Comparisons of the Pharmacokinetic Profile of Four Bioactive Components after Oral Administration of Gan-Sui-Ban-Xia Decoction Plus-Minus Gansui and Gancao Drug Combination in Normal Rats
Abstract
:1. Introduction
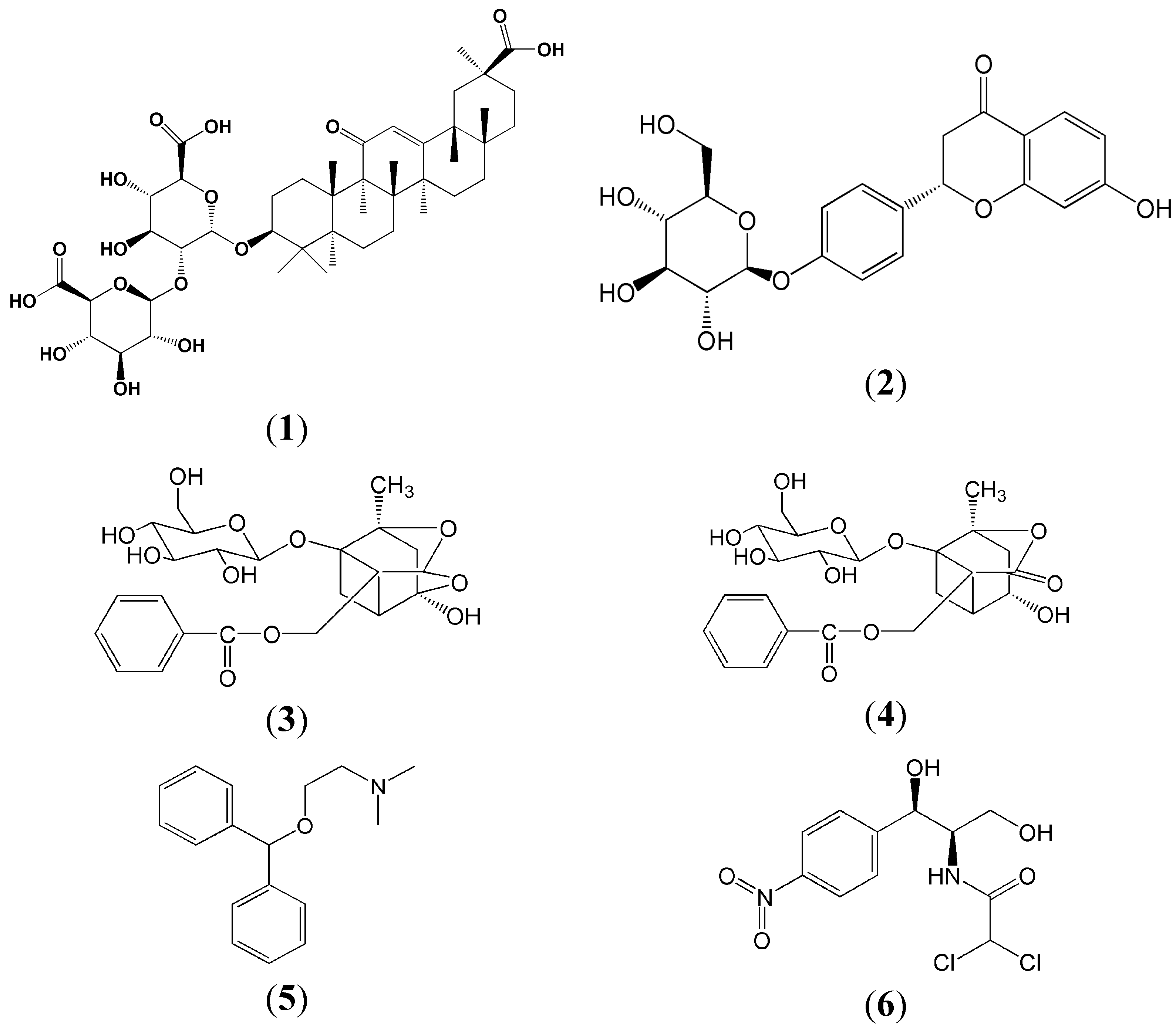
2. Results and Discussion
2.1. Sample Preparations
2.2. Method Validation
2.2.1. Specificity

2.2.2. Linearity and Lower Limits of Quantification (LLOQ)
| Compound No. | Linear Regression Equation | R2 | Range (ng/mL) | LOD (ng/mL) | LOQ (ng/mL) |
|---|---|---|---|---|---|
| 1 | y = 1861.00x + 46.77 | 0.9929 | 9.57–2450 | 2.71 | 4.50 |
| 2 | y = 14370.00x − 36.39 | 0.9956 | 4.32–2210 | 0.93 | 1.44 |
| 3 | y = 1076.80x + 32.05 | 0.9993 | 8.91–2280 | 1.66 | 3.15 |
| 4 | y = 232.76x + 33.35 | 0.9971 | 5.53–2830 | 1.98 | 2.76 |
2.2.3. Precision and Accuracy
| Compound No. | Concentration (ng/mL) | Intra-day | Inter-day | ||
|---|---|---|---|---|---|
| Accuracy (%) | Precision (RSD, %) | Accuracy (%) | Precision (RSD, %) | ||
| 1 | 1.91 × 10 | 90.88 | 10.60 | 81.91 | 8.18 |
| 3.09 × 102 | 96.45 | 8.19 | 99.13 | 8.91 | |
| 1.23 × 103 | 101.04 | 8.27 | 87.58 | 9.21 | |
| 2 | 4.32 | 90.45 | 10.56 | 73.03 | 18.70 |
| 1.38 × 102 | 91.22 | 8.37 | 99.18 | 10.22 | |
| 1.11 × 103 | 99.68 | 10.40 | 100.09 | 9.13 | |
| 3 | 1.78 × 10 | 91.09 | 12.10 | 80.15 | 16.41 |
| 1.43 × 102 | 99.48 | 9.04 | 99.28 | 8.88 | |
| 1.14 × 103 | 102.00 | 9.13 | 97.28 | 10.37 | |
| 4 | 1.11 × 10 | 92.21 | 8.91 | 77.59 | 17.27 |
| 1.77 × 102 | 99.08 | 9.33 | 93.12 | 10.32 | |
| 1.42 × 103 | 98.90 | 9.87 | 99.90 | 9.49 | |
2.2.4. Extraction Recovery and Matrix Effect
| Compound No. | Concentration (ng/mL) | Recovery | Matrix effect | ||
|---|---|---|---|---|---|
| Accuracy (%) | Precision (RSD, %) | Accuracy (%) | Precision (RSD, %) | ||
| 1 | 1.91 × 10 | 71.23 | 7.19 | 72.17 | 12.34 |
| 3.09 × 102 | 64.32 | 7.69 | 89.40 | 7.53 | |
| 1.23 × 103 | 79.01 | 5.64 | 87.08 | 3.99 | |
| 2 | 4.32 | 77.89 | 13.12 | 67.16 | 9.15 |
