Synthesis and Biological Evaluation of Hydrazone Derivatives as Antifungal Agents
Abstract
:1. Introduction
2. Results and Discussion
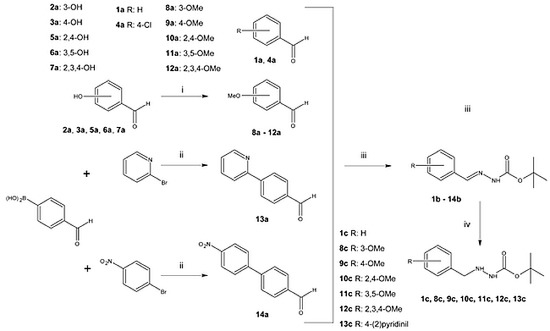
2.1. Synthesis
| R | R | R | ||||
|---|---|---|---|---|---|---|
 | 1ab | H | 6ab | 3,5-OH | 11a | 3,5-OMe |
| 2ab | 3-OH | 7ab | 2,3,4-OH | 12a | 2,3,4-OMe | |
| 3ab | 4-OH | 8a | 3-OMe | 13a | 4-(2)pyridinyl) | |
| 4ab | 4-Cl | 9a | 4-OMe | 14a | 4-(4)NO2benzyl | |
| 5ab | 2,4-OH | 10a | 2,4-OMe | |||
 | 1b | H | 6bc | 3,5-OH | 11b | 3,5-OMe |
| 2b | 3-OH | 7b | 2,3,4-OH | 12b | 2,3,4-OMe | |
| 3b | 4-OH | 8b | 3-OMe | 13b | 4-(2)pyridinyl) | |
| 4b | 4-Cl | 9b | 4-OMe | 14bc | 4-(4)NO2benzyl | |
| 5b | 2,4-OH | 10b | 2,4-OMe | |||
 | 1c | H | 10c | 2,4-OMe | 13c | 4-(2)pyridinyl) |
| 8c | 3-Ome | 11c | 3,5-OMe | 11 | ||
| 9c | 4-Ome | 12c | 2,3,4-OMe | 12 |
2.2. Antifungal Activity
| Column Heading | Aldehydes | Hydrazones | Hydrazines |
|---|---|---|---|
| C. glabrata | 2a, 5a, 7a, 13a, 14a | 7b, 12b | 12c |
| C. parapsilosis | 2a, 6a, 7a, 8a, 10a, 11a, 13a | 2b, 5b, 6b, 7b, 11b, 13b | 10c, 13c |
| C. tropicalis | 2a, 5a, 8a, 10a, 12a, 13a, 14a | 7b, 8b, 11b, 14b | 13c |
| C. krusei | 2a, 5a, 12a, 13a, 14a | 7b, 9b, 12b | 8c |
