Antioxidative and Anticanceric Activities of Magnolia (Magnolia denudata) Flower Petal Extract Fermented by Pediococcus acidilactici KCCM 11614
Abstract
:1. Introduction
2. Results and Discussion
2.1. Determination of the Total Polyphenol Content during Fermentation
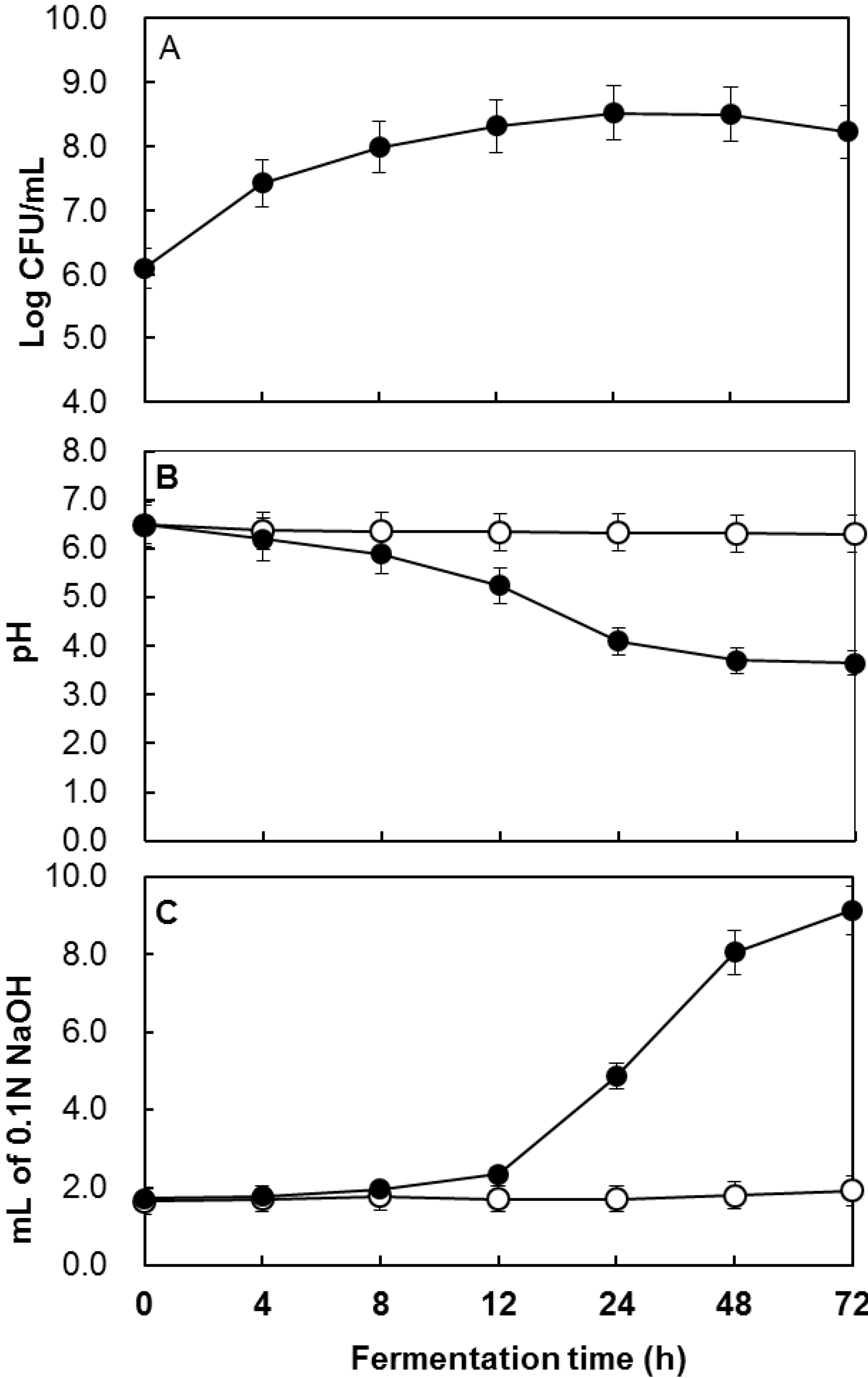
| Fermentation Time (h) | Solid Contents (mg/mL) | Total Phenolic Contents (mg GAE 1/g of Solid) | Total Flavonoids (mg Quercetin/mL) |
|---|---|---|---|
| 0 | 29.2 ± 0.4 2 | 38.1 ± 0.4 | 27.9 ± 0.1 |
| 12 | 26.5 ± 0.2 | 42.3 ± 0.8 | 31.3 ± 0.1 |
| 24 | 26.0 ± 0.1 | 42.4 ± 0.9 | 33.8 ± 0.2 |
| 48 | 24.5 ± 0.1 | 48.1 ± 0.5 | 38.1 ± 0.1 |
| 72 | 23.4 ±0.2 | 47.0 ± 0.1 | 39.6 ± 0.2 |
2.2. Antioxidative Activity of Fermented Magnolia Extract
| Fermentation Time (h) | DPPH Method (%) | β-Carotene Method (%) | FRAP Assay 1 | |||
|---|---|---|---|---|---|---|
| NFM 2 | FMPA 3 | NFM | FMPA | NFM | FMPA | |
| 0 | 80.7 ± 3.1 4,d | 85.1 ± 0.3 b,c | 84.5 ± 2.4 a | 80.7 ± 3.1 b | 1.3 ± 0.1 a | 1.3 ± 0.1 a |
| 24 | 82.3 ± 1.4 d | 91.5 ± 0.4 a | 84.0 ± 1.1 a,b | 68.7 ± 1.3 c | 1.3 ± 0.1 b | 1.2 ± 0.1 b |
| 48 | 86.0 ± 0.8 b | 91.1 ± 1.0 a | 84.2 ± 0.5 a | 60.4 ± 7.1 d | 1.3 ± 0.1 a | 1.2 ± 0.1 b |
| 72 | 83.0 ± 0.8 c,d | 91.4 ± 0.3 a | 82.6 ± 1.1 a,b | 52.5 ± 3.2 e | 1.2 ± 0.2 b | 1.3 ± 0.2 a |
| Conc. (mg/mL) | FRAP Assay 1 | |
|---|---|---|
| NFM 2 | FMPA 3 | |
| 0.125 | - | 20.4 ± 9.4 4,h |
| 0.25 | - | 118.9 ± 3.0 f |
| 0.5 | 83.2 ± 1.8 g | 226.2 ± 5.7 e |
| 1.0 | 294.1 ± 1.6 d | 537.7 ± 4.1 c |
| 2.0 | 641.5 ± 6.5 b | 974.8 ± 7.5 a |
2.3. In Vitro Determination of Anticanceric Activity
| Time 1 (h) | Anticancer Activity (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| AGS | HeLa | LoVo | MCF-7 | MRC-5 2 | ||||||
| NFM 3 | MFPA 4 | NFM | MFPA | NFM | MFPA | NFM | MFPA | NFM | MFPA | |
| 0 | 61.3 ± 9.9 5,c | 76.1 ± 3.2 b | 62.4 ± 8.4 b | 78.1 ± 3.2 a | 72.8 ± 4.1 b,c | 73.0 ± 2.7 b,c | 64.4 ± 0.9 b,c,d | 72.8 ± 1.3 d | 82.1 ± 5.1 a,b | 83.7 ± 2.1 a |
| 12 | 59.0 ± 6.3 d | 80.8 ± 2.7 a,b | 71.9 ± 7.4 a,b | 82.6 ± 7.1 a | 73.9 ± 5.1 b,c | 76.2 ± 9.3 b | 66.4 ± 7.1 b,c,d | 90.6 ± 2.0 b | 81.6 ± 1.1 a,b | 82.6 ± 1.1 a,b |
| 24 | 60.9 ± 4.9 d | 82.0 ± 2.9 a,b | 76.3 ± 3.9 a | 74.7 ± 7.4 a | 78.8 ± 5.4 b | 88.7 ± 2.1 a | 68.7 ± 2.8 b,c | 90.7 ± 1.0 a | 83.3 ± 2.7 a | 84.6 ± 3.0 a |
| 48 | 57.7 ± 3.5 c,d | 83.7 ± 0.3 a,b | 70.9 ± 5.8 a,b | 75.2 ± 6.2 a | 63.8 ± 1.8 d | 87.1 ± 0.3 a | 62.5 ± 2.6 c,d | 91.9 ± 0.2 a | 82.4 ± 5.0 a,b | 83.6 ± 2.0 a |
| 72 | 66.0 ± 4.1 c,d | 85.0 ± 2.8 a | 71.2 ± 3.2 a,b | 73.6 ± 6.3 a | 66.1 ± 4.0 c,d | 88.4 ± 1.3 a | 67.5 ± 8.5 b,c,d | 92.0 ± 0.6 a | 80.4 ± 0.4 a,b | 78.0 ± 6.2 a,b |
