Microwave-Assisted Synthesis of Novel Pyrazolo[3,4-g][1,8]naphthyridin-5-amine with Potential Antifungal and Antitumor Activity
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry

| Entries | Catalyst | Conditions | Yield (%) |
|---|---|---|---|
| 1 | – | EtOH, reflux | – |
| 2 | AlCl3 | EtOH, reflux | 30 |
| 3 | pMeC6H4SO3H | EtOH, reflux | 35 |
| 4 | ZnCl2 | EtOH, reflux | 40 |
| 5 | – | EtOH, MW (120 °C, 300 W) | – |
| 6 | AlCl3 | EtOH, MW (120 °C, 300 W) | 40 |
| 7 | pMeC6H4SO3H | EtOH, MW (120 °C, 300 W) | 45 |
| 8 | ZnCl2 | EtOH, MW (120 °C, 300 W) | 75 |
| Compound 3 | Structure | Yield (%) |
|---|---|---|
| 3a |  | 65 |
| 3b |  | 63 |
| 3c |  | 62 |
| 3d |  | 80 |
| 3e |  | 75 |
| 3f |  | 65 |
| 3g |  | 60 |
| 3h |  | 60 |
| 3i |  | 55 |
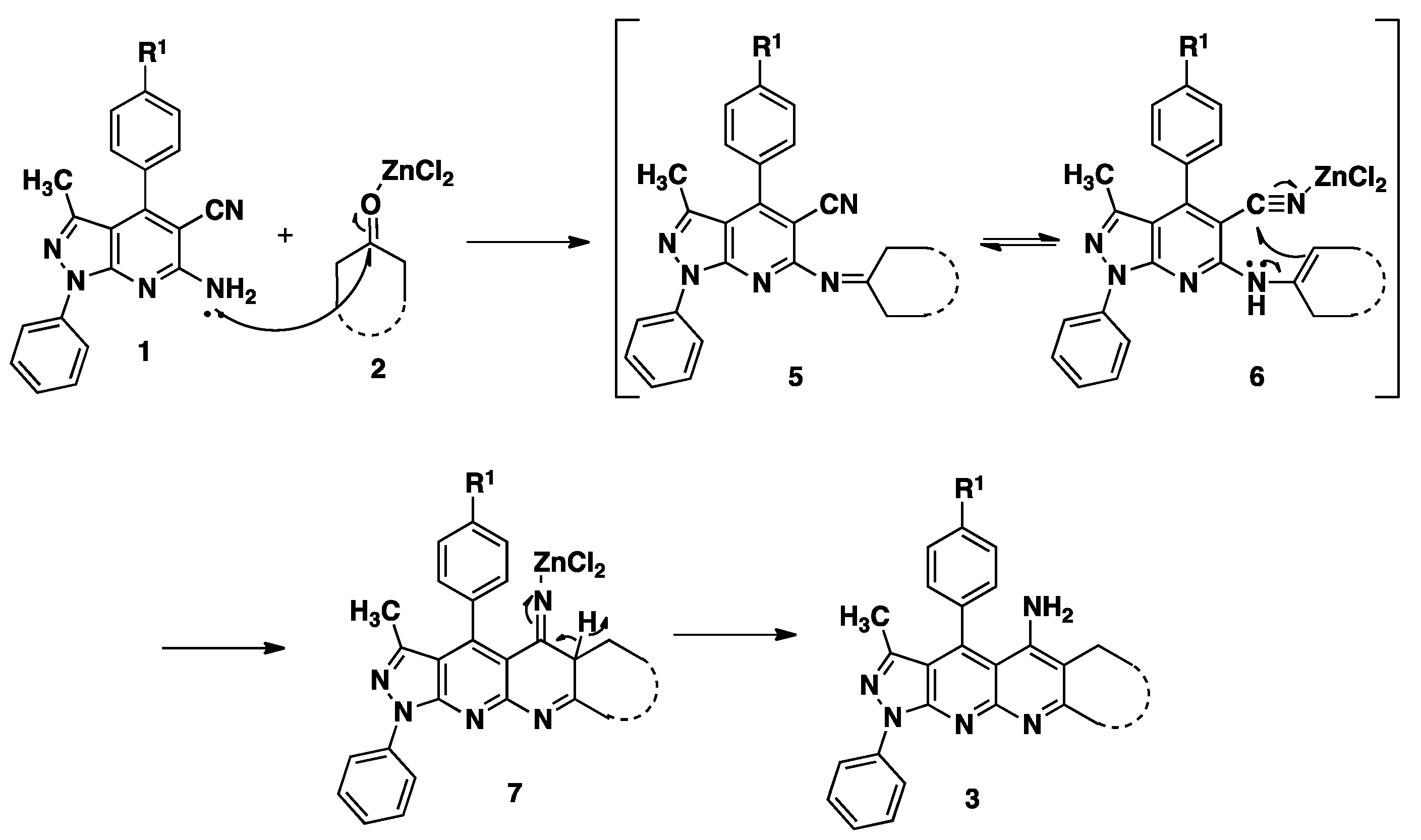
2.2. Antifungal Activity

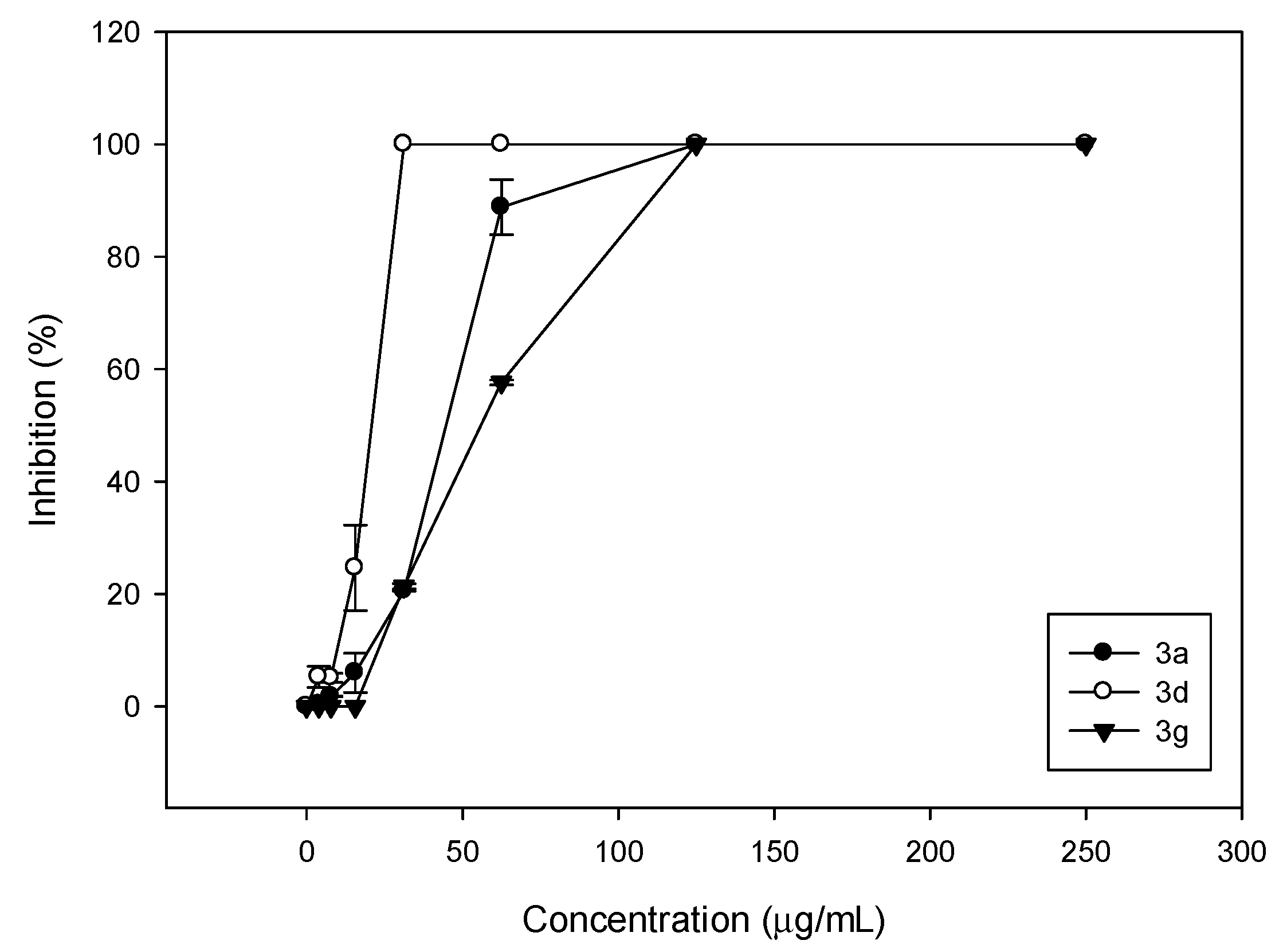
| R1 | Fused Ring | Comp | C. albicans ATCC 10231 | C. neoformans ATCC 32264 | ||||
|---|---|---|---|---|---|---|---|---|
| MIC100 | MIC80 | MIC50 | MIC100 | MIC80 | MIC50 | |||
| A | 3a | 125 | 62.5 | 62.5 | 250 | 250 | 7.8 | |
| CH3 | B | 3d | 31.2 | 31.2 | 31.2 | 250 | 125 | 125 |
| C | 3g | 125 | 62.5 | 62.5 | 125 | 125 | 125 | |
| A | 3b | >250 | 250 | 125 | >250 | 250 | 250 | |
| Cl | B | 3e | 250 | 125 | 125 | >250 | >250 | 250 |
| C | 3h | 125 | 125 | 125 | 250 | 125 | 125 | |
| A | 3c | 250 | 250 | 250 | >250 | 250 | 31.2 | |
| OCH3 | B | 3f | 250 | 125 | 125 | 250 | 125 | 125 |
| C | 3i | 250 | 125 | 125 | >250 | >250 | 250 | |
