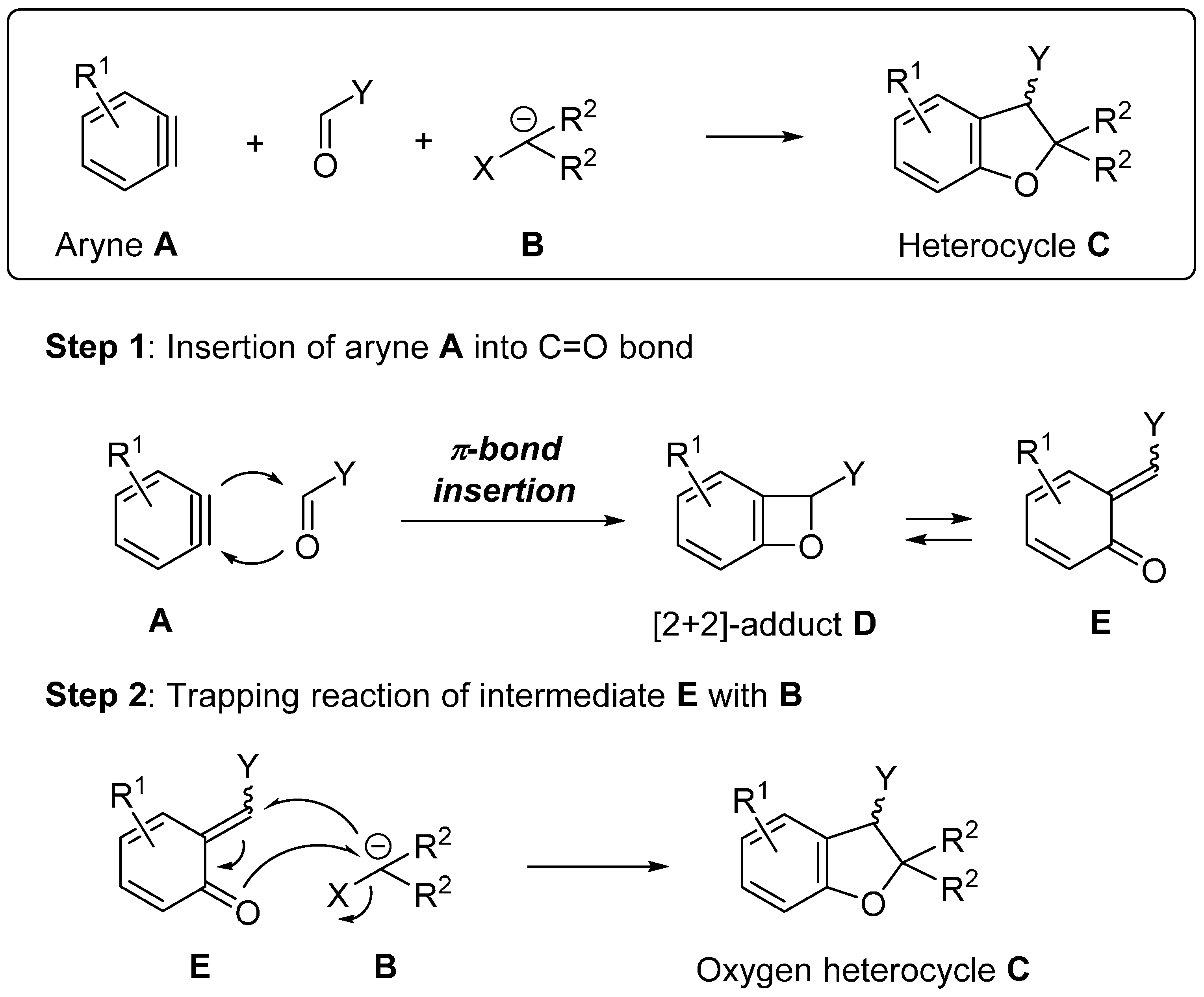
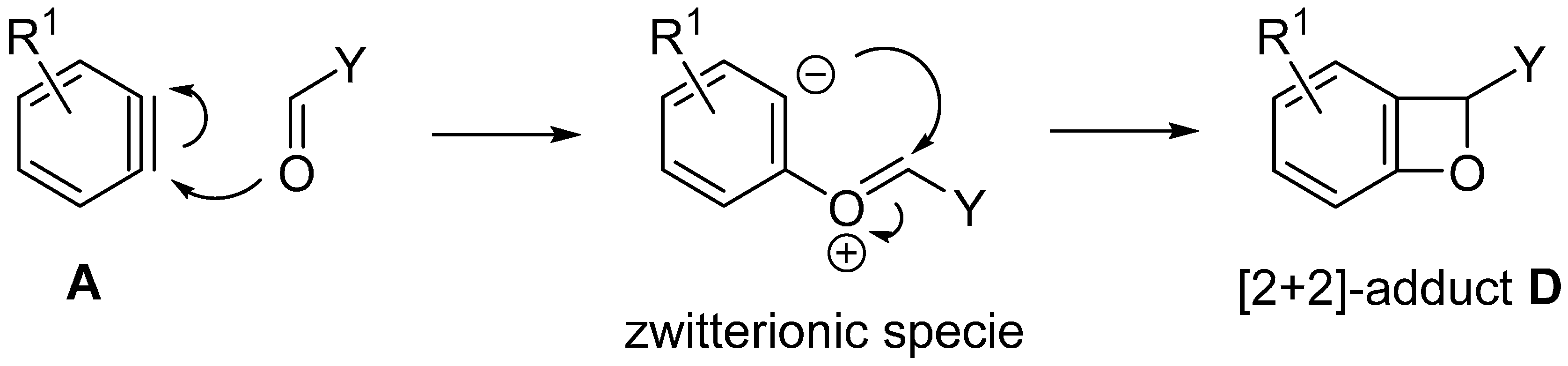
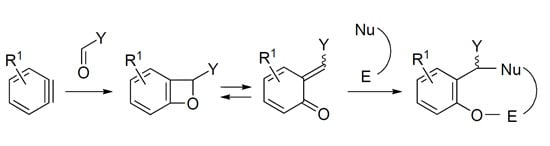
2. Syntheses of Oxygen Heterocycles Using Insertion of Arynes into C=O Bond
2.1. Domino Reaction Starting from Insertion of Arynes into Aldehydes
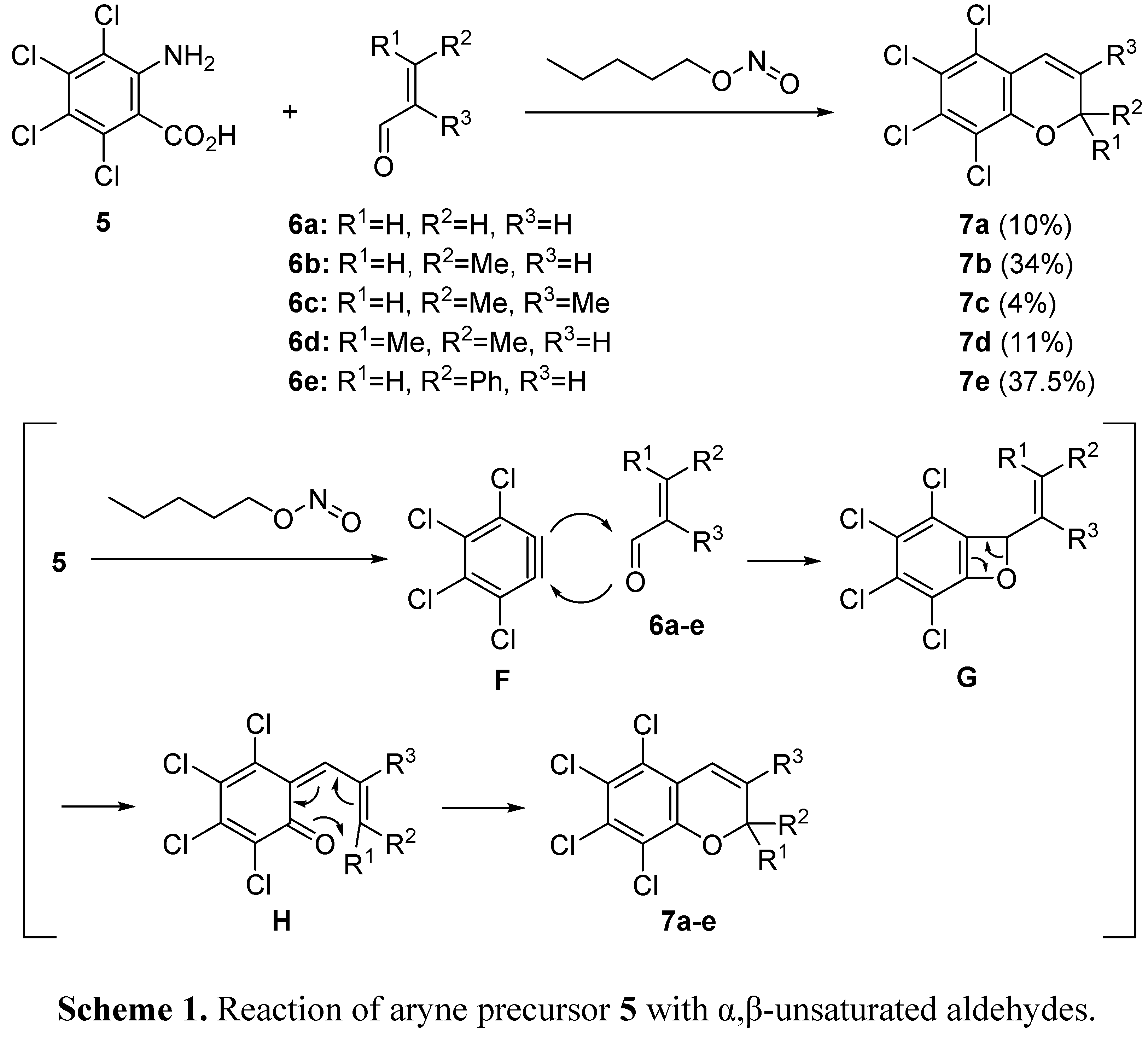

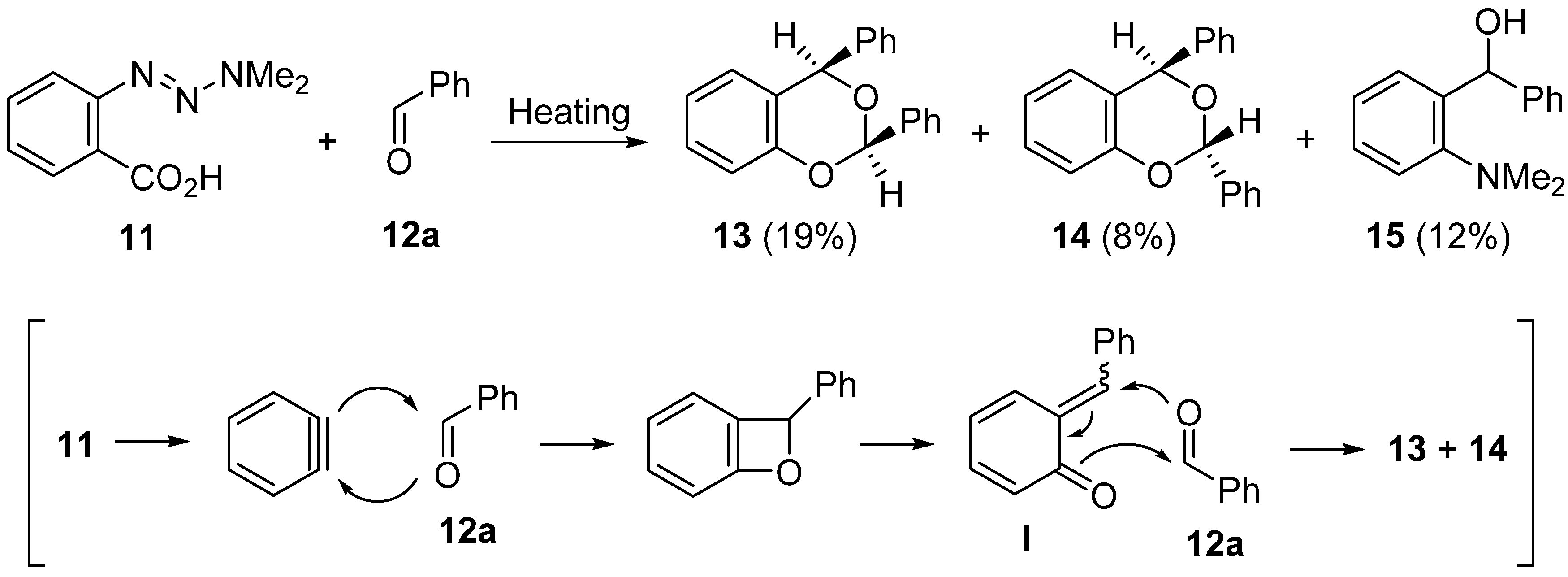

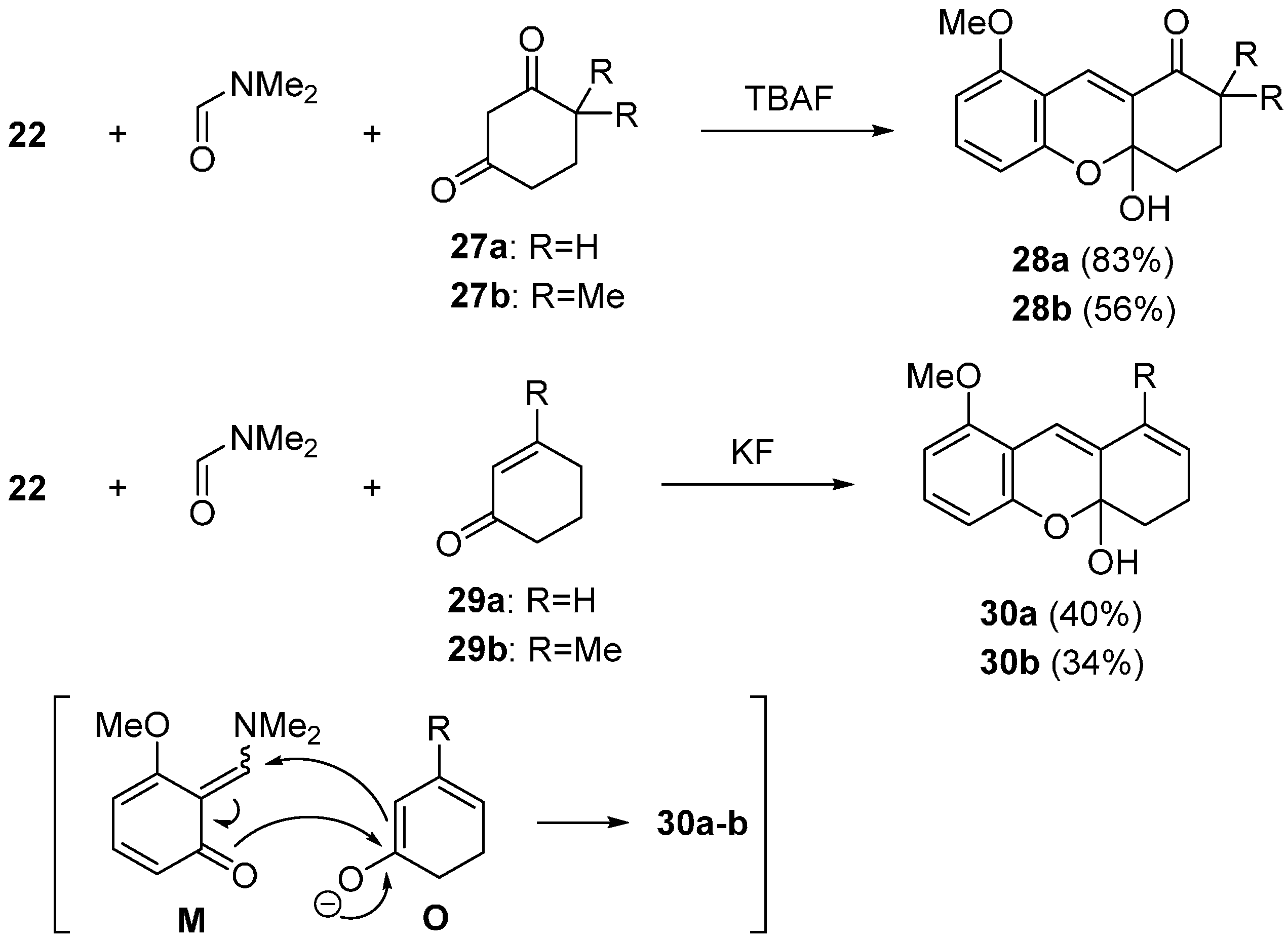
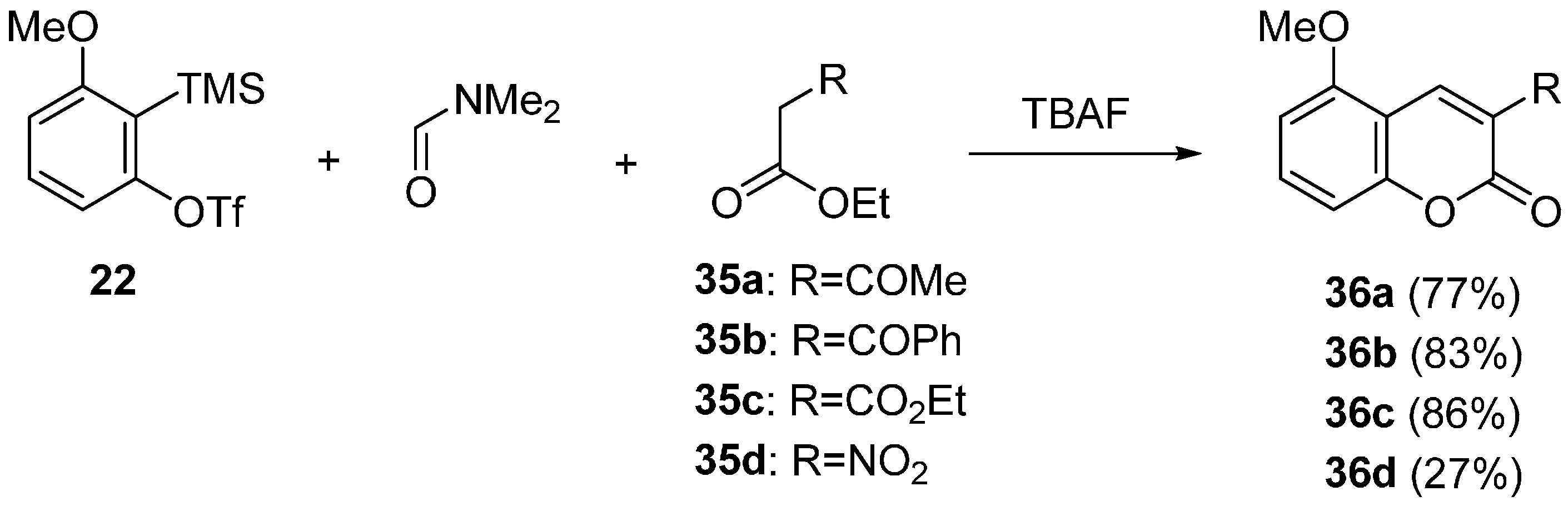
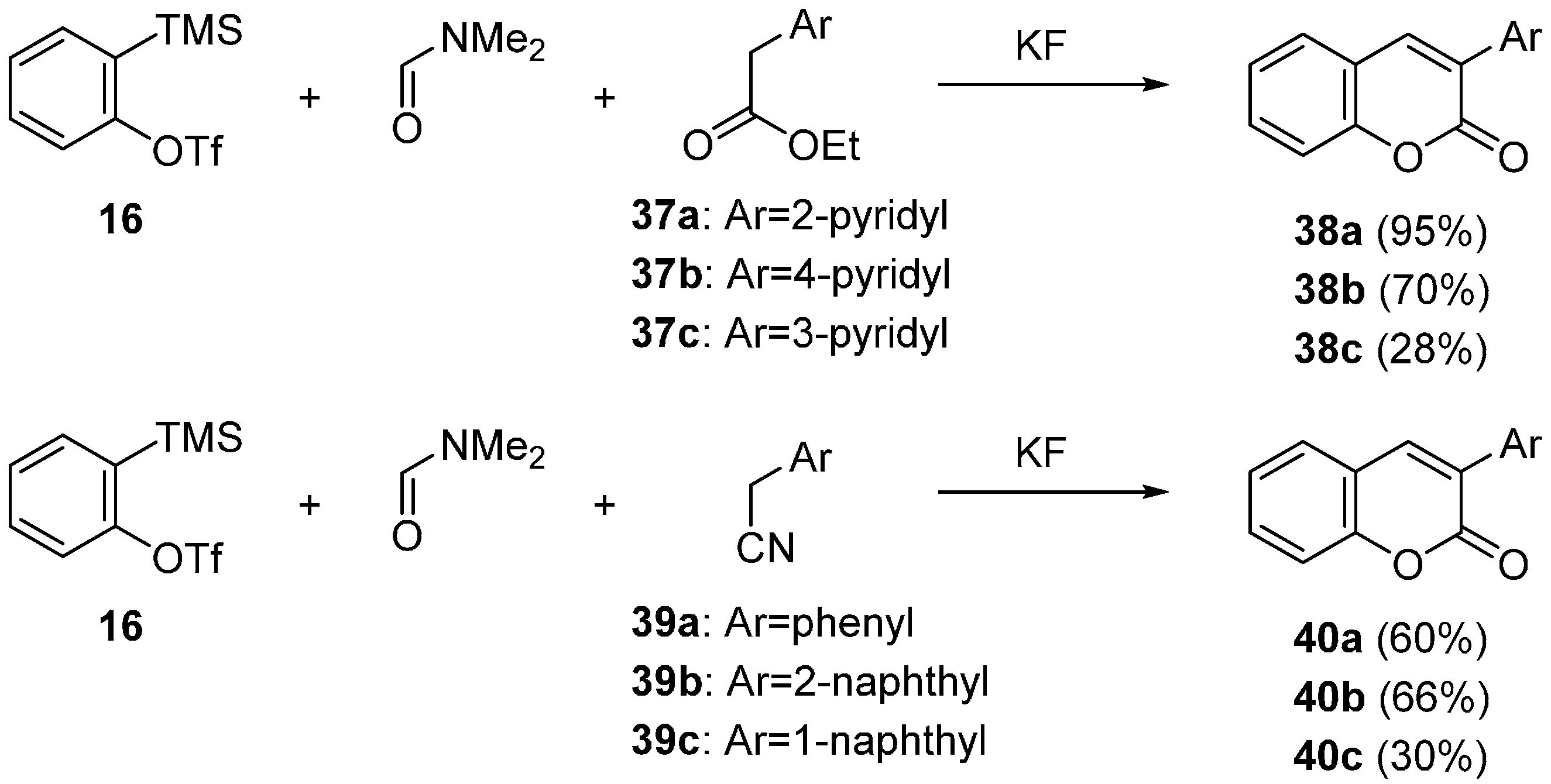
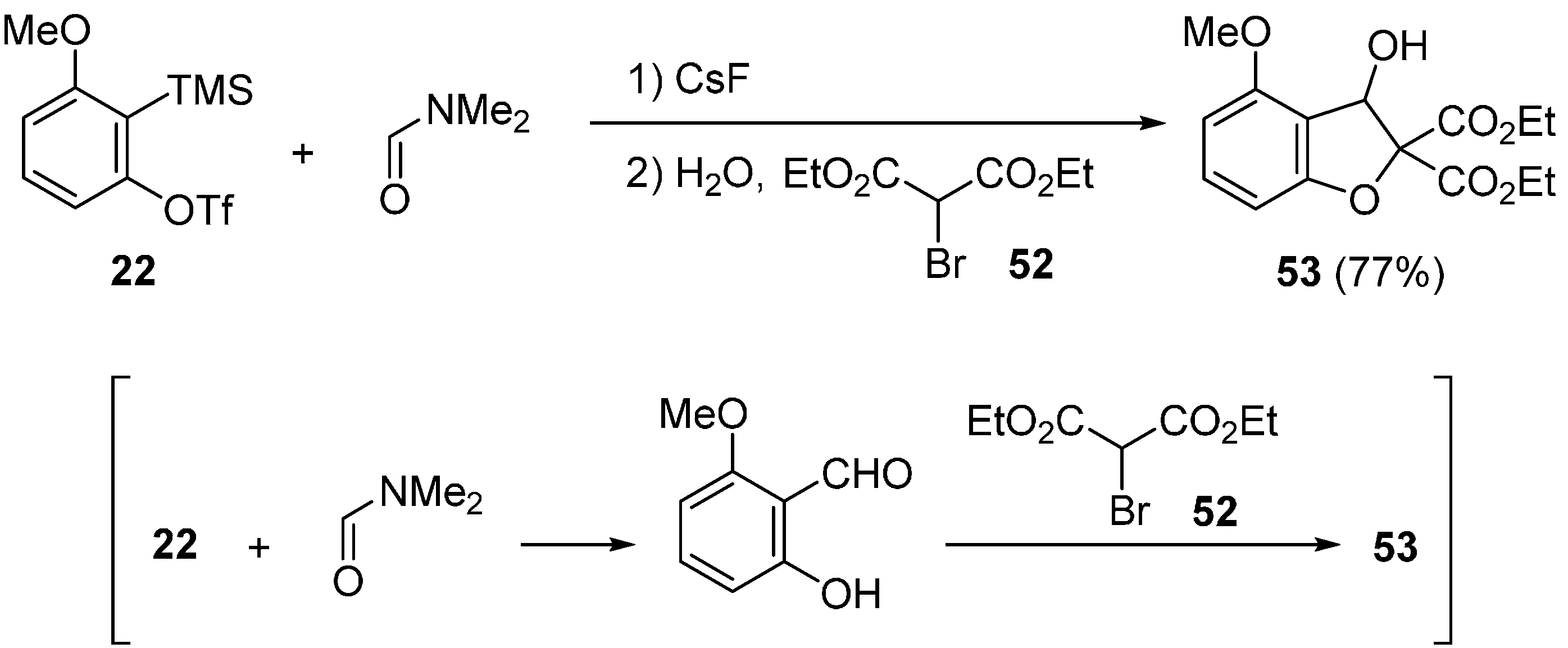
2.2. Domino Reaction Starting from Insertion of Arynes into Formamides
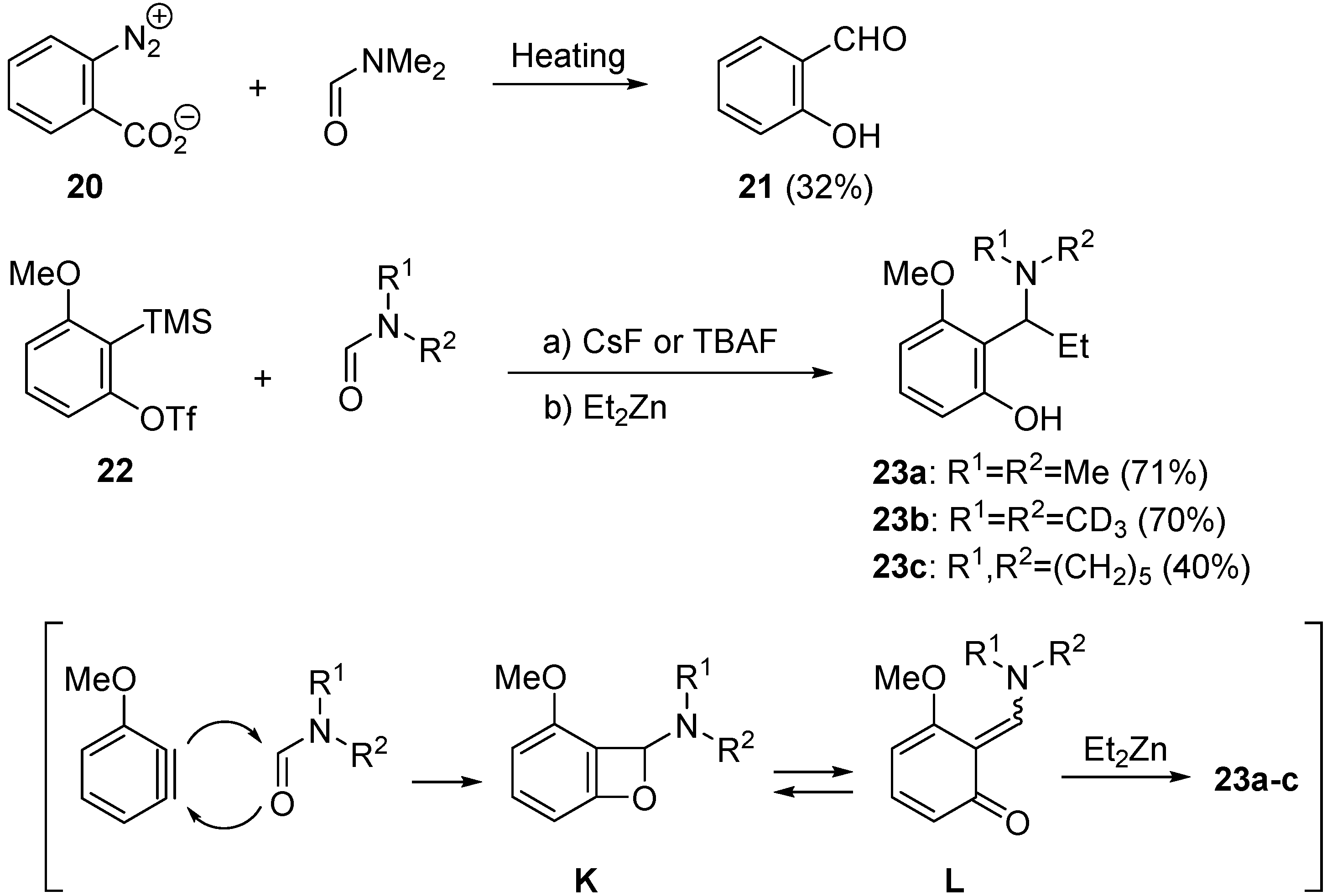

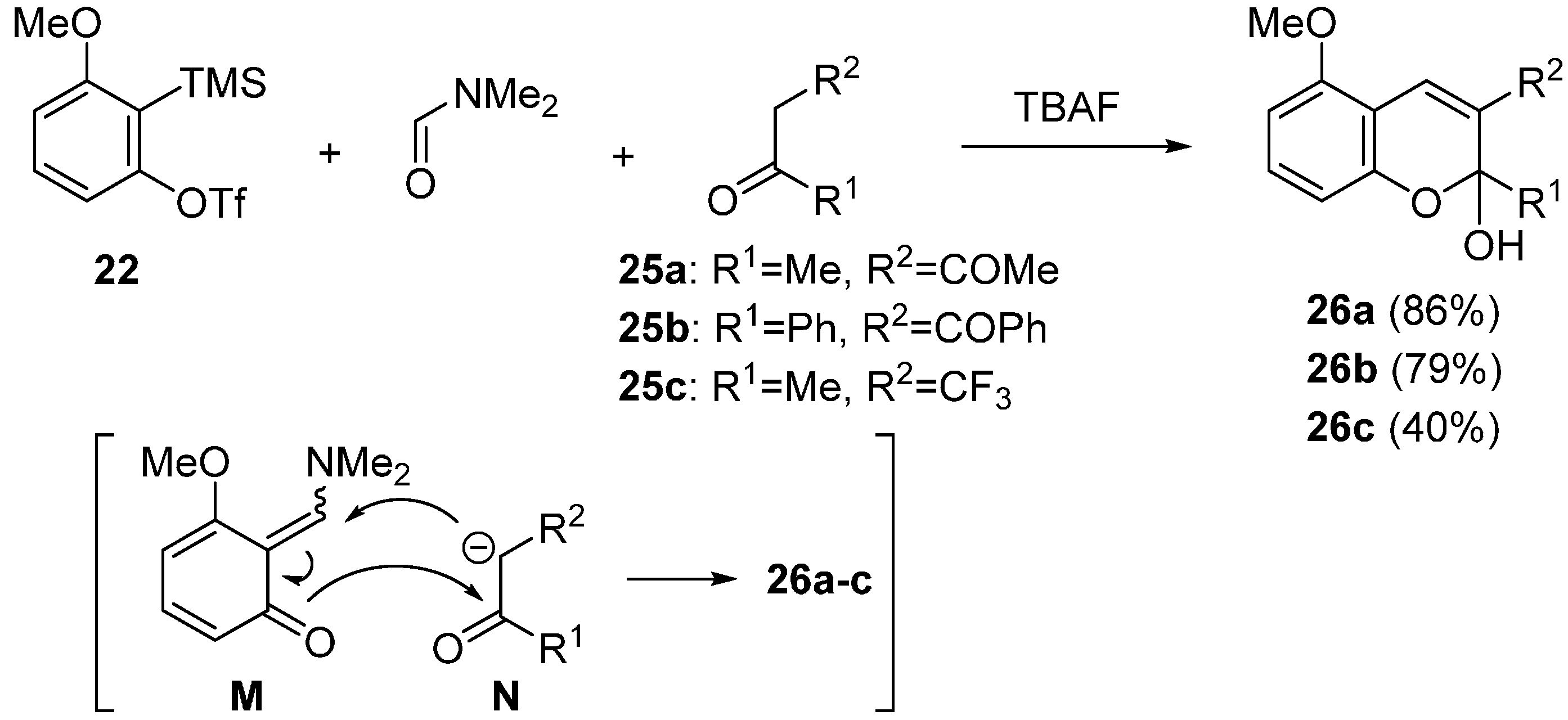






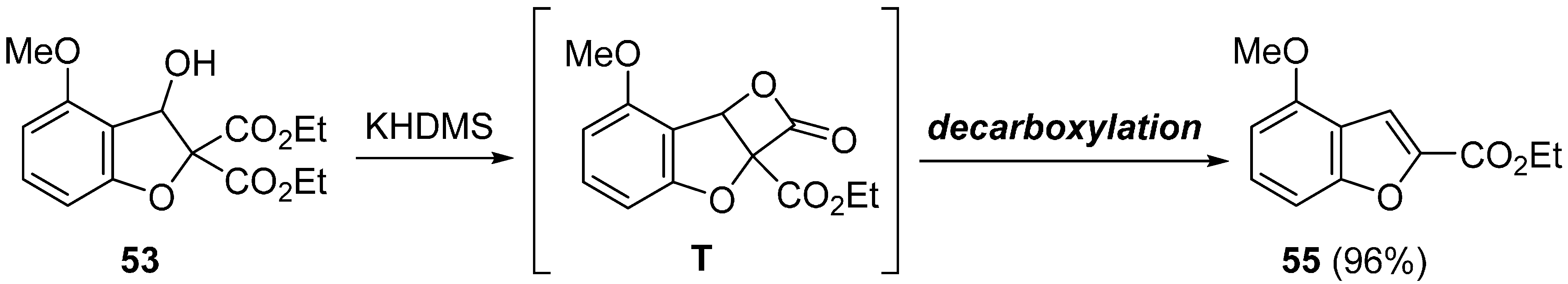


3. Concluding Remarks
Acknowledgments
Conflicts of Interest
References
- Kaur, P.; Arora, R.; Gill, N.S. Review on oxygen heterocycles. Indo Am. J. Pharm. Res. 2013, 3, 9067–9084. [Google Scholar]
- Miyabe, H.; Miyata, O.; Naito, T. Pyran and its derivatives. In Heterocycles in Natural Product Synthesis; Majumdar, K.C., Chattopadhyay, S.K., Eds.; WILEY-VCH: Weinheim, Germany, 2011; pp. 153–186. [Google Scholar]
- Atul, G.; Amit, K.; Ashutosh, R. Synthesis, stereochemistry, structural classification, and chemical reactivity of natural pterocarpans. Chem. Rev. 2013, 113, 1614–1640. [Google Scholar]
- Song, X.G.; Zhu, S.F.; Xie, X.L.; Zhou, Q.L. Enantioselective copper-catalyzed intramolecular phenolic O–H bond insertion: Synthesis of chiral 2-carboxy dihydrobenzofurans, dihydrobenzopyrans, and tetrahydrobenzooxepines. Angew. Chem. Int. Ed. 2013, 52, 2555–2558. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.W.; Cao, L.L.; Ye, Z.S.; Jiang, G.F.; Zhou, Y.G. A mild method for generation of o-quinone methides under basic conditions. The facile synthesis of trans-2,3-dihydrobenzofurans. Chem. Commun. 2013, 49, 1660–1662. [Google Scholar]