3. Experimental Section
3.1. Strains and Chemicals
3.2. Cell lines and Culture Conditions
3.3. Fermentation of Magnolia
3.4. Determination of the Total Polyphenol and Flavonoid Content
3.5. Measurement of the Antioxidative Activity Using the DPPH Method
3.6. Measurement of the Antioxidative Activity Using the β-Carotene Bleaching Activity Assay
3.7. Determination of Antioxidative Activity Using the Ferric Reducing Ability of Plasma Assay
3.8. In Vitro Determination of Anticanceric Activity
3.9. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wang, R.; Jia, H.; Wang, J.; Ahang, Z. Flowering and pollination patterns of Magnolia denudata with emphasis on anatomical changes in ovule and seed development. Flora 2010, 205, 259–265. [Google Scholar] [CrossRef]
- Seo, Y. Antioxidant activity of chemical constituents from the flower buds of Magnolia denudata. Biotechnol. Bioprocess Eng. 2010, 15, 400–406. [Google Scholar] [CrossRef]
- Noshita, T.; Kiyot, H.; Kidachi, Y.; Ryoyama, K.; Funayama, S.; Hanada, K.; Murayama, T. New cytotoxic phenolic derivatives from matured fruits of Magnolia denudata. Biosci. Biotechnol. Biochem. 2009, 73, 726–728. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Lv, G.; Pan, H.; Fan, L.; Soccol, C.R.; Pandey, A. Production of powerful antioxidant supplements via solid-state fermentation of wheat (Triticum aestivum Linn.) by Cordyceps militaris. Food Technol. Biotechnol. 2012, 50, 32–39. [Google Scholar]
- Torino, M.I.; Limon, R.I.; Martinez-Villaluenga, C.; Makinen, S.; Pihlanto, A.; Vidal-Valverde, C.; Frias, J. Antioxidant and antihypertensive properties of liquid and solid state fermented lentils. Food Chem. 2013, 136, 1030–1037. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jo, M.N.; Jung, J.E.; Lee, J.H.; Park, S.H.; Yoon, H.J.; Kim, K.T.; Paik, H.D. Cytotoxicity of the white ginseng extract and red ginseng extract treated with partially purified β-glucosidase from Aspergillus usamii KCTC 6954. Food Sci. Biotechnol. 2014, 23, 215–219. [Google Scholar] [CrossRef]
- Yoon, H.J.; Lee, K.A.; Lee, J.H.; Jin, H.J.; Kim, H.J.; Kim, K.T.; Paik, H.D. Effect of fermentation by Bacillus subtilis on antioxidant and cytotoxic activities of black rice bran. Int. J. Food Sci. Technol. 2015, 50, 612–618. [Google Scholar] [CrossRef]
- Aydemir, O.; Harth, H.; Weckx, S.; Dervisoglu, M.; de Vuyst, L. Microbial communities involved in Kasar cheese ripening. Food Microbiol. 2015, 46, 587–595. [Google Scholar] [CrossRef] [PubMed]