| 1.38 × 102 | 81.32 | 8.90 | 101.67 | 8.07 | |
| 1.11 × 103 | 69.68 | 4.71 | 80.68 | 4.93 | |
| 3 | 1.78 × 10 | 51.17 | 17.25 | 80.09 | 13.99 |
| 1.43 × 102 | 99.99 | 6.44 | 99.18 | 6.15 | |
| 1.14 × 103 | 72.93 | 5.94 | 96.51 | 3.01 | |
| 4 | 1.11 × 10 | 52.76 | 11.77 | 85.63 | 16.47 |
| 1.77 × 102 | 69.91 | 5.24 | 92.09 | 4.39 | |
| 1.42 × 103 | 68.01 | 9.11 | 94.55 | 2.13 | |
2.2.5. Stability
| Compound No. | Concentration (ng/mL) | Freeze-thaw cycles | At −80 °C for a Month | Autosampler for 24 h | |||
|---|---|---|---|---|---|---|---|
| Accuracy (%) | Precision (RSD, %) | Accuracy (%) | Precision (RSD, %) | Accuracy (%) | Precision (RSD, %) | ||
| 1 | 1.91 × 10 | 98.83 | 8.10 | 89.99 | 4.61 | 93.94 | 8.53 |
| 3.09 × 102 | 91.02 | 7.10 | 91.37 | 8.16 | 92.23 | 9.91 | |
| 1.23 × 103 | 94.33 | 10.22 | 98.18 | 9.93 | 87.36 | 7.48 | |
| 2 | 4.32 | 101.l6 | 9.83 | 91.03 | 6.51 | 88.92 | 6.22 |
| 1.38 × 102 | 89.99 | 11.03 | 99.99 | 7.83 | 97.49 | 7.42 | |
| 1.11 × 103 | 95.35 | 7.59 | 96.71 | 10.33 | 97.11 | 9.03 | |
| 3 | 1.78 × 10 | 87.63 | 4.81 | 87.54 | 9.40 | 98.03 | 9.48 |
| 1.43 × 102 | 92.10 | 10.47 | 95.32 | 6.43 | 91.28 | 4.50 | |
| 1.14 × 103 | 91.57 | 7.89 | 89.55 | 8.16 | 104.13 | 6.13 | |
| 4 | 1.11 × 10 | 93.49 | 11.36 | 101.5 | 10.01 | 97.15 | 9.33 |
| 1.77 × 102 | 102.34 | 9.68 | 79.13 | 3.93 | 91.22 | 7.06 | |
| 1.42 × 103 | 98.33 | 6.77 | 88.48 | 6.71 | 99.91 | 9.10 | |
2.3. Pharmacokinetics Study
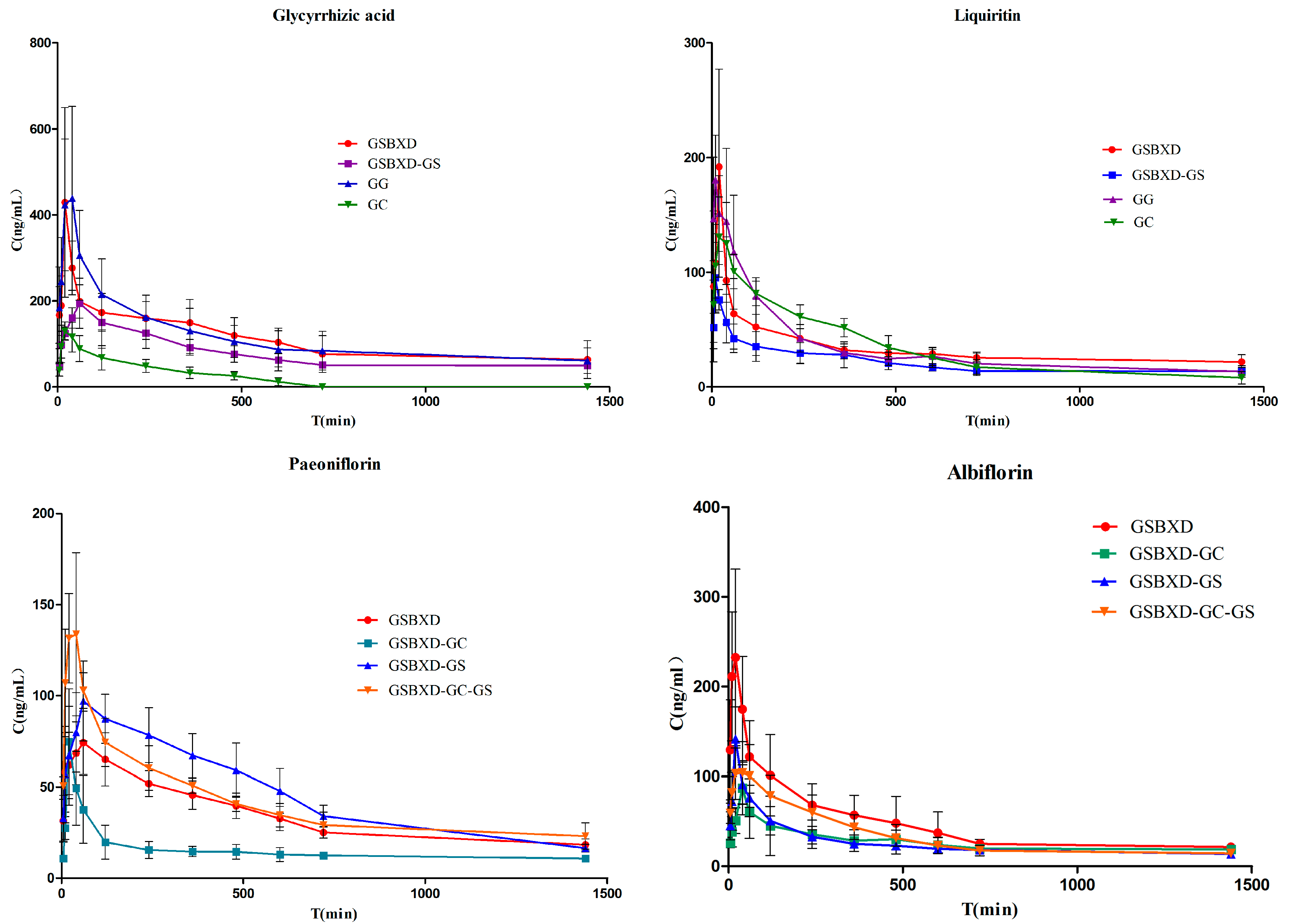
| Compound No. | Group | Cmax/ng·mL−1 | Tmax/h | T1/2/h | AUC0~t/ng·h·mL−1 |
|---|---|---|---|---|---|
| 1 | GSBXD | 503.15 ± 199.16 | 0.33 ± 0 | 5.93 ± 2.67 | 2631.14 ± 531.93 |
| GSBXD-GS | 213.90 ± 55.44 * | 1.67 ± 0.12 * | 11.56 ± 5.74 | 1278.93 ± 392.92 ** | |
| GG | 364.49 ± 46.19 | 0.64 ± 0.34 | 9.29 ± 4.70 ** | 787.72 ± 109.53 ** | |
| GC | 146.86 ± 19.86 | 0.36 ± 0.16 | 0.79 ± 0.39 | 485.51 ± 136.03 | |
| 2 | GSBXD | 192.59 ± 84.49 | 0.39 ± 0.14 | 7.04 ± 2.99 | 793.58 ± 141.51 |