- Xu, T.; Ko, H.M.; Savage, N.A.; Dong, G. Highly enantioselective Rh-catalyzed carboacylation of olefins: Efficient syntheses of chiral poly-fused rings. J. Am. Chem. Soc. 2012, 134, 20005–20008. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.-H.; Kwon, K.-H.; Yi, C.S. Dehydrative C–H alkylation and alkenylation of phenols with alcohols: Expedient synthesis for substituted phenols and benzofurans. J. Am. Chem. Soc. 2012, 134, 7325–7328. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, Ł.; Ransborg, L.K.; Lauridsen, V.; Overgaard, M.; Zweifel, T.; Jørgensen, K.A. Taming the Friedel-Crafts reaction: Organocatalytic approach to optically active 2,3-dihydrobenzofurans. Angew. Chem. Int. Ed. 2011, 50, 12496–12500. [Google Scholar] [CrossRef] [PubMed]
- Palucki, M.; Wolfe, J.P.; Buchwald, S.L. Synthesis of oxygen heterocycles via a palladium-catalyzed C–O bond-forming Reaction. J. Am. Chem. Soc. 1996, 118, 10333–10334. [Google Scholar] [CrossRef]
- Shelby, Q.; Kataoka, N.; Mann, G.; Hartwig, J. Unusual in situ ligand modification to generate a catalyst for room temperature aromatic C–O bond formation. J. Am. Chem. Soc. 2000, 122, 10718–10719. [Google Scholar] [CrossRef]
- Kuwabe, S.; Torraca, K.E.; Buchwald, S.L. Palladium-catalyzed intramolecular C–O bond formation. J. Am. Chem. Soc. 2001, 123, 12202–12206. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Dormer, P.G. Synthesis of benzo[b]furans via CuI-catalyzed ring closure. J. Org. Chem. 2005, 70, 6964–6967. [Google Scholar] [CrossRef] [PubMed]