- Kaur, B.; Chakraborty, D.; Kumar, B. Metabolic engineering of Pediococcus acidilactici BD16 for production of vanillin through ferulic acid catabolic pathway and process optimization using response surface methodology. Appl. Microbiol. Biotechnol. 2014, 98, 8539–8551. [Google Scholar] [CrossRef] [PubMed]
- Michlmayr, H.; Brandes, W.; Eder, R.; Schümann, C.; Del Hierro, A.M.; Kulbe, K.D. Characterization of two distinct glycosyl hydrolase family 78 α-l-rhamnosidases from Pediococcus acidilactici. Appl. Environ. Microbiol. 2011, 77, 6524–6530. [Google Scholar] [CrossRef] [PubMed]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef] [PubMed]
- Poyton, R.O.; Ball, K.A.; Castello, P.R. Mitochondrial generation of free radicals and hypoxic signaling. Trends Endocrinol. Metab. 2009, 20, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Durackova, Z. Some current insights into oxidative stress. Physiol. Res. 2010, 59, 459–469. [Google Scholar] [PubMed]
- Schraufstatter, I.; Hyslop, P.A.; Jackson, J.H.; Cochrane, C.G. Oxidant-induced DNA damage of target cells. J. Clin. Investig. 1988, 82, 1040–1050. [Google Scholar] [CrossRef] [PubMed]
- Mihajlovic, L.; Radosavljevic, J.; Burazer, L.; Smiljanic, K.; Velickovic, T.C. Composition of polyphenol and polyamide compounds in common ragweed (Ambrosia artemisiifolia L.) pollen and sub-pollen particles. Phytochemistry 2015, 109, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Zhao, L.; Cai, L.; Fang, H.; Chen, X.; Ding, Z. Antioxidant activities and phenolics of fermented Bletilla formosana with eight plant pathogen fungi. J. Biosci. Bioeng. 2014, 118, 396–399. [Google Scholar] [CrossRef] [PubMed]
- Duenas, M.; Fernandez, D.; Hernandez, T.; Estrella, I.; Munoz, R. Bioactive phenolic compounds of cowpeas (Vigna sinensis L.) Modifications by fermentation with natural microflora and with Lactobacillus plantarum ATCC14917. J. Sci. Food Agric. 2005, 85, 297–304. [Google Scholar] [CrossRef]
- Othman, A.; Ismail, A.; Abdul Ghani, N.; Adenan, I. Antioxidant capacity and phenolic content of cocoa beans. Food Chem. 2007, 100, 1523–1530. [Google Scholar] [CrossRef]
- Ibrahim, N.A.; Mustafa, S.; Ismail, A. Effect of lactic fermentation on the antioxidant capacity of Malaysian herbal teas. Int. Food Res. J. 2014, 21, 1483–1488. [Google Scholar]
- Hur, S.J.; Lee, S.Y.; Kim, Y.C.; Choi, I.; Kim, G.B. Effect of fermentation on the antioxidant activity in plant-based foods. Food Chem. 2014, 160, 346–356. [Google Scholar] [CrossRef] [PubMed]
