Synthesis and Biological Evaluation of N-Alkoxyphenyl-3-hydroxynaphthalene-2-carboxanilides
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry
| Comp. | R1 | log P a | MV a [cm3] | ST a [dyne/cm] | [µM] | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIC | LD50 | PET IC50 | ||||||||||
| SA | MRSA 63718 | MRSA SA 630 | MRSA 3202 | MT | MAP | |||||||
| 2 | H | 4.52 | 0 | 0 | 972 | 972 | 972 | 972 | 950 | 950 | >30 | ND |
| 3a | 2-OCH3 | 4.61 | 37.15 | 58.71 | 55 | 55 | 55 | 55 | 51 | 205 | >30 | 59.5 |
| 3b | 3-OCH3 | 4.56 | 37.15 | 58.71 | 873 | 873 | 873 | 873 | 51 | 426 | >30 | 53.4 |
| 3c | 4-OCH3 | 4.37 | 37.15 | 58.71 | 873 | 873 | 873 | 873 | 852 | 852 | >30 | ND |
| 4a | 2-OC2H5 | 4.92 | 53.66 | 56.66 | 832 | 832 | 832 | 832 | 813 | 813 | >30 | 76.1 |
| 4b | 3-OC2H5 | 4.88 | 53.66 | 56.66 | 832 | 832 | 832 | 832 | 813 | 813 | >30 | 4.5 |
| 4c | 4-OC2H5 | 4.67 | 53.66 | 56.66 | 832 | 832 | 832 | 832 | 813 | 813 | >30 | ND |
| 5a | 2-OC3H7 | 5.26 | 70.16 | 54.92 | 12.4 | 12.4 | 12.4 | 12.4 | 778 | 778 | >30 | 128 |
| 5b | 3-OC3H7 | 5.21 | 70.16 | 54.92 | 796 | 796 | 796 | 796 | 778 | 778 | >30 | 4.8 |
| 5c | 4-OC3H7 | 5.27 | 70.16 | 54.92 | 796 | 796 | 796 | 796 | 778 | 778 | >30 | ND |
| 6a | 2-OC4H9 | 5.60 | 86.67 | 53.42 | 763 | 763 | 763 | 763 | 373 | 745 | >30 | 182 |
| 6b | 3-OC4H9 | 5.54 | 86.67 | 53.42 | 763 | 763 | 763 | 763 | 745 | 745 | 16.5 ± 0.8 | 7.8 |
| 6c | 4-OC4H9 | 5.60 | 86.67 | 53.42 | 763 | 763 | 763 | 763 | 745 | 745 | >30 | ND |
| 7a | 2-OCH(CH3)2 | 5.18 | 70.54 | 53.79 | 796 | 796 | 796 | 796 | 389 | 778 | >30 | 138 |
| 7b | 3-OCH(CH3)2 | 5.13 | 70.54 | 53.79 | 796 | 796 | 796 | 796 | 24 | 778 | 27.4 ± 0.6 | 6.9 |
| 7c | 4-OCH(CH3)2 | 5.11 | 70.54 | 53.79 | 796 | 796 | 796 | 796 | 389 | 778 | >30 | ND |
| 8a | 2-OCH(CH3)C2H5 | 5.52 | 87.05 | 52.38 | 5.9 | 11.9 | 11.9 | 11.9 | 23 | 89 | >30 | 134 |
| 8b | 3-OCH(CH3)C2H5 | 5.47 | 87.05 | 52.38 | 763 | 763 | 763 | 763 | 23 | 745 | 2.7 ± 0.7 | 8.3 |
| 8c | 4-OCH(CH3)C2H5 | 5.46 | 87.05 | 52.38 | 763 | 763 | 763 | 763 | 89 | 745 | >30 | ND |
| APC | – | − | – | – | 5.7 | >46 | >46 | >46 | – | – | – | – |
| RIF | – | − | – | – | – | – | – | – | 10 | 109 | – | – |
