Preparation, Characterization and Pharmacokinetic Study of Xiangfu Siwu Decoction Essential Oil/β-Cyclodextrin Inclusion Complex
Abstract
:1. Introduction
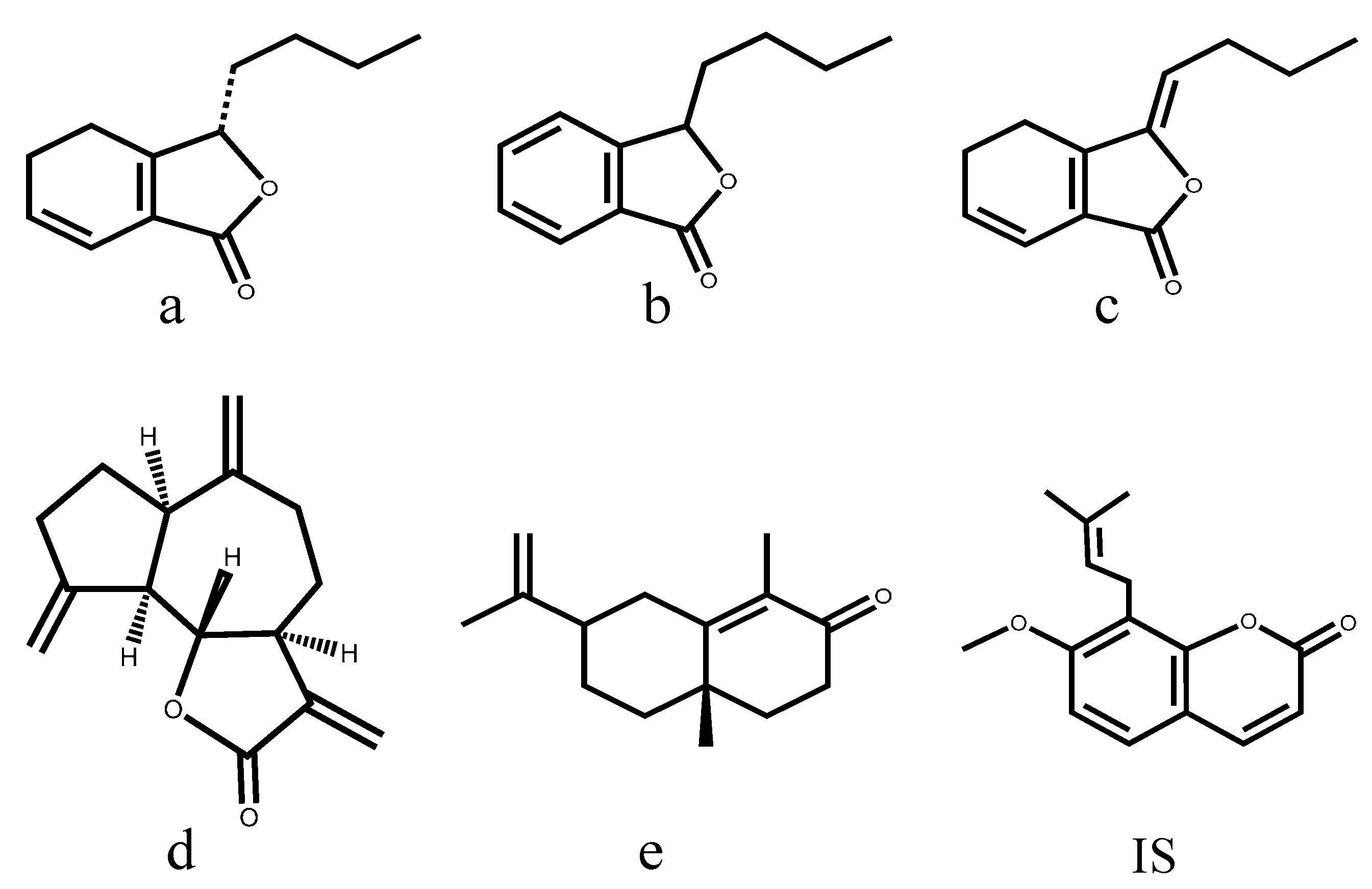
2. Results and Discussion
2.1. Characterization of EXO/β-CD Inclusion Complex
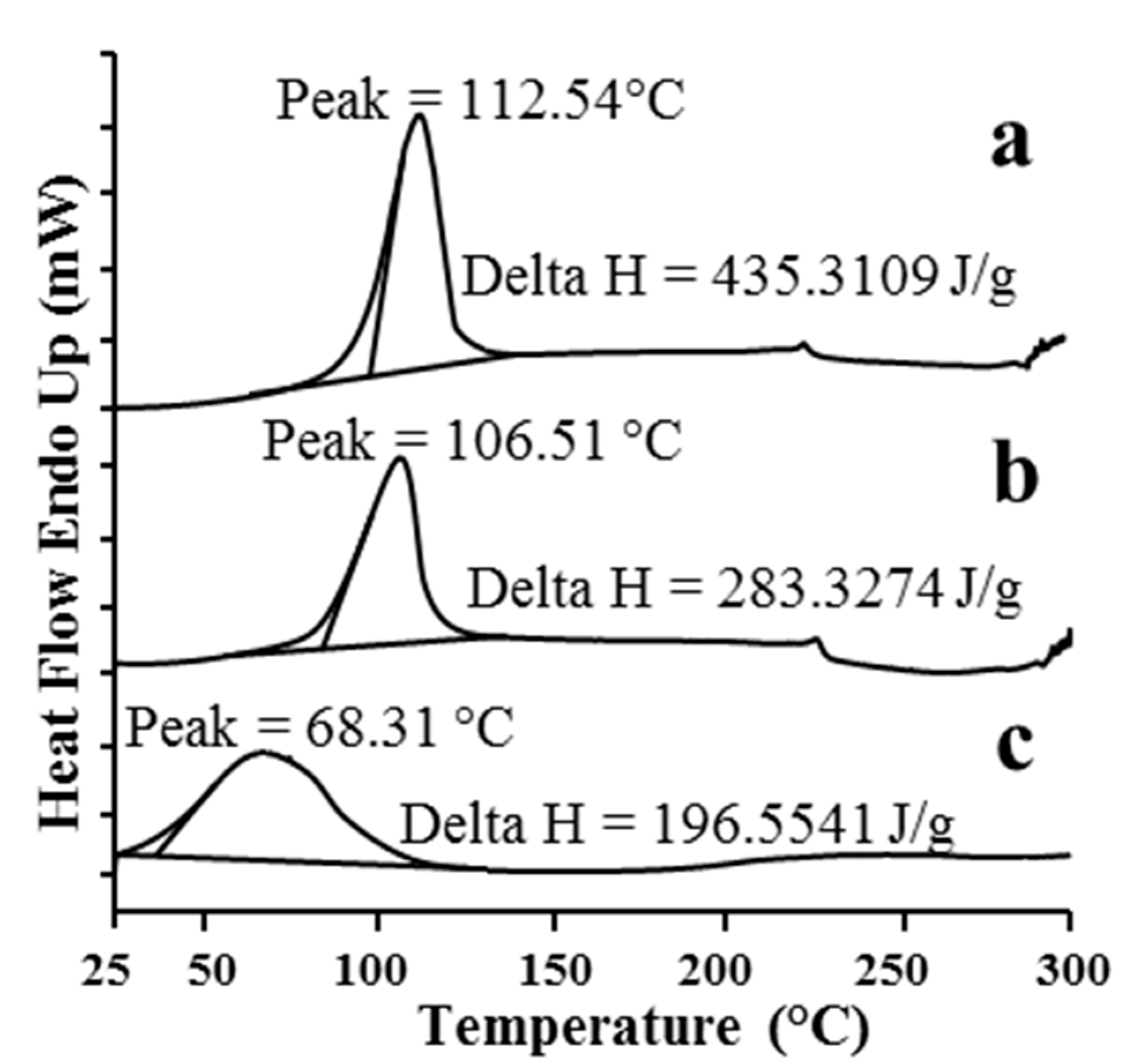
2.1.1. DSC
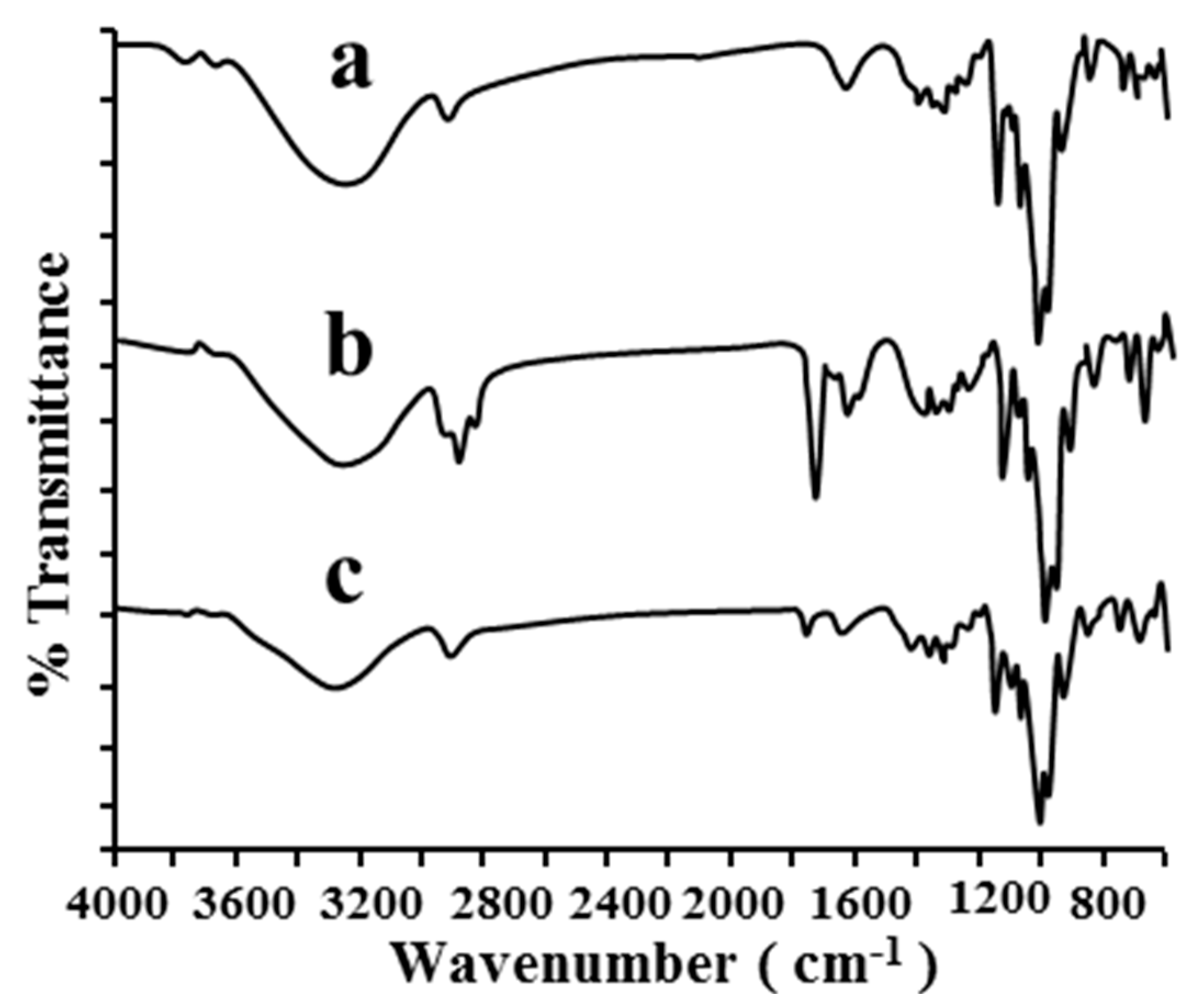
2.1.2. FT-IR
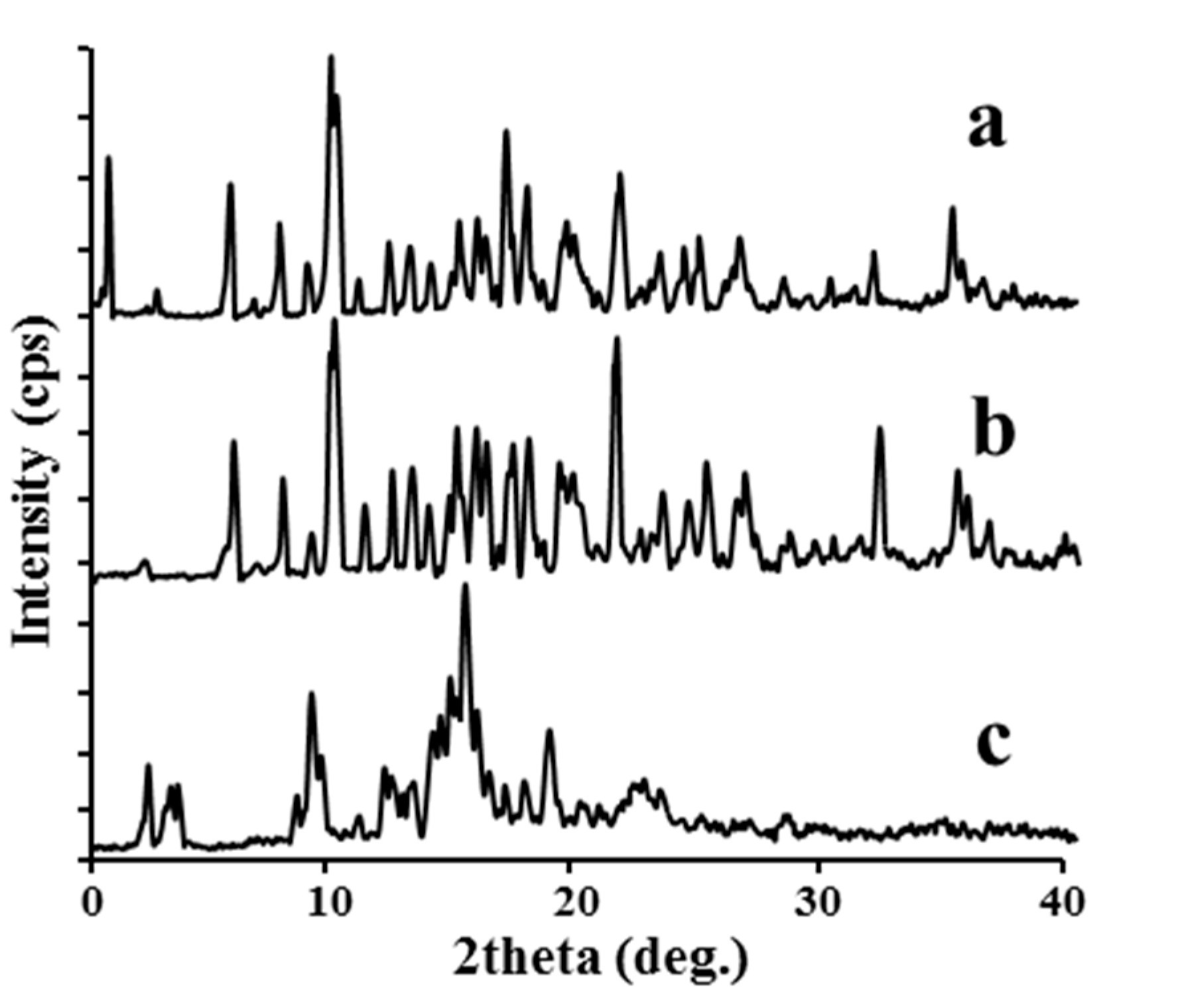
2.1.3. XRD
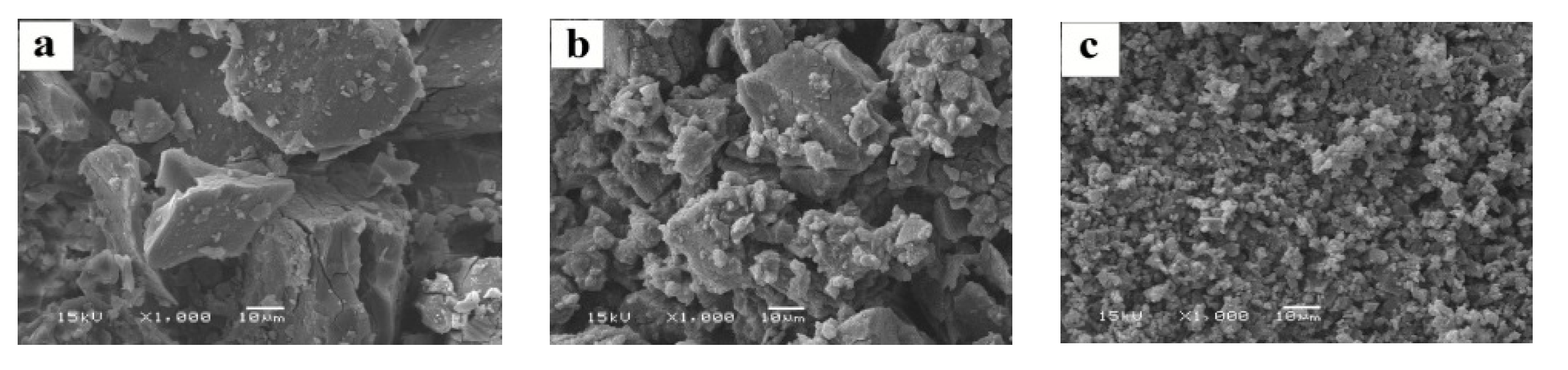
2.1.4. SEM
2.2. Liquid Chromatography and Mass Spectrometry Condition Optimization
2.3. Method Validation
2.3.1. Specificity and Selectivity

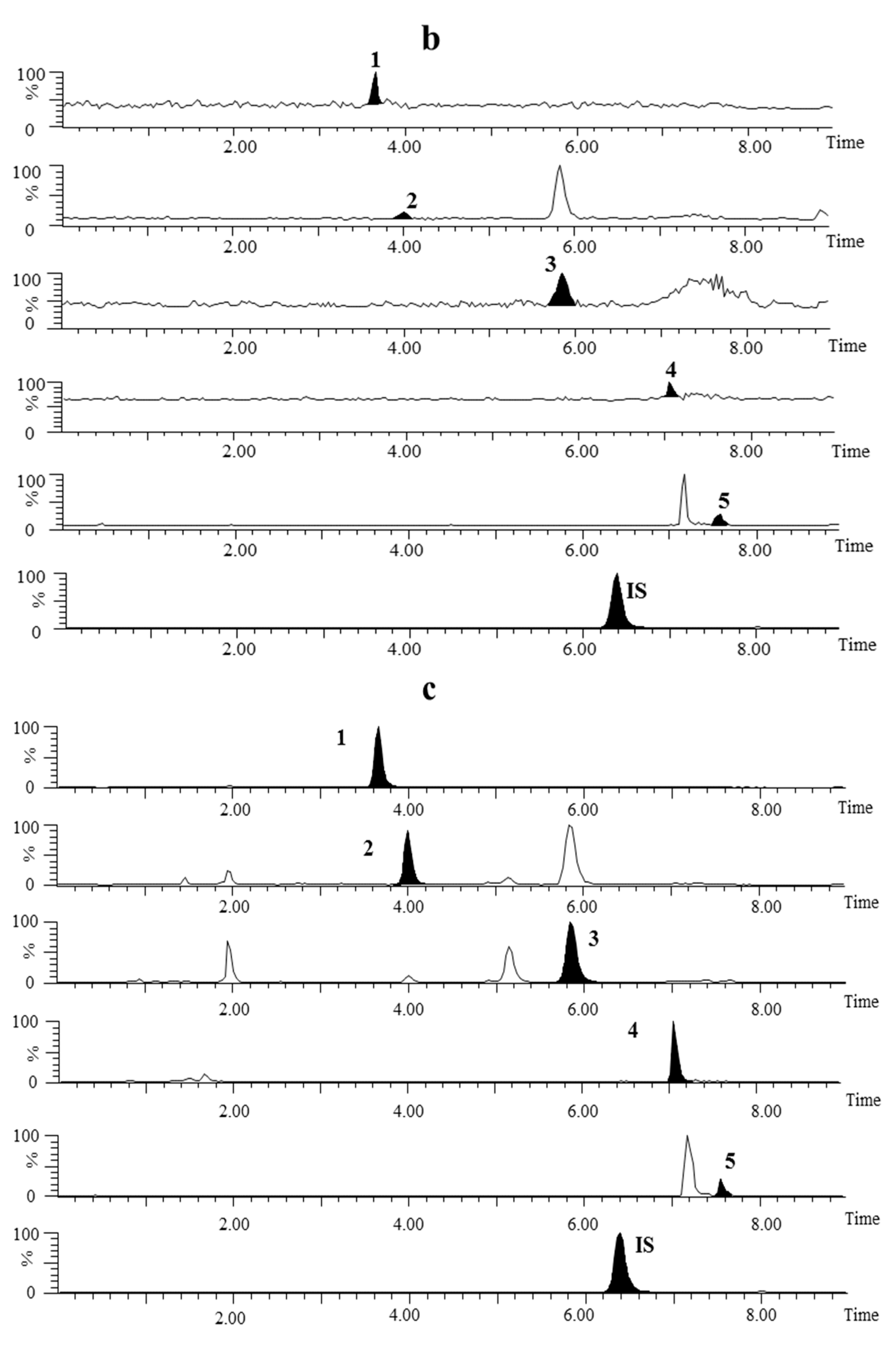
2.3.2. Linearity and LLOQ
2.3.3. Accuracy and Precision
2.3.4. Extraction Recovery and Matrix Effect
2.3.5. Stability
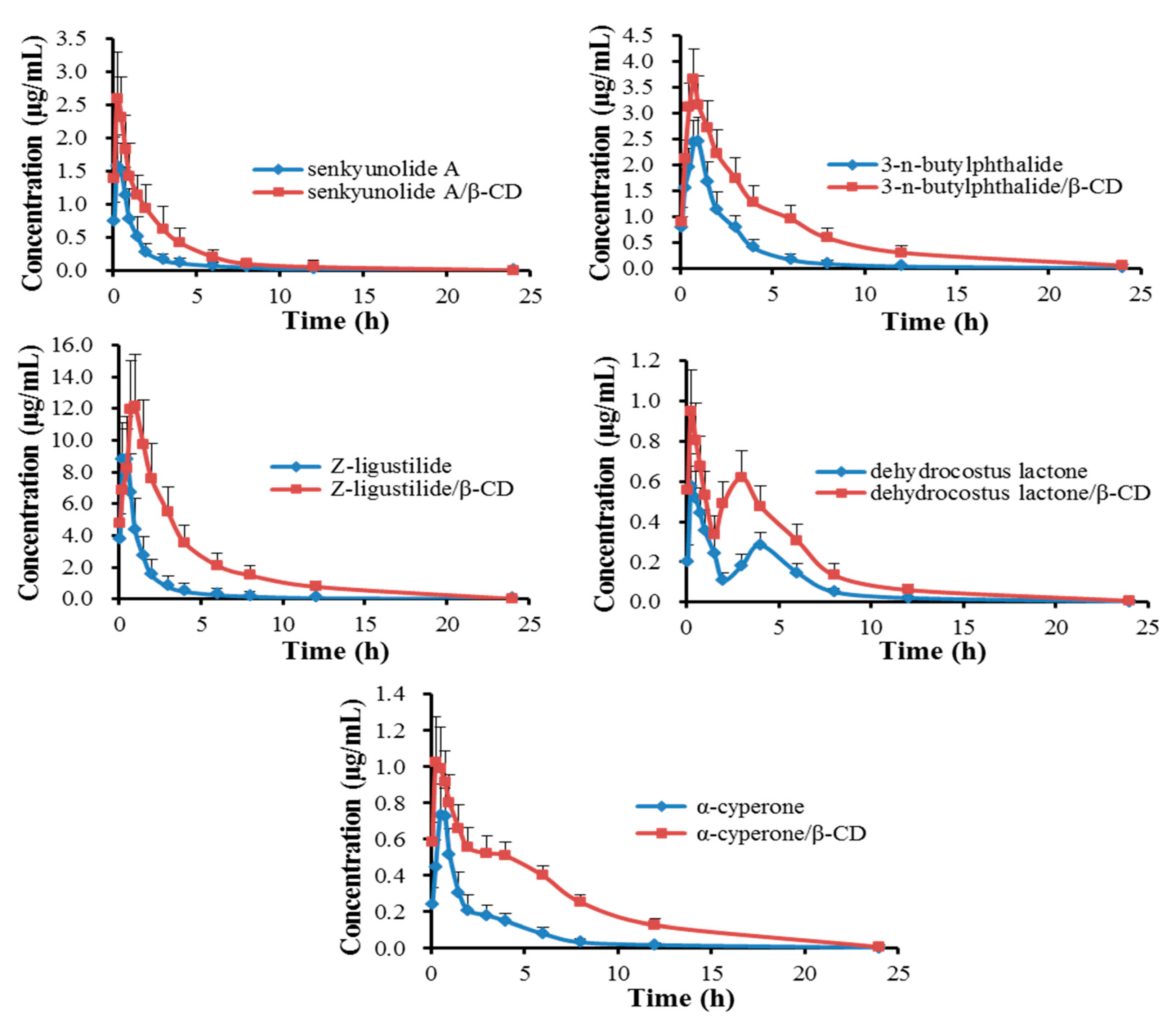
2.4. Pharmacokinetics
| Components | Groups | Dose (mg/kg) | Cmax (μg/mL) | Tmax (h) | T1/2 (h) | MRT (h) | AUC0–24 h (μg·h/mL) |
|---|---|---|---|---|---|---|---|
| Senkyunolide A | oil | 13.14 | 1.59 ± 0.21 | 0.33 ± 0.13 | 3.73 ± 0.25 | 2.76 ± 0.52 | 2.40 ± 0.36 |
| oil/β-CD | 13.41 | 2.60 ± 0.47 | 0.25 ± 0.00 | 4.43 ± 0.39 | 3.27 ± 0.25 | 6.08 ± 1.36 | |
| 3-n-Butylphthalide | oil | 34.66 | 2.48 ± 0.36 | 0.92 ± 0.13 | 4.05 ± 0.38 | 4.26 ± 0.28 | 6.51 ± 0.89 |
| oil/β-CD | 35.38 | 3.69 ± 0.25 | 0.71 ± 0.10 | 4.81 ± 0.35 | 5.02 ± 0.60 | 16.68 ± 2.96 | |
| Z-Ligustilide | oil | 116.08 | 8.80 ± 0.74 | 0.38 ± 0.14 | 3.21 ± 0.54 | 2.01 ± 0.12 | 13.29 ± 3.03 |
| oil/β-CD | 84.75 | 11.43 ± 1.90 | 0.88 ± 0.14 | 4.16 ± 0.52 | 3.98 ± 0.25 | 38.69 ± 7.30 | |