- Carril, M.; SanMartin, R.; Tellitu, I.; Domínguez, E. On-water chemistry: Copper-catalyzed straightforward synthesis of benzo[b]furan derivatives in neat water. Org. Lett. 2006, 8, 1467–1470. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Wang, B.; Zhang, Y.; Ma, D. CuI-catalyzed domino process to 2,3-disubstituted benzofurans from 1-bromo-2-iodobenzenes and β-keto esters. J. Org. Chem. 2007, 72, 5337–5341. [Google Scholar] [CrossRef] [PubMed]
- Ackermann, L.; Kaspar, L.T. TiCl4-catalyzed indirect anti-markovnikov hydration of alkynes: Application to the synthesis of benzo[b]furans. J. Org. Chem. 2007, 72, 6149–6153. [Google Scholar] [CrossRef] [PubMed]
- Tsui, G.C.; Tsoung, J.; Dougan, P.; Lautens, M. One-pot synthesis of chiral dihydrobenzofuran framework via Rh/Pd catalysis. Org. Lett. 2012, 14, 5542–5545. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Shi, B.-F. Transition-metal-catalyzed etherification of unactivated C–H bonds. Tetrahedron Lett. 2015, 56, 15–22. [Google Scholar] [CrossRef]
- Wang, X.; Lu, Y.; Dai, H.X.; Yu, J.Q. Pd(II)-catalyzed hydroxyl-directed C–H activation/C–O cyclization: Expedient construction of dihydrobenzofurans. J. Am. Chem. Soc. 2010, 132, 12203–12205. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Hou, W.; Du, Y.; Zhang, Y.; Pan, Y.; Mao, D.; Zhao, K. Oxidative aromatic C–O bond formation: Synthesis of 3-functionalized benzo[b]furans by FeCl3-mediated ring closure of α-aryl ketones. Org. Lett. 2009, 11, 4978–4981. [Google Scholar] [CrossRef] [PubMed]
- Kessar, S.V. Nucleophilic coupling with arynes. In Comprehensive Organic Synthesis; Trost, B.M., Flemming, I., Eds.; Pergamon: Oxford, UK, 1991; Volume 4, pp. 483–515. [Google Scholar]