| Amph B | 0.12 | 0.25 | ||||||
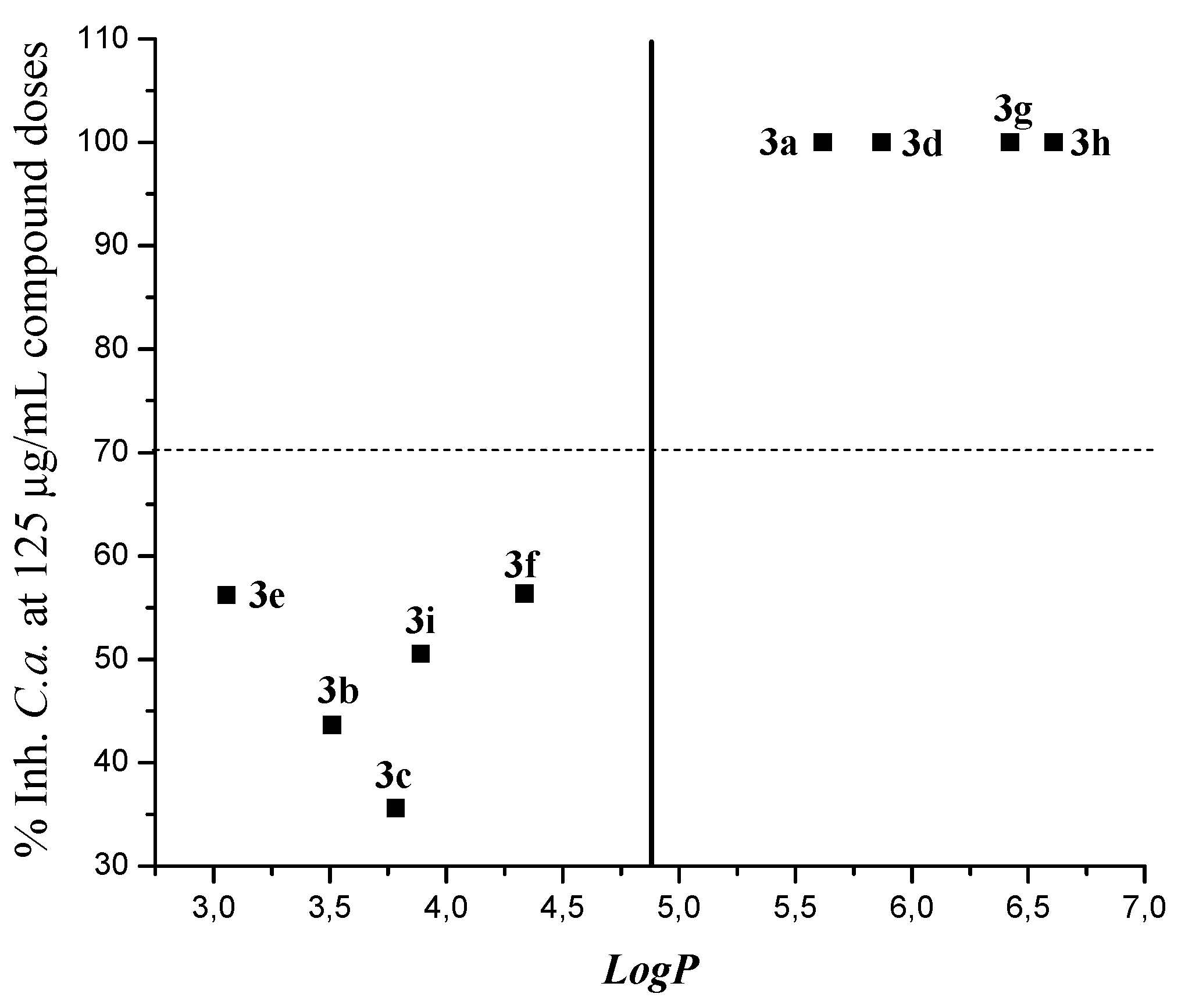
| Compound | Log P | % Ihn C.a. |
|---|---|---|
| 3a | 5.61803 | 100 |
| 3b | 3.50884 | 43.63 |
| 3c | 3.78334 | 35.58 |
| 3d | 5.87099 | 100 |
| 3e | 3.05581 | 56.19 |
| 3f | 4.33631 | 56.35 |
| 3g | 6.42396 | 100 |
| 3h | 6.60877 | 100 |
| 3i | 3.88927 | 50.55 |
Second-Order Studies with Clinical Isolates
| Strain | Voucher Specimen | 3a | 3d | 3g | Amph. | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| MIC100 | MIC80 | MIC50 | MIC100 | MIC80 | MIC50 | MIC100 | MIC80 | MIC50 | MIC100 | ||
| C. albicans | ATCC 10231 | 125 | 62.5 | 62.5 | 31.2 | 31.2 | 31.2 | 125 | 62.5 | 62.5 | 1.00 |
| C. albicans | CCC 125 | 62.5 | 62.5 | 62.5 | 31.2 | 31.2 | 31.2 | 125 | 62.5 | 31.2 | 0.78 |
| C. albicans | CCC 126 | 125 | 62.5 | 62.5 | 31.2 | 31.2 | 15.6 | 125 | 62.5 | 62.5 | 1.56 |
| C. albicans | CCC 127 | 62.5 | 31.2 | 31.2 | 31.2 | 31.2 | 31.2 | 125 | 62.5 | 31.2 | 0.78 |
| C. albicans | CCC 128 | 125 | 62.5 | 31.2 | 31.2 | 31.2 | 15.6 | 125 | 62.5 | 62.5 | 1.56 |
| C. albicans | CCC 129 | 125 | 31.2 | 62.5 | 62.5 | 31.2 | 31.2 | 250 | 62.5 | 31.2 | 0.78 |
| C. albicans | CCC 130 | 125 | 62.5 | 62.5 | 31.2 | 31.2 | 15.6 | 125 | 31.2 | 31.2 | 0.50 |
| C. glabrata | CCC 115 | >250 | >250 | >250 | >250 | >250 | >250 | >250 | >250 | 250 | 0.39 |
| C. parapsilopsis | CCC 124 | 125 | 62.5 | 62.5 | 31.2 | 31.2 | 31.2 | 125 | 125 | 62.5 | 0.78 |
| C. krusei | CCC 117 | 125 | 125 | 62.5 | 15.6 | 15.6 | 7.8 | 125 | 62.5 | 62.5 | 0.39 |