| Dehydrocostus lactone | oil | 3.64 | 0.58 ± 0.12 | 0.25 ± 0.00 | 3.81 ± 0.88 | 4.33 ± 0.30 | 1.92 ± 0.44 |
| oil/β-CD | 5.73 | 0.87 ± 0.26 | 0.25 ± 0.00 | 4.20 ± 0.37 | 4.82 ± 0.16 | 4.33 ± 0.42 | |
| α-Cyperone | oil | 8.18 | 0.75 ± 0.18 | 0.62 ± 0.14 | 3.05 ± 0.90 | 3.40 ± 0.52 | 1.81 ± 0.54 |
| oil/β-CD | 7.69 | 1.05 ± 0.21 | 0.38 ± 0.14 | 4.83 ± 0.62 | 5.35 ± 0.30 | 5.53 ± 0.95 |
3. Experimental Section
3.1. Materials and Reagents
3.2. Animals
3.3. Preparation of XEO and its β-CD Inclusion Complex
| Compound | a | b | c | d | e |
|---|---|---|---|---|---|
| XEO | 3.28 | 8.66 | 29.0 | 0.909 | 2.04 |
| β-CD inclusion complex | 0.335 | 0.884 | 2.12 | 0.143 | 0.192 |
3.4. Characterization of XEO/β-CD Inclusion Complex
3.4.1. Differential Scanning Calorimetry Study (DSC)
3.4.2. Fourier Transform Infrared Spectral Analysis (FT-IR)
3.4.3. Powder X-ray Diffraction Analysis (XRD)
3.4.4. Scanning Electron Microscopy (SEM) Image Analysis
3.5. Preparation of Calibration Standards and Quality Control Samples
3.6. UPLC-MS/MS Instrument and Conditions
3.7. Method Validation
3.7.1. Plasma Samples Preparation
3.7.2. Specificity
3.7.3. Linearity and LLOQ
3.7.4. Accuracy and precision
3.7.5. Extraction Recovery and Matrix Effect
3.7.6. Stability
3.8. Pharmacokinetic Study
4. Conclusions
Acknowledgment
Author Contributions
Conflicts of Interest
References
- Wang, J.W.; Cui, M.; Jiao, H.G.; Tong, Y.Y.; Xu, J.; Zhao, Y.K.; Han, M.; Liu, J.P. Content analysis of systematic reviews on effectiveness of Traditional Chinese Medicine. J. Tradit. Chin. Med. 2013, 33, 156–163. [Google Scholar] [CrossRef]