| C. lusitaneae | 2a, 4a, 6a, 13a, 14a | 2b, 6b, 7b, 9b, 11b | 13c |
| C. albicans | 2a, 5a, 7a, 10a, 11a, 13a | 7b, 10b, 11b, 13b | 1c, 9c, 10c, 13c |
| T. asahii | 1a, 2a, 5a, 6a, 8a, 13a | 3b, 5b, 6b, 7b, 11b, 13b | 9c, 10c, 12c, 13c |
| Isolate | T. asahii | Isolate | C. parapsilosis | ||||
|---|---|---|---|---|---|---|---|
| 13a | 7b | Fluc a | 13a | 7b | Fluc | ||
| TAH 05 | 125 | 16 | Nt b | RL 01 | 32 | 16 | 64 |
| TAH 06 | 64 | 16 | 32 | RL 05 | 32 | 16 | ≤1 |
| TAH 07 | 64 | 16 | 8 | RL 07 | 32 | 16 | ≤1 |
| TAH 09 | 250 | 8 | Nt b | RL 13 | 64 | 16 | ≤1 |
| TAH 10 | 32 | 8 | 8 | RL 20 | 16 | 16 | 4 |
| TAH 11 | 64 | 16 | 8 | RL 27 | 32 | 16 | ≤1 |
| TAH 12 | 64 | 16 | Nt b | RL 32 | 32 | 16 | ≤1 |
| TAH 13 | 64 | 16 | 4 | RL 33 | 32 | 16 | 2 |
| TAH 14 | 32 | 16 | 4 | RL 36 | 32 | 8 | 2 |
| TAH 15 | 32 | 16 | 4 | RL 38 | 32 | 8 | 4 |
2.3. Mechanism of Action
2.3.1. Evaluation of the Action on Cell Wall Stability: Sorbitol Protection Assay
| Compounds | 2 Days | 7 Days | ||
|---|---|---|---|---|
| −/Sorbitol | +/Sorbitol | −/Sorbitol | +/Sorbitol | |
| Anidulafungin | <1.0 | <1.0 | <1.0 | >125 |
| 7b | 16 | 16 | 16 | 16 |
| 13a | 32 | 32 | 32 | 32 |
2.3.2. Evaluation of the Action on Cell Membrane Stability: Cellular Leakage Assay