| C. tropicalis | CCC 131 | 125 | 125 | 62.5 | 31.2 | 15.6 | 15.6 | 125 | 125 | 62.5 | 0.50 |
2.3. Anticancer Activity
| Panel/Cell Line | Compounds | |||
|---|---|---|---|---|
| 3a | 3e | |||
| GI50 b (µM) | LC50 c (µM) | GI50 b (µM) | LC50 c (µM) | |
| Leukemia | ||||
| CCRF-CEM | 2.56 | >100 | 2.18 | >100 |
| HL-60(TB) | 2.24 | >100 | 2.12 | 8.71 |
| K-562 | 2.78 | >100 | 2.30 | 49.1 |
| MOLT-4 | 3.17 | >100 | 2.30 | 42.4 |
| RPMI-8226 | 2.25 | >100 | 1.92 | 9.72 |
| SR | 0.62 | >100 | 1.84 | 79.5 |
| Non-small Cell Lung Cancer | ||||
| A549/ATCC | 2.22 | 40.2 | 3.01 | 46.7 |
| HOP-62 | 3.32 | 58.7 | 14.1 | 60.9 |
| HOP-92 | – | – | – | – |
| NCI-H226 | 21.6 | >100 | 19.6 | >100 |
| NCI-H23 | 16.3 | 94.0 | – | – |
| NCI-H322M | 3.26 | 33.2 | 2.92 | 33.1 |
| NCI-H460 | 1.92 | 8.64 | 3.28 | 47.5 |
| NCI-H522 | 20.1 | 93.0 | 2.91 | 56.0 |
| Colon Cancer | ||||
| COLO 205 | 1.74 | 7.90 | 5.91 | 31.3 |
| HCC-2998 | – | – | 3.51 | 53.8 |
| HCT-116 | 1.68 | – | 2.15 | 25.9 |
| HCT-15 | 1.98 | >100 | 3.08 | 55.7 |
| HT29 | 14.3 | 73.2 | 2.68 | 41.9 |
| KM12 | 4.83 | 50.4 | 3.16 | 40.0 |
| SW-620 | 2.48 | 48.9 | 3.05 | 50.3 |
| CNS Cancer | ||||
| SF-268 | 3.13 | 72.8 | 5.33 | 54.6 |
| SF-295 | 2.99 | 58.3 | 10.1 | 54.0 |
| SF-539 | 1.88 | 6.49 | 14.5 | 58.8 |
| SNB-19 | 3.46 | 90.6 | 10.8 | 66.4 |
| SNB-75 | 2.43 | 46.5 | 14.0 | 72.1 |
| U251 | 1.78 | 6.85 | 1.72 | 7.36 |
| Melanoma | ||||
| LOX IMVI | 1.88 | 6.79 | 12.0 | 60.8 |
| MALME-3M | 21.5 | 74.5 | – | – |
| M14 | 3.50 | 59.0 | 3.74 | 46.6 |
| MDA-MB-435 | 17.1 | 67.3 | 3.83 | 53.4 |