- Liu, P.; Duan, J.A.; Liu, R.; Guo, J.M.; Tang, Y.P. Determination of extracellular 5-HIAA in the brains of normal and primary dysmenorrheal rats by microdialysis coupled with HPLC-ECD and its application in evaluation of Xiang-Fu-Si-Wu Decoction efficacy. Chin. J. Tradit. Chin. Med. Pharm. 2011, 26, 902–907. [Google Scholar]
- Davis, A.R.; Westhoff, C.L. Primary Dysmenorrhea in Adolescent Girls and Treatment with Oral Contraceptives. J. Pediatr. Adolesc. Gynecol. 2001, 14, 3–8. [Google Scholar] [CrossRef]
- Doubova, S.V.; Morales, H.R.; Hernández, S.F. Effect of a Psidii guajavae folium extract in the treatment of primary dysmenorrhea: a randomized clinical trial. J. Ethnopharmacol. 2007, 110, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Su, S.L.; Duan, J.A.; Zhao, X.H.; Hua, Y.Q.; Hou, P.F.; Shang, E.X.; Tang, Y.P.; Ding, A.W. Bioactive components from oils of Siwu Decoction and Xiangfu Siwu Decoction by gas chromatography: Mass spectrometry and principle components analysis. World. Sci. Technol. 2008, 10, 50–57. [Google Scholar] [CrossRef]
- Cevallos, P.A.P.; Buera, M.P.; Elizalde, B.E. Encapsulation of cinnamon and thyme essential oils components (cinnamaldehyde and thymol) in β-cyclodextrin: Effect of interactions with water on complex stability. J. Food Eng. 2010, 99, 70–75. [Google Scholar] [CrossRef]