- Pulido, R.; Bravo, L.; Saura-Calixto, F. Antioxidant activity of dietary polyphenols as determined by a modified ferric reducing/antioxidant power assay. J. Agric. Food Chem. 2000, 48, 3396–3402. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Ji, Y.; Park, H.; Lee, J.; Park, S.; Yeo, S.; Shin, H.; Holzapfel, W.H. Selection of functional lactic acid bacteria as starter cultures for the fermentation of Korean leek (Allium tuberosum Rottler ex Sprengel.). Int. J. Food Microbiol. 2014, 191, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Katina, K.; Laitila, A.; Juvonen, R.; Liukkonen, K.H.; Kariluoto, S.; Piironen, V.; Landberg, R.; Åman, P.; Poutanen, K. Bran fermentation as a means to enhance technological properties and bioactivity of rye. Food Microbiol. 2007, 24, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Đorđević, T.M.; Šiler-Marinković, S.S.; Dimitrijević-Branković, S.I. Effect of fermentation on antioxidant properties of some cereals and pseudo cereals. Food Chem. 2010, 119, 957–963. [Google Scholar] [CrossRef]
- Ng, C.C.; Wang, C.Y.; Wang, Y.P.; Tzeng, W.S.; Shyu, Y.T. Lactic acid bacterial fermentation on the production of functional antioxidant herbal Anoectochilus formosanus Hayata. J. Biosci. Bioeng. 2011, 111, 289–293. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.S.; Kim, Y.; Han, K.S.; You, S. Effects of Lactobacillus strains on cancer cell proliferation and oxidative stress in vitro. Lett. Appl. Microbiol. 2006, 42, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Hwang, W.I.; Lim, S.T. Antioxidant and anticancer activities of organic extracts from Platycodon grandiflorum A. De Candolle roots. J. Ethnopharmacol. 2004, 93, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Kawahara, T.; Tomono, T.; Hamauzu, Y.; Tanaka, K.; Yasui, H. Inhibitory effect of a hot-water extract of leaves of Japanese big-leaf magnolia (Magnolia obovata) on rotavirus-induced diarrhea in mouse pups. Evid-Based Complement. Altern. Med. 2014, 2014. [Google Scholar] [CrossRef]
- Caia, Y.; Luo, Q.; Sun, M.; Corke, H. Antioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with anticancer. Life Sci. 2004, 74, 2157–2184. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Lv, J.J.; Zhao, J.Q.; Li, Y.; Wang, D.; Yang, C.R.; Xu, M.; Zhang, Y.J. New cytotoxic lignan glycosides from Phyllanthus glaucus. Nat. Prod. Res. 2015. [CrossRef]
- Hirano, T.; Gotoh, M.; Oka, K. Natural flavonoids and lignans are potent cytostatic agents against human leukemic HL-60 cells. Life Sci. 1994, 55, 1061–1069. [Google Scholar] [CrossRef]
- Simon, H.U.; Haj-Yehia, A.; Levi-Schaffer, F. Role of reactive oxygen species (ROS) in apoptosis induction. Apoptosis 2000, 5, 415–418. [Google Scholar] [CrossRef] [PubMed]