| GSBXD-GS | 98.93 ± 26.76 | 0.17 ± 0.11 ** | 6.84 ± 3.11 | 421.44 ± 221.90 ** | |
| GG | 199.61 ± 42.40 | 0.32 ± 0.27 * | 12.86 ± 4.76 * | 787.72 ± 109.53 | |
| GC | 138.98 ± 33.54 | 0.50 ± 0.18 | 5.26 ± 1.93 | 797.39 ± 173.64 | |
| 3 | GSBXD | 82.79 ± 15.72 | 0.81 ± 0.34 | 12.55 ± 7.46 | 825.06 ± 96.02 |
| GSBXD-GS | 101.20 ± 17.96 | 1.33 ± 0.37 | 7.90 ± 1.17 | 909.502 ± 476.26 | |
| GSBXD-GC | 74.12 ± 25.99 | 0.31 ± 0.07 | 16.04 ± 2.62 | 362.33 ± 40.42 | |
| GSBXD-GG | 149.87 ± 24.51 # | 0.50 ± 0.28 | 21.41 ± 8.85 | 658.98 ± 411.01 | |
| 4 | GSBXD | 255.17 ± 98.01 | 0.33 ± 0.18 | 8.06 ± 1.44 | 1114.96 ± 346.19 |
| GSBXD-GS | 141.63 ± 39.84 | 0.28 ± 0.14 | 9.07 ± 0.87 | 513.95 ± 271.24 | |
| GSBXD-GC | 88.46 ± 18.26 | 0.81 ± 0.62 | 7.74 ± 0.74 | 618.64 ± 129.51 | |
| GSBXD-GG | 112.65 ± 25.78 # | 0.56 ± 0.27 ## | 8.73 ± 0.98 | 785.47 ± 158.14 |
2.3.1. Comparison of Pharmacokinetic Profile of Group GC and GG
2.3.2. Comparison of Pharmacokinetic Profile of Group GC and GSBXD-GS
2.3.3. Comparison of Pharmacokinetic Profile of Group GSBXD and GSBXD-GG
2.4. Influence of Gansui and Gancao Anti-drug Combination and Other Herbs in GSBXD on Pharmacokinetic Profile of Glycyrrhizinic Acid, Liquiritin, Paeoniflorin and Albiflorin
3. Experimental Section
3.1. Materials and Reagents
3.2. Animals
3.3. Chromatographic Conditions
3.4. Mass Conditions
| Analyte | Retention Time(min) | [M+H]+ (m/z) | MRM Transitions (Precursor-product) | Cone Voltage(V) | Collision Energy (eV) |
|---|---|---|---|---|---|
| Glycyrrhizinic acid | 3.48 | 823.35 | 823.35→453.35 | 18 | 30 |
| Liquiritin | 1.89 | 416.99 | 416.99→255.14 | 28 | 20 |
| Paeoniflorin | 1.77 | 502.99 | 502.99→89.11 | 40 | 22 |
| Albiflorin | 1.63 | 480.99 | 480.99→105.10 | 16 | 30 |
3.5. Preparation of GSBXD and Omitted Ingredients in GSBXD
3.6. Preparation of Calibration Standards and Quality Control Samples
3.7. Validation Procedures
3.8. Pharmacokinetic Studies