- Sköld, M.; Karlberg, A.; Matura, M.; Börje, A. The fragrance chemical β-caryophyllene-air oxidation and skin sensitization. Food Chem. Toxicol. 2006, 44, 538–545. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Cao, Y.; Sun, B.; Wang, C. Physicochemical and release characterisation of garlic oil-β-cyclodextrin inclusion complexes. Food Chem. 2011, 127, 1680–1685. [Google Scholar] [CrossRef]
- Wempe, M.F.; Wacher, V.J.; Ruble, K.M.; Ramsey, M.G.; Edgar, K.J.; Buchanan, N.L.; Buchanan, C.M. Pharmacokinetics of raloxifene in male Wistar-Hannover rats: influence of complexation with hydroxybutenyl-beta-cyclodextrin. Int. J. Pharm. 2008, 346, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Sun, J.; Fu, D.; Zhao, H.; Lan, M.; Gao, F. Preparation, characterization and pharmacokinetic studies of tacrolimus-dimethyl-β-cyclodextrin inclusion complex-loaded albumin nanoparticles. Int. J. Pharm. 2012, 427, 410–416. [Google Scholar] [CrossRef] [PubMed]
- Liao, C. Research advance on application of β-cyclodextrin. Sci. Technol. Chem. Ind. 2010, 18, 69–72. [Google Scholar]
- Liu, J.; Qiu, L.; Gao, J.; Jin, Y. Preparation, characterization and in vivo evaluation of formulation of baicalein with hydroxypropyl-β-cyclodextrin. Int. J. Pharm. 2006, 312, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Piette, M.; Evrard, B.; Frankenne, F.; Chiap, P.; Bertholet, P.; Castagne, D.; Foidart, J.M.; Delattre, L.; Piel, G. Pharmacokinetic study of a new synthetic MMP inhibitor (Ro 28–2653) after IV and oral administration of cyclodextrin solutions. Eur. J. Pharm. Sci. 2006, 28, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, G.A.; Adam, P.; Edwards, S.; Hughes, K.J.; Rendle, D.I.; Davies, N.W. Determination of pergolide in horse plasma by UPLC-MS/MS for pharmacokinetic applications. J. Pharm. Biomed. Anal. 2014, 94, 54–57. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.C.; Su, S.L.; Cui, W.X.; Liu, P.; Duan, J.A.; Guo, J.M.; Li, Z.H.; Shang, E.X.; Qian, D.W.; Huang, Z.J. Simultaneous determination of paeoniflorin, albiflorin, ferulic acid, tetrahydropalmatine, protopine, typhaneoside, senkyunolide I in Beagle dogs plasma by UPLC-MS/MS and its application to a pharmacokinetic study after Oral Administration of Shaofu Zhuyu Decoction. J. Chromatogr. B 2014, 962, 75–81. [Google Scholar]
- Jung, H.R.; Kim, S.J.; Ham, S.H.; Cho, J.H.; Lee, Y.B.; Cho, H.Y. Simultaneous determination of puerarin and its active metabolite in human plasma by UPLC-MS/MS: Application to a pharmacokinetic study. J. Chromatogr. B 2014, 971, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.F.; Liu, Y.N.; Xiong, W.; Yan, D.M.; Zhu, Y.; Gao, X.M.; Xu, Y.T.; Qi, A.D. A UPLC-MS/MS method for in vivo and in vitro pharmacokinetic studies of psoralenoside, isopsoralenoside, psoralen and isopsoralen from Psoralea corylifolia extract. J. Ethnopharmacol. 2014, 151, 609–617. [Google Scholar] [CrossRef] [PubMed]
- Yadav, V.R.; Suresh, S.; Devi, K.; Yadav, S. Effect of Cyclodextrin Complexation of Curcumin on its Solubility and Antiangiogenic and Anti-inflammatory Activity in Rat Colitis Model. AAPS PharmSciTech 2009, 10, 752–762. [Google Scholar] [CrossRef] [PubMed]