- Saito, S.; Yamamoto, Y. Recent advances in the transition-metal-catalyzed regioselective approaches to polysubstituted benzene derivatives. Chem. Rev. 2000, 100, 2901–2915. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, H.; Fukushima, H.; Ohshita, J.; Kunai, A. Arynes in a three-component coupling reaction: Straightforward synthesis of benzoannulated iminofurans. Angew. Chem. Int. Ed. 2004, 43, 3935–3938. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, H.; Fukushima, H.; Ohshita, J.; Kunai, A. Straightforward access to 2-iminoisoindolines via three-component coupling of arynes, isocyanides and imines. Tetrahedron Lett. 2004, 45, 8659–8662. [Google Scholar] [CrossRef]
- Yoshida, H.; Fukushima, H.; Morishita, T.; Ohshita, J.; Kunai, A. Three-component coupling using arynes and isocyanides: Straightforward access to benzo-annulated nitrogen or oxygen heterocycles. Tetrahedron 2007, 63, 4793–4805. [Google Scholar] [CrossRef]
- Allan, K.M.; Gilmore, C.D.; Stoltz, B.M. Benzannulated bicycles by three-component aryne reactions. Angew. Chem. Int. Ed. 2011, 50, 4488–4491. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Noyori, S.; Iwasaki, M.; Nakajima, K.; Nishihara, Y. A novel three-component coupling reaction of arynes, isocyanides, and cyanoformates: A straightforward access to cyano-substituted iminoisobenzofurans. Heterocycles 2012, 86, 933–940. [Google Scholar] [CrossRef]
- Li, J.; Noyori, S.; Nakajima, K.; Nishihara, Y. New entry to the synthesis of α-iminonitriles by Lewis acid mediated isomerization of cyano-substituted iminoisobenzofurans prepared by palladium-catalyzed three-component coupling of arynes, isocyanides, and cyanoformates. Organometallics 2014, 33, 3500–3507. [Google Scholar] [CrossRef]
- Wenk, H.H.; Winkler, M.; Sander, W. One century of aryne chemistry. Angew. Chem. Int. Ed. 2003, 42, 502–528. [Google Scholar] [CrossRef] [PubMed]