3.9. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Osborne, M.P.; Copeland, B.E. Intracavital Tadministration of radioactive colloidal gold (Au198) for the treatment of malignant effusions. N. Engl. J. Med. 1956, 225, 1122–1128. [Google Scholar] [CrossRef]
- Ayantunde, A.A.; Parsons, S.L. Pattern and prognostic factors in patients with malignant ascites: A retrospective study. Ann. Oncol. 2007, 18, 945–949. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J. Traditional medicine: A culture in the balance. Nature 2007, 448, 126–128. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.Z.; Hu, X.; Gao, W.Y.; Qu, Z.; Guo, H.M.; Liu, Z.; Liu, C.X. Pharmacokinetic study on costunolide and dehydrocostuslactone after oral administration of traditional medicine Aucklandia lappa Decne. by LC/MS/MS. J. Ethnopharmacol. 2014, 151, 191–197. [Google Scholar] [CrossRef]
- Cao, Y.; Duan, J.A.; Guo, J.M.; Li, W.X.; Tao, W.W. Pharmacokinetic properties of arsenic species after oral administration of Sargassum pallidum extract in rats using an HPLC-HG-AFS method. J. Pharm. Biomed. Anal. 2013, 96, 213–219. [Google Scholar] [CrossRef]
- Yao, Y.; Zhang, X.D.; Wang, Z.Z.; Zheng, C.L.; Li, P.; Chao, H.; Tao, W.Y.; Xiao, W.; Wang, Y.H.; Huang, L.Q.; et al. Deciphering the combination principles of Traditional Chinese Medicine from a systems pharmacology perspective based on Ma-huang Decoction. J. Ethnopharmacol. 2013, 150, 619–638. [Google Scholar]
- Liu, P.; Li, W.; Li, Z.H.; Qian, D.W.; Guo, J.M.; Shang, E.X.; Su, S.L.; Tang, Y.P.; Duan, J.A. Comparisons of pharmacokinetic and tissue distribution profile of four major bioactive components after oral administration of Xiang-Fu-Si-Wu Decoction effective fraction in normal and dysmenorrheal symptom rats. J. Ethnopharmacol. 2014, 154, 696–703. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Wei, W.; Zhang, L.; Xu, H.M. Effects and mechanisms of total glucosides of paeony on synoviocytes activities in rat collagen-induced arthritis. J. Ethnopharmacol. 