| SK-MEL-2 | 21.4 | 89.2 | 2.63 | 35.3 |
| SK-MEL-28 | 11.3 | 62.6 | 6.75 | 70.2 |
| SK-MEL-5 | 1.77 | 67.3 | 1.84 | 8.08 |
| UACC-257 | 14.3 | 61.6 | 2.43 | 33.3 |
| UACC-62 | – | – | 13.7 | 56.8 |
| Ovarian Cancer | ||||
| IGROV1 | 4.43 | 95.3 | 4.26 | 81.5 |
| OVCAR-3 | 2.06 | 9.47 | 4.17 | 43.3 |
| OVCAR-4 | 3.95 | >100 | 3.22 | 96.0 |
| OVCAR-5 | 15.6 | 72.4 | 10.1 | 53.2 |
| OVCAR-8 | 2.32 | >100 | 2.82 | 48.0 |
| NCI/ADR-RES | 15.0 | >100 | 3.41 | >100 |
| SK-OV-3 | 19.9 | 61.8 | 12.1 | 50.3 |
| Renal Cancer | ||||
| 786-0 | 2.03 | – | 4.55 | 50.4 |
| A498 | 16.2 | 64.1 | 11.9 | 62.7 |
| ACHN | 1.94 | 7.58 | 4.32 | 46.3 |
| CAKI-1 | 2.56 | 31.5 | 3.88 | 43.4 |
| RXF 393 | – | – | 8.48 | 76.0 |
| SN12C | 1.84 | 7.40 | 3.72 | 55.9 |
| TK-10 | – | – | – | – |
| UO-31 | 1.84 | 7.33 | 2.46 | 41.3 |
| Prostate Cancer | ||||
| PC-3 | 7.05 | 67.3 | 2.54 | 42.0 |
| DU-145 | 2.40 | 17.5 | 4.72 | 45.6 |
| Breast Cancer | ||||
| MCF7 | 1.62 | >100 | 2.57 | 74.0 |
| MDA-MB-231/ATCC | 1.93 | 8.47 | 4.15 | 47.5 |
| HS 578T | 12.1 | >100 | 12.5 | 100 |
| BT-549 | 24.4 | >100 | 17.2 | 66.5 |
| T-47D | 2.21 | >100 | – | – |
| MDA-MB-468 | 2.03 | 30.2 | 2.33 | 40.5 |
3. Experimental Section
3.1. General Information
3.2. Chemistry
General Procedure for the Synthesis of Pyrazolo[4,3-g][1,8]naphthyridin-5-amine
3.3. Antifungal Activity
3.3.1. Microorganisms and Media
3.3.2. Fungal Growth Inhibition Percentage Determination
3.3.3. MIC100, MIC80 and MIC50 Determinations
3.4. Anticancer Activity