- Nerome, H.; Machmudah, S.; Wahyuidiono; Fukuzato, R.; Higashiura, T.; Youn, Y.S.; Lee, Y.W.; Goto, M. Nanoparticle formation of lycopene/β-cyclodextrin inclusion complex using supercritical antisolvent precipitation. J. Supercrit. Fluids 2013, 83, 97–103. [Google Scholar] [CrossRef]
- Al Omari, M.M.; Daraghmeh, N.H.; El-Barghouthi, M.I.; Zughul, M.B.; Chowdhry, B.Z.; Leharne, S.A.; Badwan, A.A. Novel inclusion complex of ibuprofen tromethamine with cyclodextrins: Physico-chemical characterization. J. Pharm. Biomed. Anal. 2009, 50, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Eid, E.M.; Abdul, A.B.; Suliman, F.E.O.; Sukari, M.A.; Rasedeo, A.; Fatah, S.S. Characterization of the inclusion complex of zerumbone with hydroxypropyl-β-cyclodextrin. Carbohydr. Polym. 2011, 83, 1707–1714. [Google Scholar] [CrossRef] [Green Version]
- Periasamy, R.; Kothainayaki, S.; Rajamohan, R.; Sivakumar, K. Spectral investigation and characterization of host-guest inclusion complex of 4,4′-methylene-bis (2-chloroaniline) with beta- cyclodextrin. Carbohydr. Polym. 2014, 114, 558–566. [Google Scholar] [CrossRef] [PubMed]
- Periasamy, R.; Kothainayaki, S.; Sivakumar, K. Investigation on inter molecular complexation between 4,4′-methylene-bis (N,N-dimethylaniline) and β-cyclodextrin: Preparation and characterization in aqueous medium and solid state. J. Mol. Struct. 2015, 1080, 69–79. [Google Scholar] [CrossRef]
- Yin, L.F.; Huang, S.J.; Zhu, C.L.; Zhang, S.H.; Zhang, Q.; Chen, X.J.; Liu, Q.W. In vitro and in vivo studies on a novel solid dispersion of repaglinide using polyvinylpyrrolidone as the carrier. Drug Dev. Ind. Pharm. 2012, 38, 1371–1380. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, K.; Sivakumar, K.; Stalin, T. 2,6-Dinitroaniline and β-cyclodextrin inclusion complex properties studied by different analytical methods. Carbohydr. Polym. 2014, 113, 577–587. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Bharti, N.; Madan, J.; Hiremath, S.N. Characterization of Cyclodextrin Inclusion Complexes—A Review. J. Pharm. Sci. Technol. 2010, 2, 171–183. [Google Scholar]
- Tayade, P.T.; Vavia, P.R. Inclusion complexes of Ketoprofen with β-cyclodextrins: Oral pharmacokinetics of Ketoprofen in human. Indian J. Pharm. Sci. 2006, 68, 164–170. [Google Scholar] [CrossRef]
- Loftsson, T.; Hreinsdottir, D.; Masson, M. Evaluation of cyclodextrin solubilization of drugs. Int. J. Pharm. 2005, 302, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Loftsson, T.; Vogensen, S.B.; Brewster, M.E. Effects of cyclodextrins on drug delivery through biological membranes. J. Pharm. Sci. 2007, 96, 2532–2546. [Google Scholar] [CrossRef] [PubMed]
- Loftsson, T.; Brewster, M.E. Pharmaceutical applications of cyclodextrins: Effects on drug permeation through biological membranes. J. Pharm. Pharmacol. 2011, 63, 1119–1135. [Google Scholar] [CrossRef] [PubMed]
- Loftsson, T.; Duchene, D. Cyclodextrins and their pharmaceutical applications. Int. J. Pharm. 2007, 329, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Brewster, M.E.; Noppe, M.; Peeters, J.; Loftsson, T. Effect of the unstirred water layer on permeability enhancement by hydrophilic cyclodextrins. Int. J. Pharm. 2007, 342, 250–253. [Google Scholar] [CrossRef] [PubMed]