2009, 121, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xiang, J.; Liu, M. Protective effects of glycyrrhizic acid by rectal treatment on a TNBS-induced rat colitis model. J. Pharm. Pharmacol. 2011, 63, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Michaelis, M.; Geiler, J.; Naczk, P. Glycyrrhizin inhibits highly pathogenic H5N1 influenza a virus-induced pro-inflammatory cytokine and chemokine expression in human macrophages. Med. Microbiol. Immun. 2010, 199, 291–297. [Google Scholar] [CrossRef]
- Rahman, S.; Sultana, S. Chemopreventive activity of glycyrrhizin on lead acetate mediated hepatic oxidative stress and its hyperproliferative activity in wistar rats. Chem. Bio. Interact. 2006, 160, 61–69. [Google Scholar] [CrossRef]
- Wu, H.; Li, W.; Wang, T.S.; Shu, Y.Q.; Liu, P. Paeoniflorin suppress NF-κB activation through modulation of Iκ Bα and enhances 5-fluorouracil-induced apoptosis in human gastric carcinoma cells. Biomed. Pharmacother. 2008, 62, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Qiu, F.M.; Zhong, X.M.; Mao, Q.Q.; Huang, Z. Antidepressant-like effects of paeoniflorin on the behavioural, biochemical, and neurochemical patterns of rats exposed to chronic unpredictable stress. Neurosci. Lett. 2013, 541, 209–213. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.Y.; Wang, W.X.; Guo, H.Z.; Zhou, D.F. Antidepressant-like effect of liquiritin from Glycyrrhizauralensis in chronicvariable stress induced depression model rats. Behav. Brain Res. 2008, 194, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Abe, K.; Ikeda, T.; Wake, K. Glycyrrhizin prevents of lipopolysaccharide/d-galactosamine-induced liver injury through down-regulation of matrix metalloproteinase-9 in mice. J. Pharm. Pharmacol. 2008, 60, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.B.; Li, G.F.; Tang, Z.K.; Wu, B.Y. Modulation on the P-glycoprotein in the jejunum by combined use of Glycyrrhiza inflata and Kansui. Yao Xue Xue Bao. 2010, 45, 510–516. [Google Scholar] [PubMed]