| DCMU | – | − | − | − | – | – | – | – | – | – | – | 1.9 |
2.2. In Vitro Antibacterial Susceptibility Testing
2.3. In Vitro Antimycobacterial Evaluation
2.4. In Vitro Cytotoxicity Assay
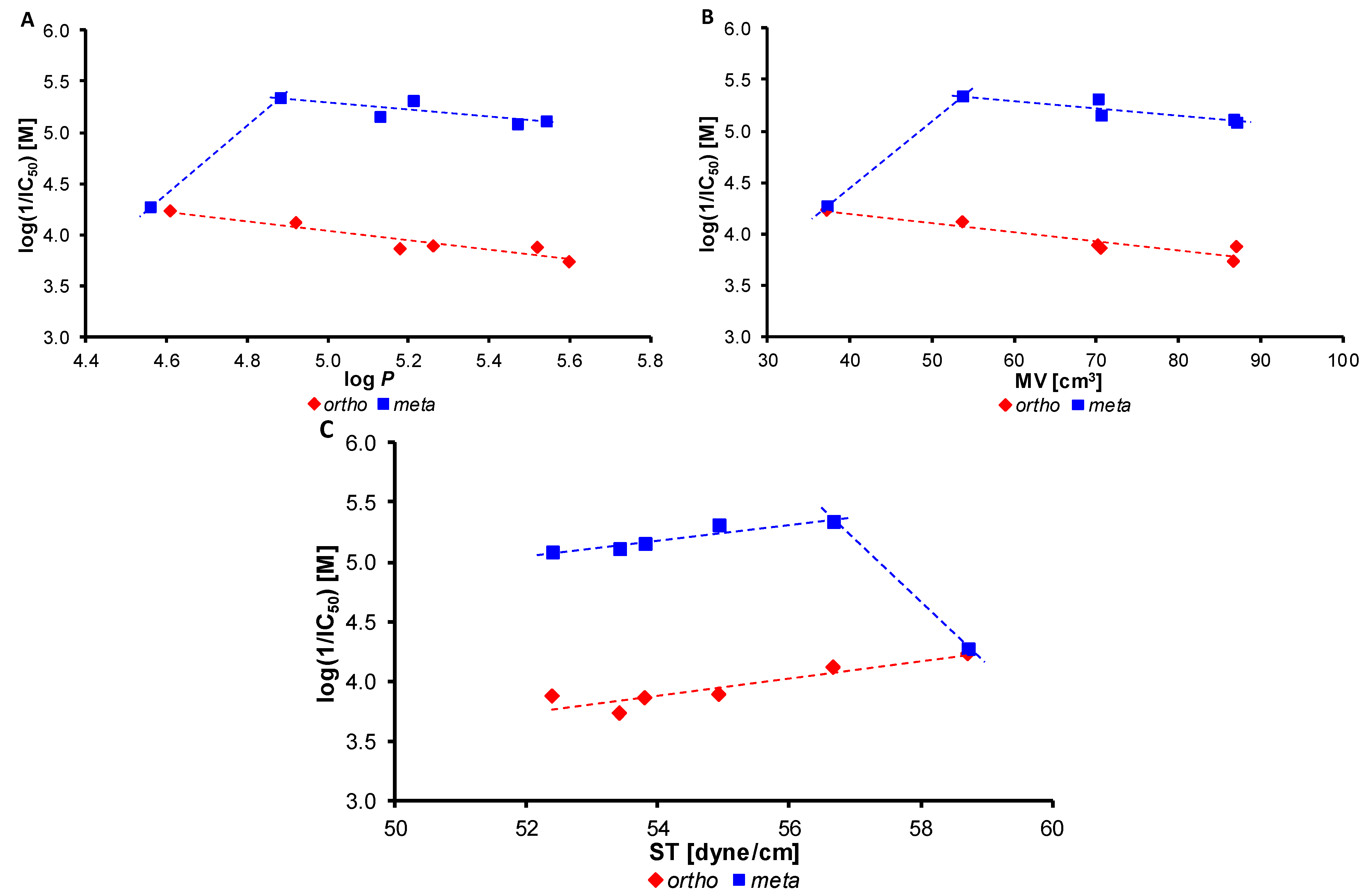
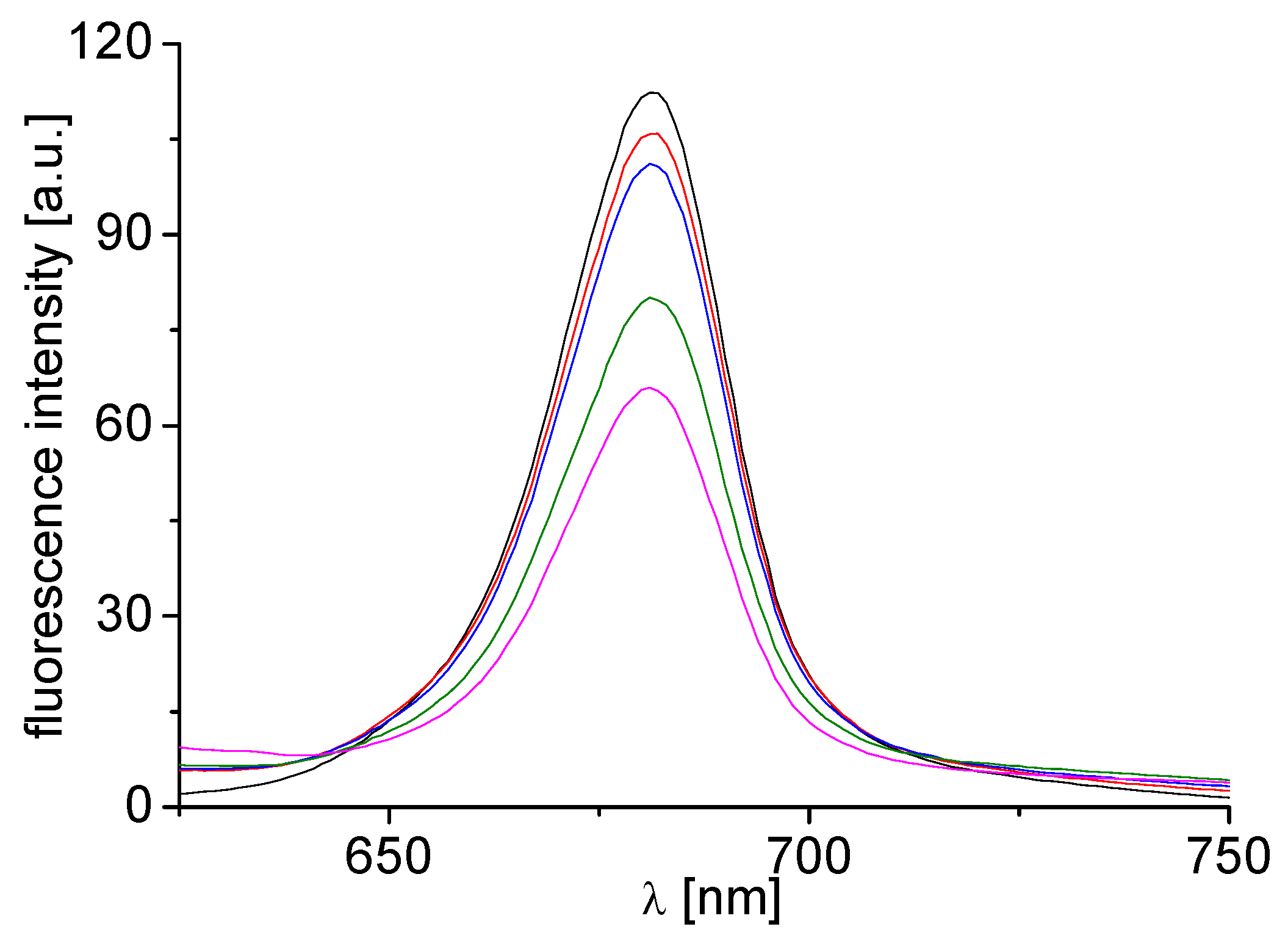
2.5. Inhibition of Photosynthetic Electron Transport (PET) in Spinach Chloroplasts
3. Experimental Section
3.1. General Information
3.2. Synthesis
3.2.1. General Procedure for the Synthesis of Anilines 1a–1o
3.2.2. General Procedure for the Synthesis of Carboxamide Derivatives 2–8c
3.3. In Vitro Antibacterial Susceptibility Testing
3.4. In Vitro Antimycobacterial Evaluation
3.5. In Vitro Cytotoxicity Assay
3.6. Study of Inhibition Photosynthetic Electron Transport (PET) in Spinach Chloroplasts
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kaku, N.; Yanagihara, K.; Morinaga, Y.; Yamada, K.; Harada, Y.; Migiyama, Y.; Nagaoka, K.; Matsuda, J.; Uno, N.; Hasegawa, H.; et al. Influence of antimicrobial regimen on decreased in-hospital mortality of patients with MRSA bacteremia. J. Infect. Chemother. 2014, 20, 350–355. [Google Scholar] [CrossRef]
- Lodise, T.P.; McKinnon, P.S. Clinical and economic impact of methicillin resistance in patients with Staphylococcus aureus bacteremia. Diagn. Microbiol. Infect. Dis. 2005, 52, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Stefani, S.; Chung, D.R.; Lindsay, J.A.; Friedrich, A.W.; Kearns, A.M.; Westh, H.; Mackenzie, F.M. Meticillin-resistant Staphylococcus aureus (MRSA): Global epidemiology and harmonisation of typing methods. Int. J. Antimicrob. Agents 2012, 39, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Wilcox, M.H. MRSA new treatments on the horizon: Current status. Injury 2011, 42, S42–S44. [Google Scholar] [CrossRef]
- World Health Organization. Global Tuberculosis Report 2014; WHO Press: Geneva, Switzerland, 2014. [Google Scholar]
- Ioachimescu, O.C.; Tomford, J.W. Nontuberculous mycobacterial disorders. In Disease Management Project; Carey, W., Ed.; Cleveland Clinic—Centre for Continuing Education: Cleveland, OH, USA, 2015; Available online: http://www.clevelandclinicmeded.com/medicalpubs/diseasemanagement/infectious-disease/nontuberculous-mycobacterial-disorders/Default.htm (accessed on 15 April 2015).