- Pellissier, H.; Santelli, M. The use of arynes in organic synthesis. Tetrahedron 2003, 59, 701–730. [Google Scholar] [CrossRef]
- Winkler, M.; Wenk, H.H.; Sander, W. Arynes. In Reactive Intermediate Chemistry; Moss, R.A., Platz, M.S., Jones, M.J., Eds.; John Wiley & Sons: Hoboken, New Jersey, NJ, USA, 2004; pp. 741–794. [Google Scholar]
- Peña, D.; Pérez, D.; Guitián, E. Insertion of arynes into σ bonds. Angew. Chem. Int. Ed. 2006, 45, 3579–3581. [Google Scholar] [CrossRef] [PubMed]
- Dyke, A.M.; Hester, A.J.; Lloyd-Jones, G.C. Organometallic generation and capture of ortho-arynes. Synthesis 2006, 2006, 4093–4112. [Google Scholar] [CrossRef]
- Peña, D.; Pérez, D.; Guitián, E. Aryne-mediated synthesis of heterocycles. Heterocycles 2007, 74, 89–100. [Google Scholar]
- Sanz, R. Recent applications of aryne chemistry to organic synthesis. Org. Prep. Proced. Int. 2008, 40, 215–291. [Google Scholar] [CrossRef]
- Kitamura, T. Synthetic methods for the generation and preparative application of benzyne. Aust. J. Chem. 2010, 63, 987–1001. [Google Scholar] [CrossRef]
- Yoshida, H.; Ohshita, J.; Kunai, A. Aryne, ortho-quinone methide, and ortho-quinodimethane: Synthesis of multisubstituted arenes using the aromatic reactive intermediates. Bull. Chem. Soc. Jpn. 2010, 83, 199–219. [Google Scholar] [CrossRef]
- Okuma, K. Reaction of arynes with carbon-heteroatom double bonds. Heterocycles 2012, 85, 515–544. [Google Scholar] [CrossRef]
- Yoshida, H.; Kunai, A. Multicomponent coupling reaction of arynes for construction of heterocyclic skeletons. Heterocycles 2012, 85, 1333–1349. [Google Scholar] [CrossRef]
- Bhunia, A.; Yetra, S.R.; Biju, A.T. Recent advances in transition-metal-free carbon-carbon and carbon-heteroatom bond-forming reactions using arynes. Chem. Soc. Rev. 2012, 41, 3140–3152. [Google Scholar] [CrossRef] [PubMed]
