Studies on Transition Metal-Quercetin Complexes Using Electrospray Ionization Tandem Mass Spectrometry
Abstract
:1. Introduction
2. Results and Discussion
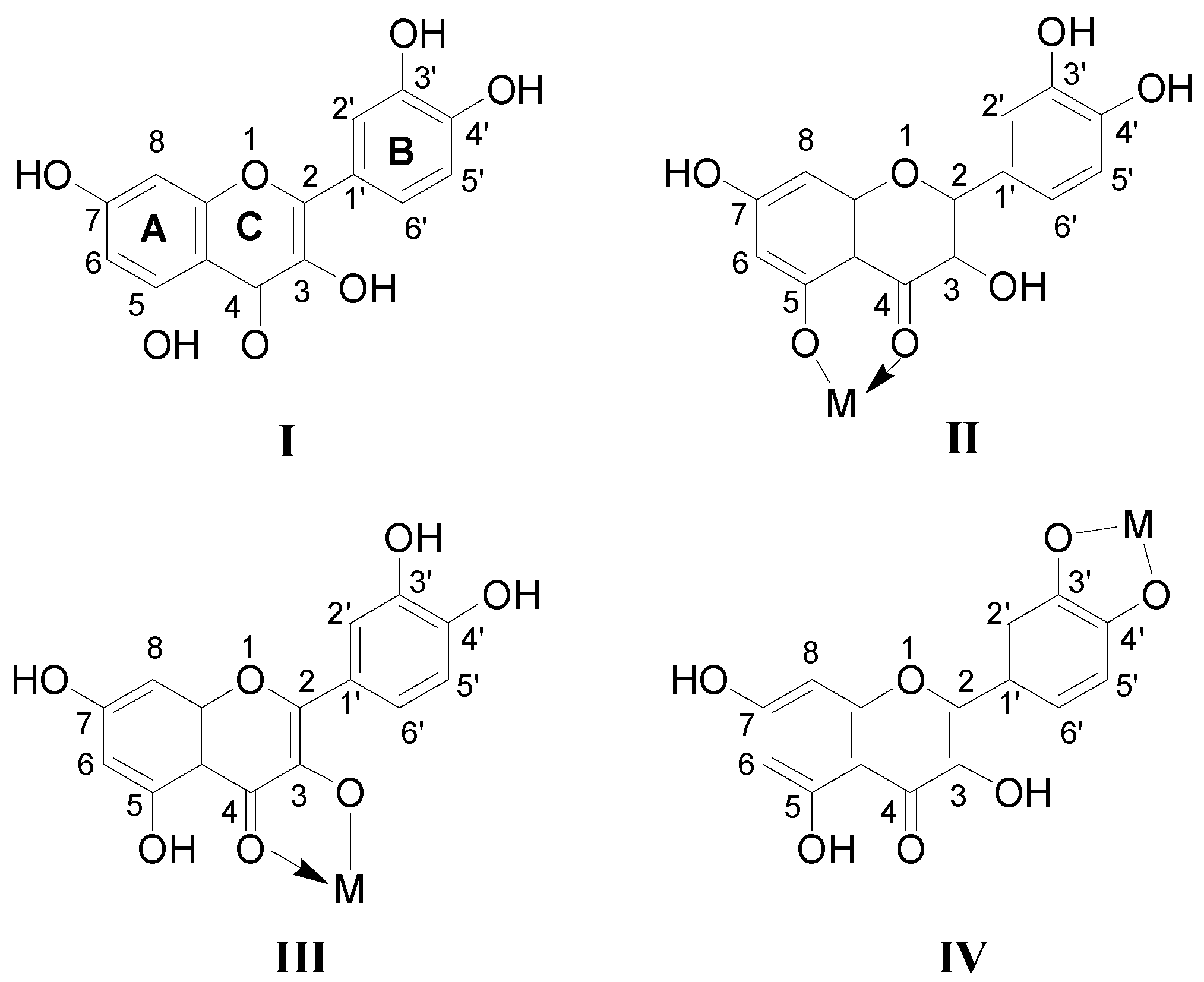
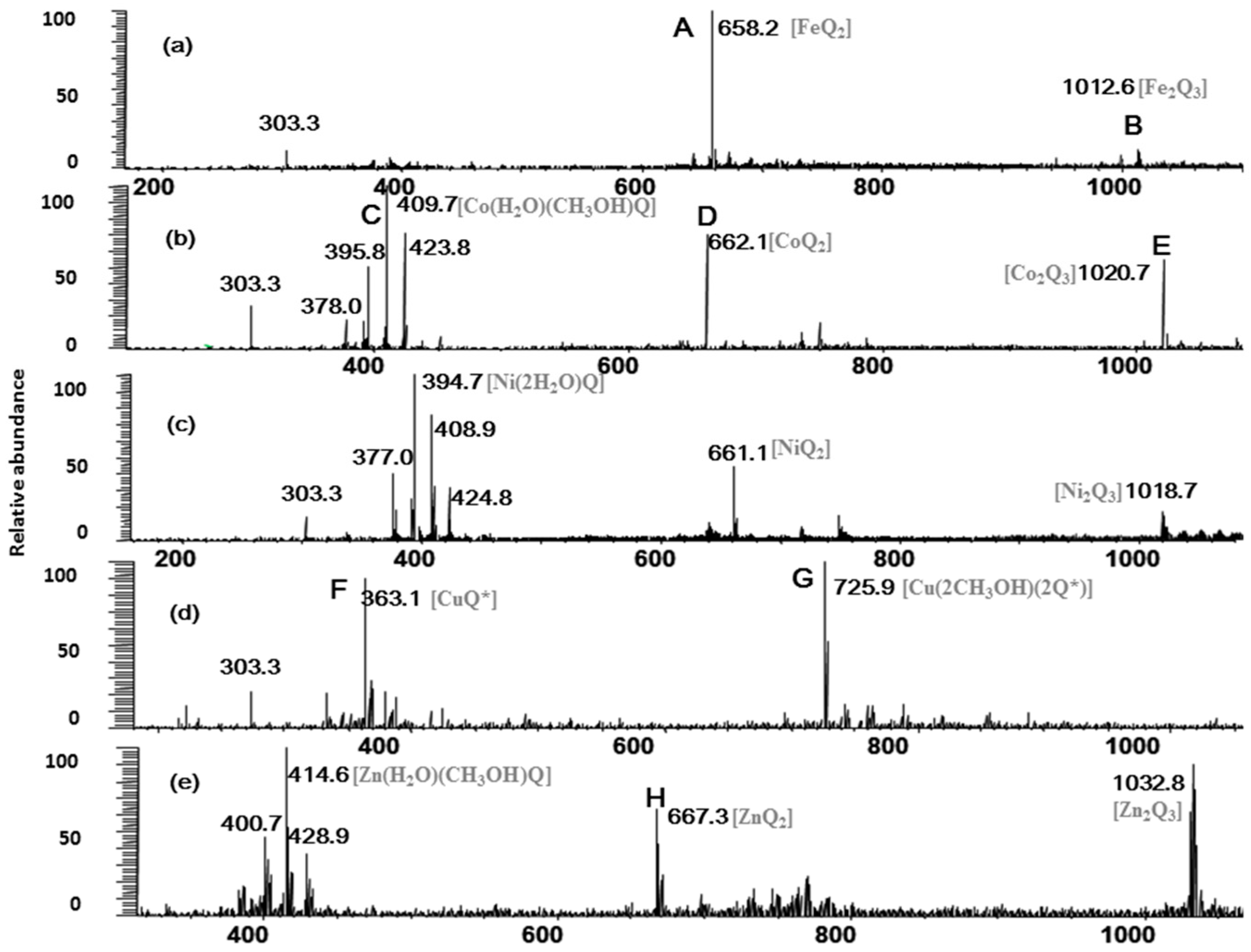
2.1. Full-Scan ESI-MS Analysis of M2+/Quercetin Complexes
| Metal Ion | Number of d-electrons | Metal R. | Space Configuration | CFSE(Dq) |
|---|---|---|---|---|
| Fe2+ | d6 | 75 pm | Octahedron | −4 |
| Co2+ | d7 | 72 pm | Octahedron | −8 |
| Ni2+ | d8 | 70 pm | Octahedron | −12 |
| Cu2+ | d9 | 69 pm | Plane square | −12.3 |
| Zn2+ | d10 | 74 pm | Tetrahedron | 0 |
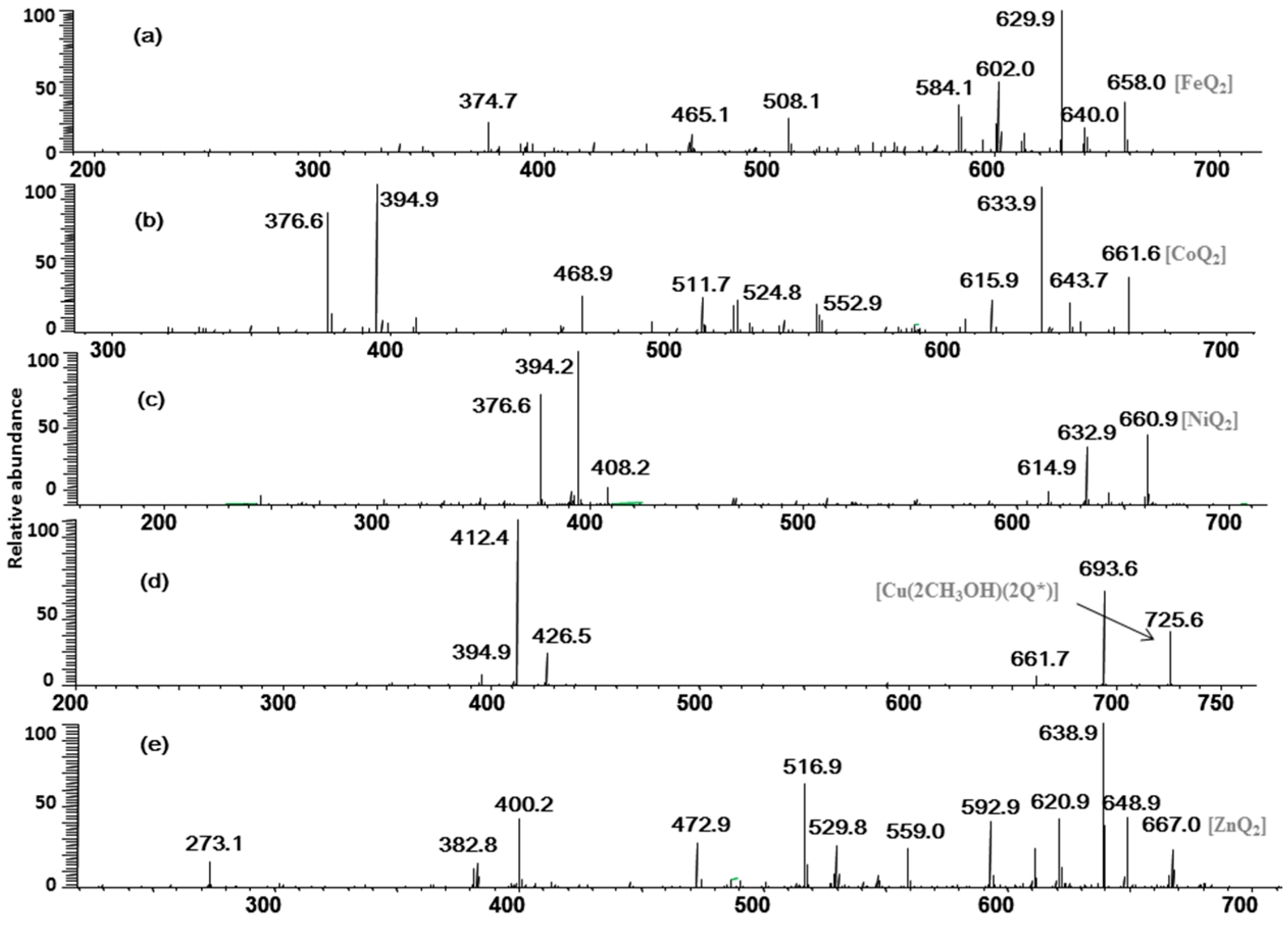
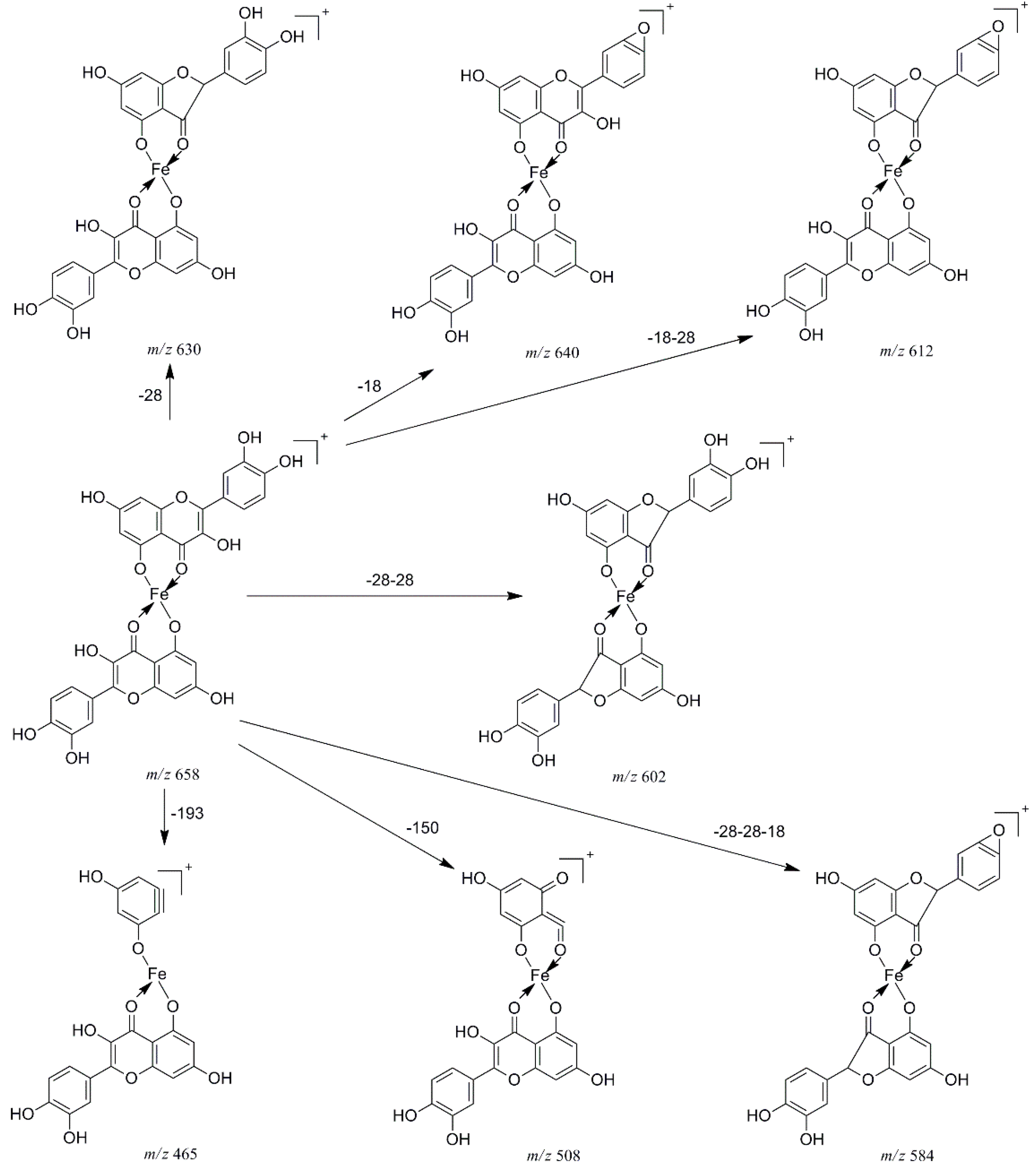
2.2. Multistage Tandem Mass Spectrometry Analysis of M2+/Quercetin Complexes
| Atom | Net Charge (q) | Charge Density Edge (Q) | Energy-Weighted Charge Density Edge (QR) |
|---|---|---|---|
| O-3 | −0.3712 | 0.0709 | −0.1814 |
| O-5 | −0.3948 | 0.0018 | −0.0046 |
| O-3' | −0.3708 | 0.0422 | −0.1081 |
| O-4' | −0.3705 | 0.0169 | −0.0432 |
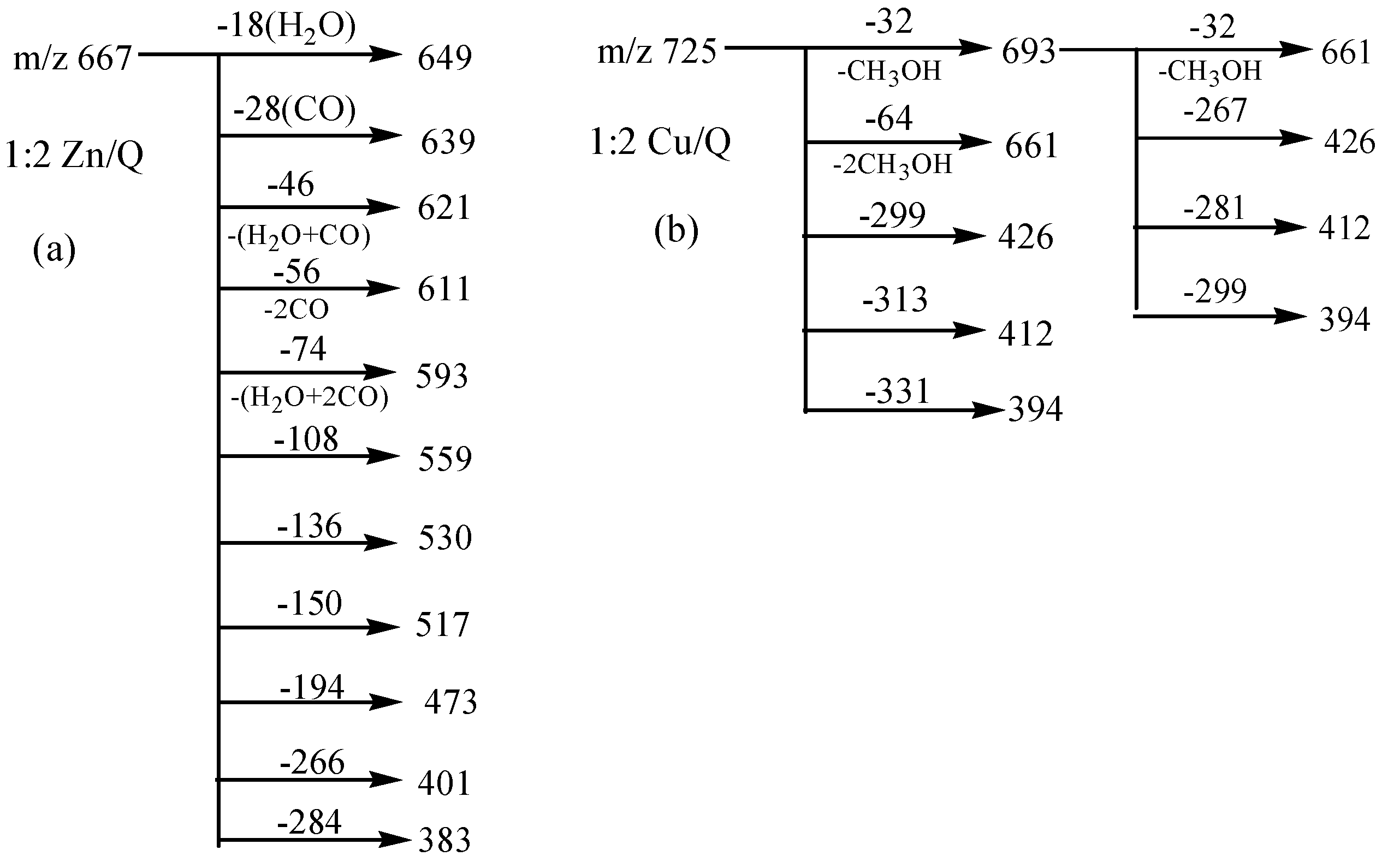
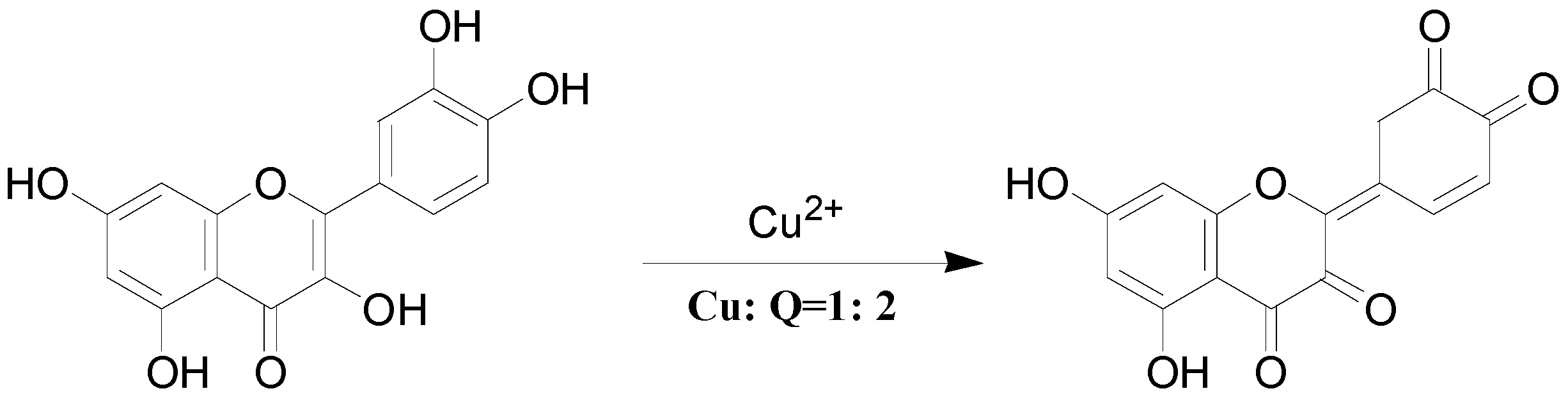