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Vanov, A.S.; Tugusheva, N.Z.; Granik, V.G. Benzo[b]naphthyridines. Russ. Chem. Rev. 2005, 74, 915–936. [Google Scholar] [CrossRef]
- Litvinov, V.P. Chemistry and biological activities of 1,8-naphthyridines. Russ. Chem. Rev. 2004, 73, 637–669. [Google Scholar] [CrossRef]
- Litvinov, V.P.; Roman, S.V.; Dyachenko, V.D. Naphthyridines. Structure, physicochemical properties and general methods of synthesis. Russ. Chem. Rev. 2000, 69, 201–220. [Google Scholar] [CrossRef]
- Roma, G.; Grossi, G.; Braccio, M.D.; Piras, D.; Ballabeni, V.; Tognolini, M.; Bertoni, S.; Barocelli, E. 1,8-Naphthyridines VII. New substituted 5-amino[1,2,4]triazolo[4,3-a][1,8]naphthyridine-6-carboxamides and their isosteric analogues, exhibiting notable anti-inflammatory and/or analgesic activities, but no acute gastrolesivity. Eur. J. Med. Chem. 2008, 43, 1665–1680. [Google Scholar] [CrossRef] [PubMed]
- Roma, G.; Braccio, M.D.; Grossi, G.; Mattioli, F.; Ghia, M. 1,8-Naphthyridines IV. 9-Substituted N,N-dialkyl-5-(alkylamino or cycloalkylamino) [1,2,4]triazolo[4,3-a][1,8]naphthyridine-6-carboxamides, new compounds with anti-aggressive and potent anti-inflammatory activities. Eur. J. Med. Chem. 2000, 35, 1021–1035. [Google Scholar] [CrossRef] [PubMed]
- Atanasova, M.; Ilieva, S.; Galabov, B. QSAR analysis of 1,4-dihydro-4-oxo-1-(2-thiazolyl)-1,8-naphthyridines with anticancer activity. Eur. J. Med. Chem. 2007, 42, 1184–1192. [Google Scholar] [CrossRef] [PubMed]