- Kratky, M.; Vinsova, J. Salicylanilide ester prodrugs as potential antimicrobial agents—A review. Curr. Pharm. Des. 2011, 17, 3494–3505. [Google Scholar] [CrossRef] [PubMed]
- Zadrazilova, I.; Pospisilova, S.; Pauk, K.; Imramovsky, A.; Vinsova, J.; Cizek, A.; Jampilek, J. In vitro bactericidal activity of 4- and 5-chloro-2-hydroxy-N-[1-oxo-1-(phenylamino)alkan-2-yl]benzamides against MRSA. BioMed. Res. Int. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Liechty, C.H.; Sequin, U.; Bold, G.; Furet, P.; Meyer, T.; Traxler, P. Salicylanilides as inhibitors of the protein tyrosine kinase epidermal growth factor receptor. Eur. J. Med. Chem. 2004, 39, 11–26. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.E.; Fitzner, J.N.; Stevens, T.; Chin, W.; Wright, C.D.; Boyce, J.P. Salicylanilides: Selective inhibitors of interleukin-12p40 production. Bioorg. Med. Chem. 2008, 16, 8760–8764. [Google Scholar] [CrossRef] [PubMed]
- Kos, J.; Zadrazilova, I.; Pesko, M.; Keltosova, S.; Tengler, J.; Gonec, T.; Bobal, P.; Kauerova, T.; Oravec, M.; Kollar, P.; et al. Antibacterial and herbicidal activity of ring-substituted 3-hydroxynaphthalene-2-carboxanilides. Molecules 2013, 18, 7977–7997. [Google Scholar] [CrossRef] [PubMed]
- Shaner, D.L. Herbicide safety relative to common targets in plants and mammals. Pest. Manag. Sci. 2004, 60, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Delaney, J.; Clarke, E.; Hughes, D.; Rice, M. Modern agrochemical research: A missed opportunity for drug discovery? Drug Discov. Today 2006, 11, 839–845. [Google Scholar] [CrossRef] [PubMed]
- Duke, S.O. Herbicide and pharmaceutical relationships. Weed Sci. 2010, 58, 334–339. [Google Scholar] [CrossRef]
- Myung, K.; Klittich, C.J. Can agricultural fungicides accelerate the discovery of human antifungal drugs? Drug Discov. Today 2015, 20, 7–10. [Google Scholar] [CrossRef] [PubMed]
- Imramovsky, A.; Pesko, M.; Monreal-Ferriz, J.; Kralova, K.; Vinsova, J.; Jampilek, J. Photosynthesis-Inhibiting efficiency of 4-chloro-2-(chlorophenylcarbamoyl)phenyl alkylcarbamates. Bioorg. Med. Chem. Lett. 2011, 21, 4564–4567. [Google Scholar] [CrossRef] [PubMed]