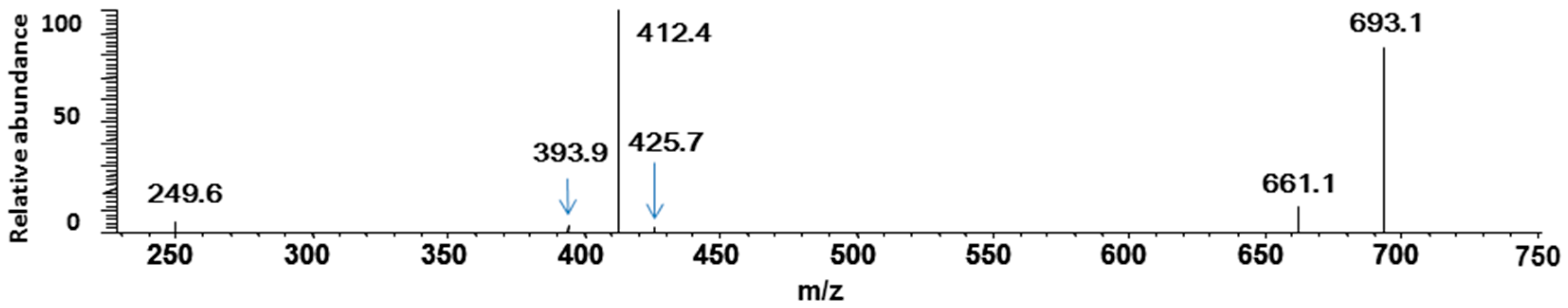
3. Experimental Section
3.1. Reagents and Chemicals
3.2. Sample Preparation
3.3. Mass Spectrometry
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Corradini, E.; Foglia, P.; Giansanti, P.; Gubbiotti, R.; Samperi, R.; Lagana, A. Flavonoids: Chemical properties and analytical methodologies of identification and quantitation in foods and plants. Nat. Prod. Res. 2011, 25, 469–495. [Google Scholar] [CrossRef] [PubMed]
- Guardia, T.; Rotelli, A.E.; Juarez, A.O.; Pelzer, L.E. Anti-inflammatory properties of plant flavonoids. Effects of rutin, quercetin and hesperidin on adjuvant arthritis in rat. Il Farmaco 2001, 56, 683–687. [Google Scholar] [CrossRef] [PubMed]
- Mira, L.; Fernandez, M.T.; Santos, M.; Rocha, R.; Florencio, M.H.; Jennings, K.R. Interactions of flavonoids with iron and copper ions: A mechanism for their antioxidant activity. Free Radic. Res. 2002, 36, 1199–208. [Google Scholar] [CrossRef] [PubMed]
- Grazul, M.; Budzisz, E. Biological activity of metal ions complexes of chromones, coumarins and flavones. Coord. Chem. Rev. 2009, 253, 2588–2598. [Google Scholar] [CrossRef]