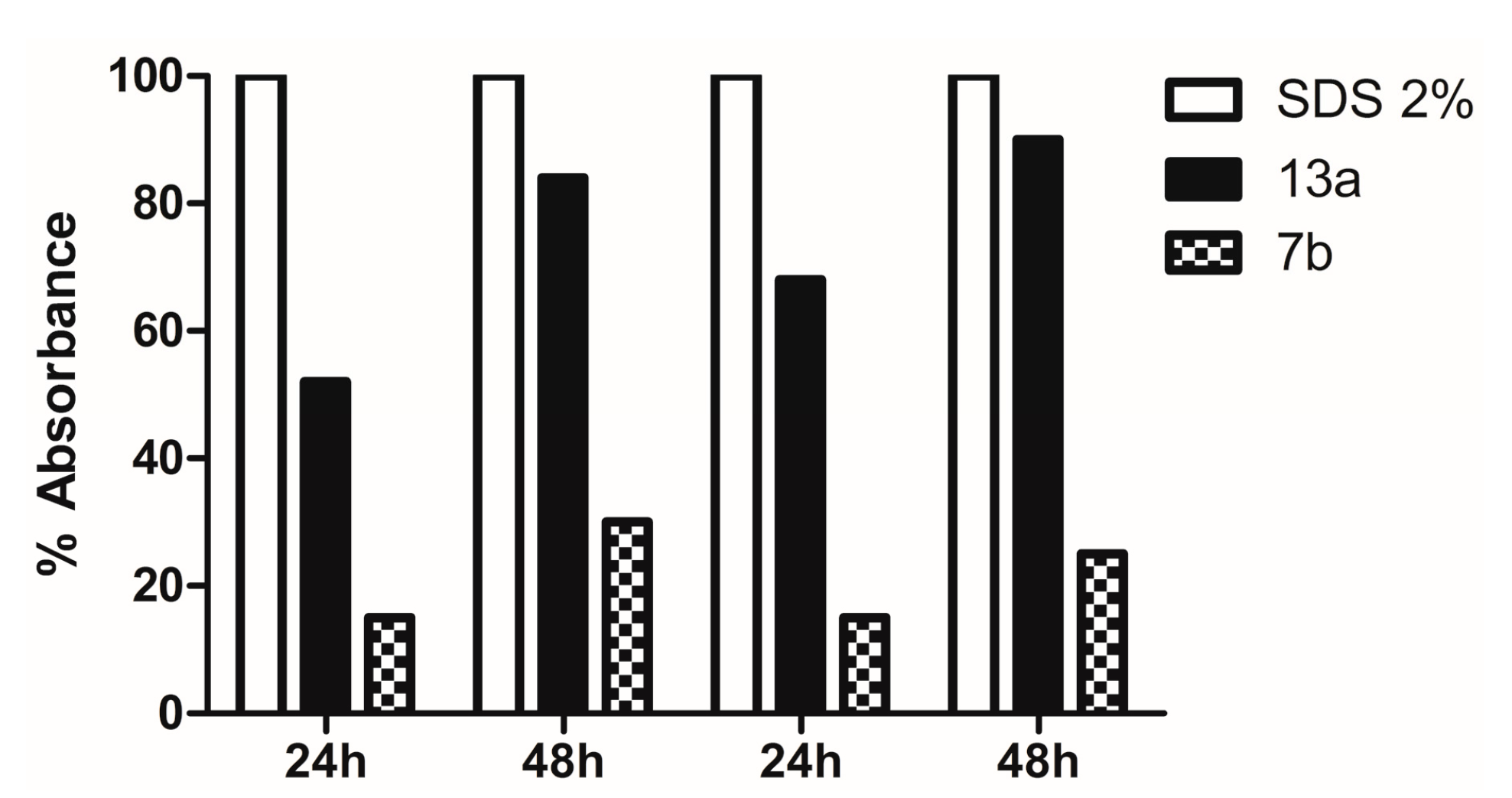
2.3.3. Evaluation of Action on Membrane Ergosterol: Ergosterol Effect Assay
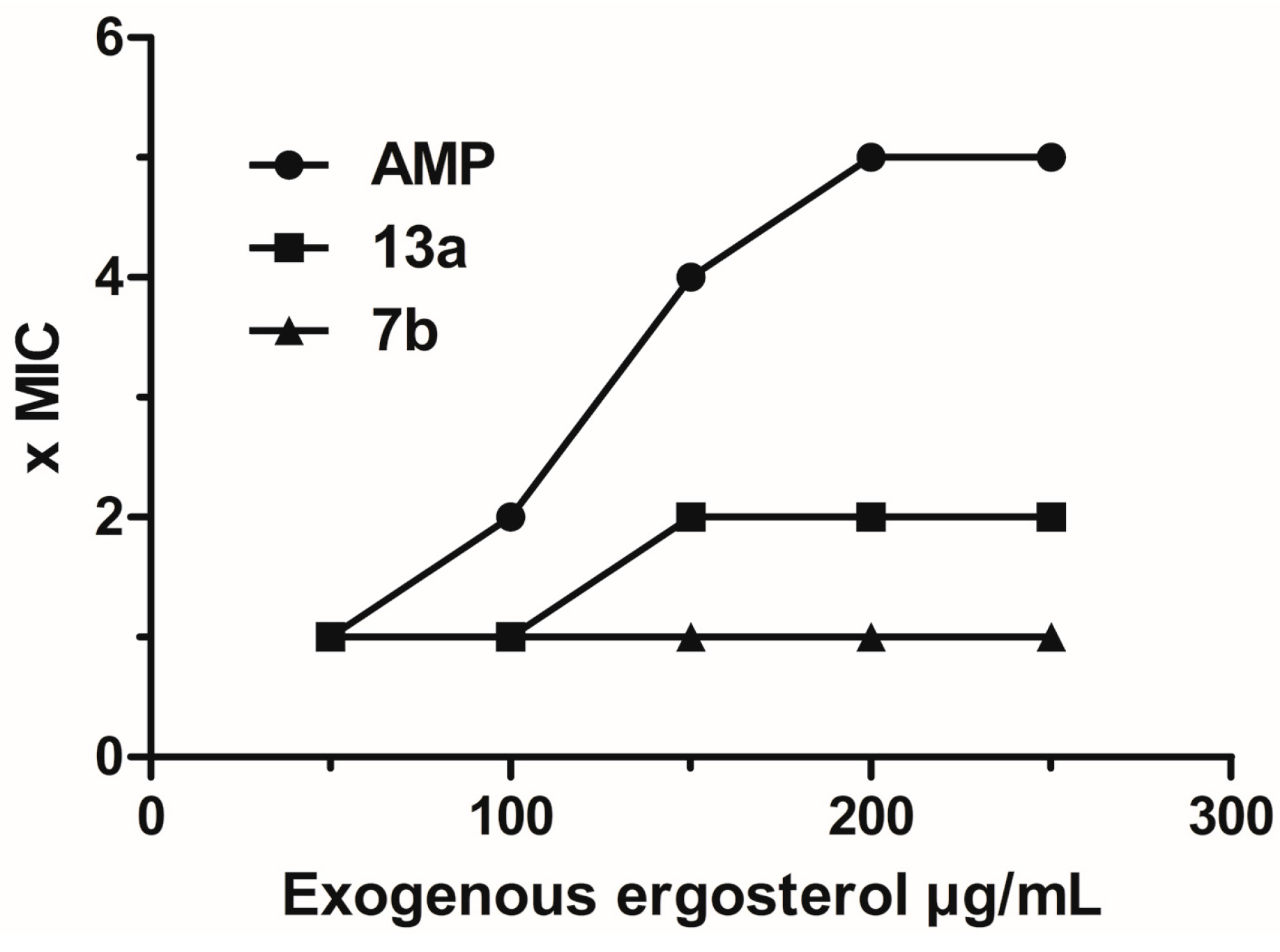
2.4. Cell Viability and Genotoxicity

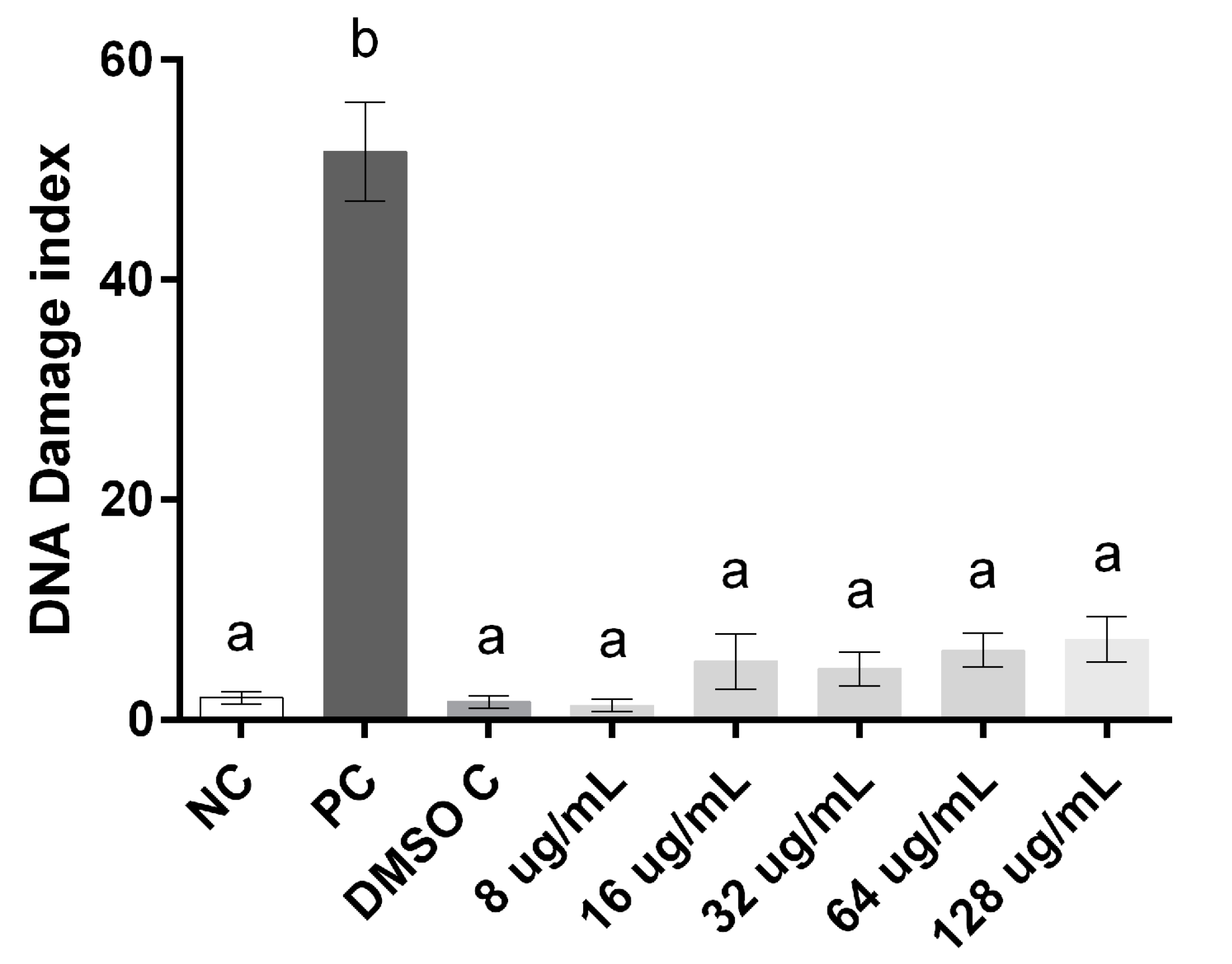
3. Experimental Section
3.1. General Information
3.2. Chemistry
3.2.1. General Procedure for the Preparation of Derivatives 8a–12a
3.2.2. General Procedure for the Preparation of Derivatives 13a–14a
3.2.3. General Procedure for the Preparation of Derivatives 1b–14b
3.2.4. General Procedure for the Preparation of Derivatives 1c, 8c–14c
3.3. Antifungal Activity
3.3.1. Isolates
3.3.2. Antifungal Activity Screening Assay
3.3.3. Minimal Inhibitory Concentration
3.4. Mechanisms of Action
3.4.1. Sorbitol Protection Assay
3.4.2. Cellular Leakage Assay
3.4.3. Ergosterol Effect Assay
3.5. Cytotoxicity
3.5.1. Cell Viability
3.5.2. Genotoxicity
3.6. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Pappas, P.G.M.D. Opportunistic fungi: A view to the future. Am. J. Med. Sci. 2010, 340, 253–257. [Google Scholar] [CrossRef] [PubMed]
- Romani, L. Immunity to fungal infections. Nat. Rev. Immunol. 2011, 11, 275–288. [Google Scholar] [CrossRef] [PubMed]