- Gonec, T.; Kos, J.; Zadrazilova, I.; Pesko, M.; Keltosova, S.; Tengler, J.; Bobal, P.; Kollar, P.; Cizek, A.; Kralova, K.; et al. Antimycobacterial and herbicidal activity of ring-substituted 1-hydroxynaphthalene-2-carboxanilides. Bioorg. Med. Chem. 2013, 21, 6531–6541. [Google Scholar] [CrossRef] [PubMed]
- Gonec, T.; Kos, J.; Zadrazilova, I.; Pesko, M.; Govender, R.; Keltosova, S.; Kollar, B.; Imramovsky, A.; O’Mahony, J.; Coffey, A.; et al. Antibacterial and herbicidal activity of ring-substituted 2-hydroxynaphthalene-1-carboxanilides. Molecules 2013, 18, 9397–9419. [Google Scholar] [CrossRef] [PubMed]
- Kralova, K.; Sersen, F.; Cizmarik, J. Inhibitory effect of piperidinoethylesters of alkoxyphenylcarbamic acids on photosynthesis. Gen. Physiol. Biophys. 1992, 11, 261–267. [Google Scholar]
- Kralova, K.; Bujdakova, H.; Kuchta, T.; Loos, D. Correlation between biological activity and the structure of 6-amino-2-R-thiobenzothiazoles. Anti-yeast activity and inhibition of photochemical activity of chloroplasts. Pharmazie 1994, 49, 460–461. [Google Scholar] [PubMed]
- Kralova, K.; Kallova, J.; Loos, D.; Devinsky, F. Correlation between biological activity and the structure of N,N'-bis(alkyldimethyl)-1,6-hexanediammonium dibromides. Antibacterial activity and inhibition of photochemical activity of chloroplasts. Pharmazie 1994, 49, 857–858. [Google Scholar] [PubMed]
- Kralova, K.; Bujdakova, H.; Cizmarik, J. Antifungal and antialgal activity of piperidinopropyl esters of alkoxy substituted phenylcarbamic acids. Pharmazie 1995, 50, 440–441. [Google Scholar] [PubMed]
- Kralova, K.; Perina, M.; Waisser, K.; Jampilek, J. Structure-activity relationships of N-benzylsalicylamides for inhibition of photosynthetic electron transport. Med. Chem. 2015, 11, 156–164. [Google Scholar] [CrossRef] [PubMed]
- De Marco, A.; de Candia, M.; Carotti, A.; Cellamare, S.; de Candia, E.; Altomare, C. Lipophilicity-related inhibition of blood platelet aggregation by nipecotic acid anilides. Eur. J. Pharm. Sci. 2004, 22, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Lu, L.; Wang, B.; Pu, S.; Zhang, X.; Zhu, G.; Shi, W.; Zhang, L.; Wang, H.; Wang, S.; et al. Genetic basis of virulence attenuation revealed by comparative genomic analysis of Mycobacterium tuberculosis strain H37Ra versus H37Rv. PLoS ONE 2008, 3. [Google Scholar] [CrossRef] [PubMed]