- Vijayababu, M.R.; Kanagaraj, P.; Arunkumar, A.; Ilangvan, R.; Dharmarajan, A.; Arunakaran, J. Quercetin induces p53-independent apoptosis in human prostate cancer cells by modulating Bcl-2-related proteins: A possible mediation by IGFBP-3. Oncol. Res. 2006, 16, 67–74. [Google Scholar] [PubMed]
- Lim, Y.; Jeong, T.; Tyner, A.L.; Park, J.H. Induction of cell cycle arrest and apoptosis in HT-29 human colon cancer cells by the dietary compounds luteolin. Am. J. Physiol. Gastrointest. Liver Physiol. 2007, 292, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.; Mi, M.; Ling, W.; Zhu, J.; Zhang, Q.; Wei, N.; Zhou, Y.; Tang, Y.; Yuan, J. Structurally related cytotoxic effects of flavonoids on human cancer cells in vitro. Arch. Pharm. Res. 2008, 31, 1137–1144. [Google Scholar] [CrossRef] [PubMed]
- Moreno, M.I.N.; Isla, M.I.; Sampietro, A.R.; Vattuone, M.A. Comparison of the free radical-scavenging activity of propolis from several regions of Argentina. J. Ethnopharmacol. 2000, 71, 109–114. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wei, Y.; Yuan, S.; Liu, G.; Zhang, Y.L.; Wang, W. Potential anticancer activity of litchi fruit pericarp extract against hepatocellular carcinoma in vitro and in vivo. Cancer Lett. 2006, 239, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Not available.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, H.; Kim, H.-S.; Eom, S.J.; Kim, K.-T.; Paik, H.-D. Antioxidative and Anticanceric Activities of Magnolia (Magnolia denudata) Flower Petal Extract Fermented by Pediococcus acidilactici KCCM 11614. Molecules 2015, 20, 12154-12165. https://doi.org/10.3390/molecules200712154
Park H, Kim H-S, Eom SJ, Kim K-T, Paik H-D. Antioxidative and Anticanceric Activities of Magnolia (Magnolia denudata) Flower Petal Extract Fermented by Pediococcus acidilactici KCCM 11614. Molecules. 2015; 20(7):12154-12165. https://doi.org/10.3390/molecules200712154
Chicago/Turabian StylePark, Hye, Hyun-Suk Kim, Su Jin Eom, Kee-Tae Kim, and Hyun-Dong Paik. 2015. "Antioxidative and Anticanceric Activities of Magnolia (Magnolia denudata) Flower Petal Extract Fermented by Pediococcus acidilactici KCCM 11614" Molecules 20, no. 7: 12154-12165. https://doi.org/10.3390/molecules200712154
APA StylePark, H., Kim, H.-S., Eom, S. J., Kim, K.-T., & Paik, H.-D. (2015). Antioxidative and Anticanceric Activities of Magnolia (Magnolia denudata) Flower Petal Extract Fermented by Pediococcus acidilactici KCCM 11614. Molecules, 20(7), 12154-12165. https://doi.org/10.3390/molecules200712154