- Barchiesi, F.; Arzeni, D.; Fothergill, A.W.; Di Francesco, L.F.; Caselli, F.; Rinaldi, M.G.; Scalise, G. In vitro activities of the new antifungal triazole sch 56592 against common and emerging yeast pathogens. Antimicrob. Agents Chemother. 2000, 44, 226–229. [Google Scholar] [CrossRef] [PubMed]
- Van Asbeck, E.C.; Clemons, K.V.; Stevens, D.A. Candida parapsilosis: A review of its epidemiology, pathogenesis, clinical aspects, typing and antimicrobial susceptibility. Crit. Rev. Microbiol. 2009, 35, 283–309. [Google Scholar] [CrossRef] [PubMed]
- Miceli, M.H.; Díaz, J.A.; Lee, S.A. Emerging opportunistic yeast infections. Lancet Infect. Dis. 2011, 11, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.W.T.; Takemoto, J.Y. Antifungal amphiphilic aminoglycosides. Med. Chem. Commun. 2014, 5, 1048–1057. [Google Scholar] [CrossRef]
- Colombo, A.L.; Nucci, M.; Park, B.J.; Nouér, S.A.; Arthington-Skaggs, B.; da Matta, D.A.; Warnock, D.; Morgan, J.; Brazilian Network Candidemia Study. Epidemiology of candidemia in Brazil: A nationwide sentinel surveillance of candidemia in eleven medical centers. J. Clin. Microbiol. 2006, 44, 2816–2823. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajeshkumar, R.; Sundararaman, M. Emergence of Candida spp. and exploration of natural bioactive molecules for anticandidal therapy—status quo. Mycoses 2012, 55, e60–e73. [Google Scholar] [CrossRef] [PubMed]