- Afanas’ev, I.B.; Ostrakhovitch, E.A.; Mikhal’chik, E.V.; Ibragimova, G.A.; Korkina, L.G. Enhancement of antioxidant and anti-inflammatory activities of bioflavonoid rutin by complexation with transition metals. Biochem. Pharmacol. 2001, 61, 677–684. [Google Scholar] [CrossRef] [PubMed]
- Cheng, I.F.; Breen, K. On the ability of four flavonoids, baicilein, luteolin, naringenin, and quercetin, to suppress the Fenton reaction of the iron-ATP complex. Biometals 2000, 13, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Kostyuk, V.A.; Potapovich, A.I.; Strigunova, E.N.; Kostyuk, T.V.; Afanas’ev, I.B. Experimental evidence that flavonoid metal complexes may act as mimics of superoxide dismutase. Arch. Biochem. Biophys. 2004, 428, 204–208. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.J.; Sun, S.F.; Cao, W.; Liang, Y.; Song, J.R. Antioxidant property of quercetin-Cr(III) complex: The role of Cr(III) ion. J. Mol. Struct. 2009, 918, 194–197. [Google Scholar] [CrossRef]
- Bravo, A.; Anacona, J.R. Metal complexes of the flavonoid quercetin: Antibacterial properties. Transit. Metal Chem. 2001, 26, 20–23. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, L.F.; Wang, J.Y.; Tang, N. Antioxidative and anti-tumour activities of solid quercetin metal(II) complexes. Transit. Metal Chem. 2001, 26, 57–63. [Google Scholar] [CrossRef]
- Mendoza, E.E.; Burd, R. Quercetin as a Systemic Chemopreventative Agent: Structural and Functional Mechanisms. Mini-Rev. Med. Chem. 2011, 11, 1216–1221. [Google Scholar] [PubMed]
- Malesev, D.; Kuntic, V. Investigation of metal-flavonoid chelates and the determination of flavonoids via metal-flavonoid complexing reactions. J. Serb. Chem. Soc. 2007, 72, 921–939. [Google Scholar] [CrossRef]
- Cornard, J.P.; Merlin, J.C. Spectroscopic and structural study of complexes of quercetin with Al(III). J. Inorg. Biochem. 2002, 92, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, N.; Tanemura, H.; Kawanishi, S. Mechanism of oxidative DNA damage induced by quercetin in the presence of Cu(II). Mutat. Res. 1999, 425, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Torreggiani, A.; Tamba, M.; Trinchero, A.; Bonora, S. Copper(II)-quercetin complexes in aqueous solutions: Spectroscopic and kinetic properties. J. Mol. Struct. 2005, 744, 759–766. [Google Scholar] [CrossRef]
- El Hajji, H.; Nkhili, E.; Tomao, V.; Dangles, O. Interactions of quercetin with iron and copper ions: Complexation and autoxidation. Free Radic. Res. 2006, 40, 303–320. [Google Scholar] [CrossRef] [PubMed]
- Leopoldini, M.; Russo, N.; Chiodo, S.; Toscano, M. Iron chelation by the powerful antioxidant flavonoid quercetin. J. Agric. Food Chem. 2006, 54, 6343–6351. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.R.; In, Y.M.; Yuan, Y.; Lee, J.J.; Kim, S. In situ spectroelectrochemical study of quercetin oxidation and complexation with metal ions in acidic solutions. Bull. Korean Chem. Soc. 2007, 28, 889–892. [Google Scholar] [CrossRef]
- Jurasekova, Z.; Torreggiani, A.; Tamba, M.; Sanchez-Cortes, S.; Garcia-Ramos, J.V. Raman and surface-enhanced Raman scattering (SERS) investigation of the quercetin interaction with metals: Evidence of structural changing processes in aqueous solution and on metal nanoparticles. J. Mol. Struct. 2009, 918, 129–137. [Google Scholar] [CrossRef]