- He, Y.J.; Shi, S.Y.; Jin, K.T. The modulation effect of Glycyrrhiza in combination with Euphorbia pekinensis, Euphorbia kansui, and Daphne genkwa on the enzyme activity of CYP1A2 in rat livers. Chin. Remed. Clin. 2007, 7, 278–280. [Google Scholar]
- Sample Availability: Samples of the compounds glycyrrhizinic acid, liquiritin, paeoniflorin and albiflorin are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Qian, D.; Pan, Y.; Zhu, Z.; Huang, J.; Xi, J.; Guo, J.; Zhou, X.; Zhong, G.; Duan, J. Comparisons of the Pharmacokinetic Profile of Four Bioactive Components after Oral Administration of Gan-Sui-Ban-Xia Decoction Plus-Minus Gansui and Gancao Drug Combination in Normal Rats. Molecules 2015, 20, 9295-9308. https://doi.org/10.3390/molecules20059295
Zhang Y, Qian D, Pan Y, Zhu Z, Huang J, Xi J, Guo J, Zhou X, Zhong G, Duan J. Comparisons of the Pharmacokinetic Profile of Four Bioactive Components after Oral Administration of Gan-Sui-Ban-Xia Decoction Plus-Minus Gansui and Gancao Drug Combination in Normal Rats. Molecules. 2015; 20(5):9295-9308. https://doi.org/10.3390/molecules20059295
Chicago/Turabian StyleZhang, Yang, Dawei Qian, Ying Pan, Zhenghua Zhu, Jing Huang, Junzuan Xi, Jianming Guo, Xueping Zhou, Gansheng Zhong, and Jinao Duan. 2015. "Comparisons of the Pharmacokinetic Profile of Four Bioactive Components after Oral Administration of Gan-Sui-Ban-Xia Decoction Plus-Minus Gansui and Gancao Drug Combination in Normal Rats" Molecules 20, no. 5: 9295-9308. https://doi.org/10.3390/molecules20059295
APA StyleZhang, Y., Qian, D., Pan, Y., Zhu, Z., Huang, J., Xi, J., Guo, J., Zhou, X., Zhong, G., & Duan, J. (2015). Comparisons of the Pharmacokinetic Profile of Four Bioactive Components after Oral Administration of Gan-Sui-Ban-Xia Decoction Plus-Minus Gansui and Gancao Drug Combination in Normal Rats. Molecules, 20(5), 9295-9308. https://doi.org/10.3390/molecules20059295