- Imramovský, A.; Polanc, S.; Vinšová, J.; Kočevar, M.; Jampílek, J.; Rečková, Z.; Kaustová, J. A new modification of anti-tubercular active molecules. Bioorgan. Med. Chem. 2007, 15, 2551–2559. [Google Scholar] [CrossRef]
- Dimmock, J.R.; Vashishtha, S.C.; Stables, J.P. Anticonvulsant properties of various acetylhydrazones, oxamoylhydrazones and semicarbazones derived from aromatic and unsaturated carbonyl compounds. Eur. J. Med. Chem. 2000, 35, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Lima, P.C.; Lima, L.M.; da Silva, K.C.M.; Léda, P.H.O.; de Miranda, A.L.P.; Fraga, C.A.M.; Barreiro, E.J. Synthesis and analgesic activity of novel n-acylarylhydrazones and isosters, derived from natural safrole. Eur. J. Med. Chem. 2000, 35, 187–203. [Google Scholar] [CrossRef] [PubMed]
- Silva, G.A.; Costa, L.M.M.; Brito, F.C.F.; Miranda, A.L.P.; Barreiro, E.J.; Fraga, C.A.M. New class of potent antinociceptive and antiplatelet 10h-phenothiazine-1-acylhydrazone derivatives. Bioorgan. Med. Chem. 2004, 12, 3149–3158. [Google Scholar] [CrossRef]