- Dehghan, G.; Dolatabadi, J.E.N.; Jouyban, A.; Zeynali, K.A.; Ahmadi, S.M.; Kashanian, S. Spectroscopic Studies on the Interaction of Quercetin-Terbium(III) Complex with Calf Thymus DNA. DNA Cell Biol. 2011, 30, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Dolatabadi, J.E.N. Molecular aspects on the interaction of quercetin and its metal complexes with DNA. Int. J. Biol. Macromol. 2011, 48, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.P.; Shi, S.Y.; Sun, X.R.; Xiong, X.; Peng, M.J. The effect of Cu2+ on interaction between flavonoids with different C-ring substituents and bovine serum albumin: Structure-affinity relationship aspect. J. Inorg. Biochem. 2011, 105, 1529–1537. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, M.T.; Mira, M.L.; Florencio, M.H.; Jennings, K.R. Iron and copper chelation by flavonoids: An electrospray mass spectrometry study. J. Inorg. Biochem. 2002, 92, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Satterfield, M.; Brodbelt, J.S. Enhanced detection of flavonoids by metal complexation and electrospray ionization mass spectrometry. Anal. Chem. 2000, 72, 5898–5906. [Google Scholar] [CrossRef] [PubMed]
- Pikulski, M.; Brodbelt, J.S. Differentiation of flavonoid glycoside isomers by using metal complexation and electrospray ionization mass spectrometry. J. Am. Soc. Mass Spectrom. 2003, 14, 1437–1453. [Google Scholar] [CrossRef] [PubMed]
- Dowling, S.; Regan, F.; Hughes, H. The characterisation of structural and antioxidant properties of isoflavone metal chelates. J. Inorg. Biochem. 2010, 104, 1091–1098. [Google Scholar] [CrossRef] [PubMed]
- Pekal, A.; Biesaga, M.; Pyrzynska, K. Interaction of quercetin with copper ions: Complexation, oxidation and reactivity towards radicals. Biometals 2011, 24, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Housecroft, C.E.; Sharpe, A.G. Inorganic Chemistry, 2nd ed.; Ashford Colour Press: London, UK, 2005. [Google Scholar]
- Katyal, M. Flavones as Analytical Reagents—A Review. Talanta 1968, 15, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Katyal, M.; Prakash, S. Analytical Reactions of Hydroxyflavones. Talanta 1977, 24, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Satterfield, M.; Brodbelt, J.S. Structural characterization of flavonoid glycosides by collisionally activated dissociation of metal complexes. J. Am. Soc. Mass Spectrom. 2001, 12, 537–549. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples are not available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Guo, M. Studies on Transition Metal-Quercetin Complexes Using Electrospray Ionization Tandem Mass Spectrometry. Molecules 2015, 20, 8583-8594. https://doi.org/10.3390/molecules20058583
Liu Y, Guo M. Studies on Transition Metal-Quercetin Complexes Using Electrospray Ionization Tandem Mass Spectrometry. Molecules. 2015; 20(5):8583-8594. https://doi.org/10.3390/molecules20058583
Chicago/Turabian StyleLiu, Yuanzhen, and Mingquan Guo. 2015. "Studies on Transition Metal-Quercetin Complexes Using Electrospray Ionization Tandem Mass Spectrometry" Molecules 20, no. 5: 8583-8594. https://doi.org/10.3390/molecules20058583