- Tadross, P.M.; Stoltz, B.M. A comprehensive history of arynes in natural product total synthesis. Chem. Rev. 2012, 112, 3550–3577. [Google Scholar] [CrossRef] [PubMed]
- Bhojgude, S.S.; Biju, A.T. Arynes in transition-metal-free multicomponent coupling reactions. Angew. Chem. Int. Ed. 2012, 51, 1520–1522. [Google Scholar] [CrossRef] [PubMed]
- Gampe, C.M.; Carreira, E.M. Arynes and cyclohexyne in natural product synthesis. Angew. Chem. Int. Ed. 2012, 51, 3766–3778. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, H.; Kunai, A. Aryne insertion reactions into carbon–carbon σ-bonds. Synlett 2012, 23, 1725–1732. [Google Scholar] [CrossRef]
- Pérez, D.; Peña, D.; Guitián, E. Aryne cycloaddition reactions in the synthesis of large polycyclic aromatic compounds. Eur. J. Org. Chem. 2013, 2013, 5981–6013. [Google Scholar] [CrossRef]
- Goetz, A.E.; Garg, N.K. Enabling the use of heterocyclic arynes in chemical synthesis. J. Org. Chem. 2014, 79, 846–851. [Google Scholar] [CrossRef] [PubMed]
- Miyabe, H. Insertion of arynes into the π-bond giving [2 + 2] cycloaddition-type adducts. Curr. Org. Chem. 2015, in press. [Google Scholar] [CrossRef]
- Van De Water, R.W.; Pettus, T.R.R. o-Quinone methides: Intermediates underdeveloped and underutilized in organic synthesis. Tetrahedron 2002, 28, 5367–5405. [Google Scholar] [CrossRef]
- Heaney, H.; Jablonski, J.M.; McCarty, C.T. Aryne chemistry. Part XXXI. Reactions of arynes with αβ-unsaturated aldehydes. J. Chem. Soc. Perkin Trans. 1 1972, 2903–2910. [Google Scholar] [CrossRef]
- Heaney, H.; Jablonski, J.M. Reactions of arynes in the synthesis of 2H-chromens. Chem. Commun. 1968, 1139. [Google Scholar] [CrossRef]