- Loncle, C.; Brunel, J.M.; Vidal, N.; Dherbomez, M.; Letourneux, Y. Synthesis and antifungal activity of cholesterol-hydrazone derivatives. Eur. J. Med. Chem. 2004, 39, 1067–1071. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Aal, M.T.; El-Sayed, W.A.; El-Ashry, E.S.H. Synthesis and antiviral evaluation of some sugar arylglycinoylhydrazones and their oxadiazoline derivatives. Arch. Pharm. 2006, 339, 656–663. [Google Scholar] [CrossRef]
- El-Hawash, S.A.M.; Abdel Wahab, A.E.; El-Demellawy, M.A. Cyanoacetic acid hydrazones of 3-(and 4-)acetylpyridine and some derived ring systems as potential antitumor and anti-HCV agents. Arch. Pharm. 2006, 339, 14–23. [Google Scholar] [CrossRef]
- Walcourt, A.; Loyevsky, M.; Lovejoy, D.B.; Gordeuk, V.R.; Richardson, D.R. Novel aroylhydrazone and thiosemicarbazone iron chelators with anti-malarial activity against chloroquine-resistant and -sensitive parasites. Int. J. Biochem. Cell Biol. 2004, 36, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Dalazen, D.; Zanrosso, D.; Wanderley, L.; da SIlva, N.L.; Fuentefria, A.M. Comparação do perfil de suscetibilidade entre isolados clínicos de Candida spp. orais e vulvovaginais no sul do brasil. J. Bras. Patol. Med. Lab. 2011, 47, 33–38. [Google Scholar] [CrossRef]
- Reference Method for Broth Dilution Antifungal, 3rd ed; Document m27-a3; CLSI: Wayne, PA, USA, 2008.