- Rath, T.; Roderfeld, M.; Blocher, S.; Rhode, A.; Basler, T.; Akineden, O.; Abdulmawjood, A.; Halwe, J.M.; Goethe, R.; Bulte, M.; et al. Presence of intestinal Mycobacterium avium subspecies paratuberculosis (MAP) DNA is not associated with altered MMP expression in ulcerative colitis. BMC Gastroenterol. 2011, 11. [Google Scholar] [CrossRef] [PubMed]
- National Committee for Clinical Laboratory Standards. Susceptibility Testing of Mycobacteria, Nocardiae, and Other Aerobic Actinomycetes; Approved Standard, M24-A2, 2nd ed.; NCCLS: Wayne, PA, USA, 2011. [Google Scholar]
- Imramovsky, A.; Vinsova, J.; Monreal-Ferriz, J.; Dolezal, R.; Jampilek, J.; Kaustova, J.; Kunc, F. New antituberculotics originated from salicylanilides with promising in vitro activity against atypical mycobacterial strains. Bioorg. Med. Chem. 2009, 17, 3572–3579. [Google Scholar] [CrossRef] [PubMed]
- Pauk, K.; Zadrazilova, I.; Imramovsky, A.; Vinsova, J.; Pokorna, M.; Masarikova, M.; Cizek, A.; Jampilek, J. New derivatives of salicylamides: Preparation and antimicrobial activity against various bacterial species. Bioorg. Med. Chem. 2013, 21, 6574–6581. [Google Scholar] [CrossRef] [PubMed]
- Bueno, J. Antitubercular in vitro drug discovery: Tools for begin the search. In Understanding Tuberculosis-New Approaches to Fighting against Drug Resistance; Cardona, P.J., Ed.; In Tech: Rijeka, Croatia, 2012; pp. 147–168. [Google Scholar]
- Janin, Y.L. Antituberculosis drugs: Ten years of research. Bioorg. Med. Chem. 2007, 15, 2479–2513. [Google Scholar] [CrossRef] [PubMed]
- Zumla, A.; Nahid, P.; Cole, S.T. Advances in the development of new tuberculosis drugs and treatment regimens. Nat. Rev. Drug Discov. 2013, 12, 388–404. [Google Scholar] [CrossRef] [PubMed]
- Suffness, M.; Douros, J. Current status of the NCI plant and animal product program. J. Nat. Prod. 1982, 45, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Izawa, S. Acceptors and donors for chloroplast electron transpor. In Methods in Enzymology; Colowick, P., Kaplan, N.O., Eds.; Academic Press: New York, NY, USA; London, UK, 1980; Volume 69, part C; pp. 413–434. [Google Scholar]