- Heaney, H.; McCarty, C.T. Reactions of arynes with carbonyl compounds. J. Chem. Soc. Chem. Commun. 1970, 123a. [Google Scholar] [CrossRef]
- Nakayama, J.; Yoshida, M.; Simamura, O. Reaction of benzyne generated from 1-(2-carboxyphenyl)-3,3-dimethyltriazene with benzaldehyde and some other carbonyl compounds. Chem. Lett. 1973, 2, 451–454. [Google Scholar] [CrossRef]
- Yoshida, H.; Watanabe, M.; Fukushima, H.; Ohshita, J.; Kunai, A. A 2:1 coupling reaction of arynes with aldehydes via o-quinone methides: Straightforward synthesis of 9-arylxanthenes. Org. Lett. 2004, 6, 4049–4051. [Google Scholar] [CrossRef] [PubMed]
- Yaroslavsky, S. Reaction of aryldiazonium salts with dimethylformamide. Tetrahedron Lett. 1965, 6, 1503–1507. [Google Scholar] [CrossRef]
- Yoshioka, E.; Kohtani, S.; Miyabe, H. Sequential reaction of arynes via insertion into the π-bond of amides and trapping reaction with dialkylzincs. Org. Lett. 2010, 12, 1956–1959. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, E.; Miyabe, H. Insertion of arynes into the carbon-oxygen double bond of amides and its application into the sequential reactions. Tetrahedron 2012, 68, 179–189. [Google Scholar] [CrossRef]
- Okuma, K.; Nojima, A.; Nakamura, Y.; Matsunaga, N.; Nagahora, N.; Shioji, K. Reaction of benzyne with formamides and acetylimidazole. Bull. Chem. Soc. Jpn. 2011, 84, 328–332. [Google Scholar] [CrossRef]
- Yoshioka, E.; Kohtani, S.; Miyabe, H. A multicomponent coupling reaction induced by insertion of arynes into C=O bond of formamide. Angew. Chem. Int. Ed. 2011, 50, 6638–6642. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, E.; Tamenaga, H.; Miyabe, H. [4 + 2] cycloaddition of intermediates generated from arynes and DMF. Tetrahedron Lett. 2014, 55, 1402–1405. [Google Scholar] [CrossRef]