- Gu, F.; Li, W.; Men, G.; Wang, Y.; Wu, C.; Zhang, A. Pharmacokinetic study of norfloxaein- sulfobutylether-β-cyelodextrin complex in rat. Chin. J. Mod. Appl. Pharm. 2012, 29, 346–349. [Google Scholar]
- Samples Availability: Samples of the compounds senkyunolide A, 3-n-butylphthalide, Z-ligustilide, dehydrocostus lactone and α-cyperone are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xi, J.; Qian, D.; Duan, J.; Liu, P.; Zhu, Z.; Guo, J.; Zhang, Y.; Pan, Y. Preparation, Characterization and Pharmacokinetic Study of Xiangfu Siwu Decoction Essential Oil/β-Cyclodextrin Inclusion Complex. Molecules 2015, 20, 10705-10720. https://doi.org/10.3390/molecules200610705
Xi J, Qian D, Duan J, Liu P, Zhu Z, Guo J, Zhang Y, Pan Y. Preparation, Characterization and Pharmacokinetic Study of Xiangfu Siwu Decoction Essential Oil/β-Cyclodextrin Inclusion Complex. Molecules. 2015; 20(6):10705-10720. https://doi.org/10.3390/molecules200610705
Chicago/Turabian StyleXi, Junzuan, Dawei Qian, Jinao Duan, Pei Liu, Zhenhua Zhu, Jianming Guo, Yang Zhang, and Ying Pan. 2015. "Preparation, Characterization and Pharmacokinetic Study of Xiangfu Siwu Decoction Essential Oil/β-Cyclodextrin Inclusion Complex" Molecules 20, no. 6: 10705-10720. https://doi.org/10.3390/molecules200610705
APA StyleXi, J., Qian, D., Duan, J., Liu, P., Zhu, Z., Guo, J., Zhang, Y., & Pan, Y. (2015). Preparation, Characterization and Pharmacokinetic Study of Xiangfu Siwu Decoction Essential Oil/β-Cyclodextrin Inclusion Complex. Molecules, 20(6), 10705-10720. https://doi.org/10.3390/molecules200610705