- Borse, T.H.; Maheswarim, V.L.; Baviskar, M.P. Effect of diphenyl carbazide on the metribuzin induced inhibition of photosystem-II photochemistry. J. Plant Biochem. Biotechnol. 2000, 9, 119–121. [Google Scholar] [CrossRef]
- Purcell, M.; Leroux, G.; Carpentier, R. Interaction of the electron donor diphenylcarbazide with the herbicide-binding niche of photosystem II. Biochim. Biophys. Acta 1991, 1058, 374–378. [Google Scholar] [CrossRef]
- Otevrel, J.; Mandelova, Z.; Pesko, M.; Guo, J.; Kralova, K.; Sersen, F.; Vejsova, M.; Kalinowski, D.; Kovacevic, Z.; Coffey, A.; et al. Investigating the spectrum of biological activity of ring-substituted salicylanilides and carbamoylphenylcarbamates. Molecules 2010, 15, 8122–8142. [Google Scholar] [CrossRef] [PubMed]
- Otevrel, J.; Bobal, P.; Zadrazilova, I.; Govender, R.; Pesko, M.; Keltosova, S.; Koleckarova, P.; Marsalek, P.; Imramovsky, A.; Coffey, A.; et al. Antimycobacterial and photosynthetic electron transport inhibiting activity of ring-substituted 4-arylamino-7-chloroquinolinium chlorides. Molecules 2013, 18, 10648–10670. [Google Scholar] [CrossRef] [PubMed]
- Kralova, K.; Sersen, F.; Miletin, M.; Dolezal, M. Inhibition of photosynthetic electron transport in spinach chloroplasts by 2,6-disubstituted pyridine-4-thiocarboxamides. Chem. Pap. 2002, 56, 214–217. [Google Scholar]
- Servusova, B.; Eibinova, D.; Dolezal, M.; Kubicek, V.; Paterova, P.; Pesko, M.; Kralova, K. Substituted N-benzylpyrazine-2-carboxamides: Synthesis and biological evaluation. Molecules 2012, 17, 13183–13198. [Google Scholar] [CrossRef] [PubMed]
- Govindjee, A. Sixty-three years since Kautsky: Chlorophyll a fluorescence. Aust. J. Plant Physiol. 1995, 22, 131–160. [Google Scholar] [CrossRef]
- Kralova, K.; Sersen, F.; Pesko, M.; Waisser, K.; Kubicova, L. 5-Bromo- and 3,5-dibromo-2-hydroxy-N-phenylbenzamides—Inhibitors of photosynthesis. Chem. Pap. 2014, 68, 46–52. [Google Scholar] [CrossRef]
- Rupe, H.; Seiberth, M.; Kussmaul, H. Stereoisomere Abkömmlinge des Aminomethylencamphers. Helv. Chim. Acta 1920, 3, 50–70. [Google Scholar] [CrossRef]