- Yoshida, H.; Ito, Y.; Ohshita, J. Three-component coupling using arynes and DMF: Straightforward access to coumarins via ortho-quinone methides. Chem. Commun. 2011, 47, 8512–8514. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, E.; Miyabe, H. Three-component coupling reactions of arynes for the synthesis of benzofurans and coumarins. Molecules 2014, 19, 863–880. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, E.; Kohtani, S.; Miyabe, H. 2,3,4,9-Tetrahydro-9-(3-hydroxy-1,4-dioxo-1H-dihydronaphthalen-2-yl)-8-methoxy-3,3-dimethyl-1H-xanthen-1-one. Molbank 2015, M841, 1–6. [Google Scholar]
- Yoshioka, E.; Tanaka, H.; Kohtani, S.; Miyabe, H. Straightforward synthesis of dihydrobenzofurans and benzofurans from arynes. Org. Lett. 2013, 15, 3938–3941. [Google Scholar] [CrossRef] [PubMed]
- Witiak, D.T.; Newman, H.A.I.; Poochikian, G.K.; Fogt, S.W.; Baldwin, J.B.; Sober, C.L.; Feller, D.R. Diethyl (4bα,4cα,9aα,9bα)-3,6-dichlorocyclobuta [1,2-b:3,4-b]bisbenzofuran-9a,9b(4bH,4cH)-dicarboxylate: The cis,syn photodimer of ethyl 5-chlorobenzofuran-2-carboxylatea, an analogue related to the antilipidemic drug clofibrate. J. Med. Chem. 1978, 21, 833–837. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miyabe, H. Synthesis of Oxygen Heterocycles via Aromatic C-O Bond Formation Using Arynes. Molecules 2015, 20, 12558-12575. https://doi.org/10.3390/molecules200712558
Miyabe H. Synthesis of Oxygen Heterocycles via Aromatic C-O Bond Formation Using Arynes. Molecules. 2015; 20(7):12558-12575. https://doi.org/10.3390/molecules200712558
Chicago/Turabian StyleMiyabe, Hideto. 2015. "Synthesis of Oxygen Heterocycles via Aromatic C-O Bond Formation Using Arynes" Molecules 20, no. 7: 12558-12575. https://doi.org/10.3390/molecules200712558