- Escalante, A.; Gattuso, M.; Pérez, P.; Zacchino, S. Evidence for the mechanism of action of the antifungal phytolaccoside b isolated from phytolacca tetramera hauman. J. Nat. Prod. 2008, 71, 1720–1725. [Google Scholar] [CrossRef] [PubMed]
- Khatib, S.; Nerya, O.; Musa, R.; Shmuel, M.; Tamir, S.; Vaya, J. Chalcones as potent tyrosinase inhibitors: The importance of a 2,4-substituted resorcinol moiety. Bioorgan. Med. Chem. 2005, 13, 433–441. [Google Scholar] [CrossRef]
- Fan, X.; Song, Y.L.; Long, Y.Q. An efficient and practical synthesis of the hiv protease inhibitor atazanavir via a highly diastereoselective reduction approach. Org. Proc. Res. Dev. 2008, 12, 69–75. [Google Scholar] [CrossRef]
- Xu, Z.; Singh, J.; Schwinden, M.D.; Zheng, B.; Kissick, T.P.; Patel, B.; Humora, M.J.; Quiroz, F.; Dong, L.; Hsieh, D.M.; et al. Process research and development for an efficient synthesis of the HIV protease inhibitor bms-232632. Org. Proc. Res. Dev. 2002, 6, 323–328. [Google Scholar] [CrossRef]
- Innocente, A.; Casanova, B.B.; Klein, F.; Lana, A.D.; Pereira, D.; Muniz, M.N.; Sonnet, P.; Gosmann, G.; Fuentefria, A.M.; Gnoatto, S.C.B. Synthesis of isosteric triterpenoid derivatives and antifungal activity. Chem. Biol. Drug Des. 2014, 83, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Frost, D.J.; Brandt, K.D.; Cugier, D.; Goldman, R. A whole-cell Candida albicans assay for the detection of inhibitors towards fungal cell wall synthesis and assembly. J. Antibiot. 1995, 48, 306–310. [Google Scholar] [CrossRef] [PubMed]
- Carrasco, H.; Raimondi, M.; Svetaz, L.; Liberto, M.D.; Rodriguez, M.V.; Espinoza, L.; Madrid, A.; Zacchino, S. Antifungal activity of eugenol analogues. Influence of different substituents and studies on mechanism of action. Molecules 2012, 17, 1002–1024. [Google Scholar] [CrossRef] [PubMed]
- Burow, M.E.; Weldon, C.B.; Tang, Y.; Navar, G.L.; Krajewski, S.; Reed, J.C.; Hammond, T.G.; Clejan, S.; Beckman, B.S. Differences in susceptibility to tumor necrosis factor α-induced apoptosis among mcf-7 breast cancer cell variants. Cancer Res. 1998, 58, 4940–4946. [Google Scholar] [PubMed]
- Dos Santos Montagner, G.F.F.; Sagrillo, M.; Machado, M.M.; Almeida, R.C.; Mostardeiro, C.P.; Duarte, M.M.M.F.; da Cruz, I.B.M. Toxicological effects of ultraviolet radiation on lymphocyte cells with different manganese superoxide dismutase ala16val polymorphism genotypes. Toxicol. In Vitro 2010, 24, 1410–1416. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.P.; McCoy, M.T.; Tice, R.R.; Schneider, E.L. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp. Cell Res. 1988, 175, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: All samples of the compounds are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Casanova, B.B.; Muniz, M.N.; De Oliveira, T.; De Oliveira, L.F.; Machado, M.M.; Fuentefria, A.M.; Gosmann, G.; Gnoatto, S.C.B. Synthesis and Biological Evaluation of Hydrazone Derivatives as Antifungal Agents. Molecules 2015, 20, 9229-9241. https://doi.org/10.3390/molecules20059229
Casanova BB, Muniz MN, De Oliveira T, De Oliveira LF, Machado MM, Fuentefria AM, Gosmann G, Gnoatto SCB. Synthesis and Biological Evaluation of Hydrazone Derivatives as Antifungal Agents. Molecules. 2015; 20(5):9229-9241. https://doi.org/10.3390/molecules20059229
Chicago/Turabian StyleCasanova, Bruna B., Mauro N. Muniz, Thayse De Oliveira, Luís Flavio De Oliveira, Michel M. Machado, Alexandre M. Fuentefria, Grace Gosmann, and Simone C. B. Gnoatto. 2015. "Synthesis and Biological Evaluation of Hydrazone Derivatives as Antifungal Agents" Molecules 20, no. 5: 9229-9241. https://doi.org/10.3390/molecules20059229