- National Committee for Clinical Laboratory Standards. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically; Approved Standard, 5th ed.; CLSI Document M7-A5; NCCLS: Wayne, PA, USA, 2000. [Google Scholar]
- National Committee for Clinical Laboratory Standards. Performance Standards for Antimicrobial Susceptibility Testing; 12th Informational Supplement M100-S12; NCCLS: Wayne, MI, USA, 2002. [Google Scholar]
- Gonec, T.; Kos, J.; Nevin, E.; Govender, R.; Pesko, M.; Tengler, J.; Kushkevych, I.; Stastna, V.; Oravec, M.; Kollar, P.; et al. Preparation and biological properties of ring-substituted naphthalene-1-carboxanilides. Molecules 2014, 19, 10386–10409. [Google Scholar] [CrossRef] [PubMed]
- Masarovicova, E.; Kralova, K. Approaches to measuring plant photosynthesis activity. In Handbook of Photosynthesis, 2nd ed.; Pessarakli, M., Ed.; Taylor & Francis Group: Boca Raton, FL, USA, 2005; pp. 617–656. [Google Scholar]
- Kralova, K.; Sersen, F.; Sidoova, E. Photosynthesis inhibition produced by 2-alkylthio-6-R-benzothiazoles. Chem. Pap. 1992, 46, 348–350. [Google Scholar]
- Sample Availability: Samples of compounds 1a–8c are available from the authors T. Gonec, J. Kos and J. Jampilek.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonec, T.; Zadrazilova, I.; Nevin, E.; Kauerova, T.; Pesko, M.; Kos, J.; Oravec, M.; Kollar, P.; Coffey, A.; O'Mahony, J.; et al. Synthesis and Biological Evaluation of N-Alkoxyphenyl-3-hydroxynaphthalene-2-carboxanilides. Molecules 2015, 20, 9767-9787. https://doi.org/10.3390/molecules20069767
Gonec T, Zadrazilova I, Nevin E, Kauerova T, Pesko M, Kos J, Oravec M, Kollar P, Coffey A, O'Mahony J, et al. Synthesis and Biological Evaluation of N-Alkoxyphenyl-3-hydroxynaphthalene-2-carboxanilides. Molecules. 2015; 20(6):9767-9787. https://doi.org/10.3390/molecules20069767
Chicago/Turabian StyleGonec, Tomas, Iveta Zadrazilova, Eoghan Nevin, Tereza Kauerova, Matus Pesko, Jiri Kos, Michal Oravec, Peter Kollar, Aidan Coffey, Jim O'Mahony, and et al. 2015. "Synthesis and Biological Evaluation of N-Alkoxyphenyl-3-hydroxynaphthalene-2-carboxanilides" Molecules 20, no. 6: 9767-9787. https://doi.org/10.3390/molecules20069767