- Kuramoto, Y.; Ohshita, Y.; Yoshida, J.; Yazaki, A.; Shiro, M.; Koike, T. A novel antibacterial 8-chloroquinolone with a distorted orientation of the N-1-(5-Amino-2,4-difluorophenyl) group. J. Med. Chem. 2003, 46, 1905–1917. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Kuo, S.C.; Hsieh, M.C.; Mauger, A.; Lin, C.M.; Hamel, E.; Lee, K.H. Antitumor agents. 174. 2',3',4',5,6,7-substituted 2-phenyl-1,8-naphthyridin-4-ones: Their synthesis, cytotoxicity, and inhibition of tubulin polymerization. J. Med. Chem. 1997, 40, 2266–2275. [Google Scholar] [CrossRef] [PubMed]
- Ferrarini, P.L.; Mori, C.; Badawneh, M.; Calderone, V.; Greco, R.; Manera, C.; Martinelli, A.; Nieri, P.; Saccomanni, G. Synthesis and β-blocking activity of (R,S)-(E)-oximeethers of 2,3-dihydro-1,8-naphthyridine and 2,3-dihydrothiopyrano[2,3-b]pyridine: Potential antihypertensive agents Part IX. Eur. J. Med. Chem. 2000, 35, 815–826. [Google Scholar] [CrossRef] [PubMed]
- Sherlock, M.H.; Kaminski, J.J.; Tom, W.C.; Lee, J.F.; Wong, S.C.; Kreutner, W.; Bryant, R.W.; Mcphail, A.T. Antiallergy agents. 1. Substituted 1,8-naphthyridin-2(1H)-ones as inhibitors of SRS-A release. J. Med. Chem. 1988, 31, 2108–2121. [Google Scholar] [CrossRef] [PubMed]
- Barlin, G.; Tan, W. 2-Chloro-7-methyl-12-phenyldibenzo[b,g][1,8]naphthyridin-11(6H)-one. Aust. J. Chem. 1984, 37, 1065–1073. [Google Scholar] [CrossRef]
- Sampathkumar, N.; Kumar, N.V.; Rajendran, S.P. A simple synthesis of dibenzo[b,g][1,8]naphthyridines. Synth. Commun. 2004, 34, 2019–2014. [Google Scholar] [CrossRef]
- Naik, T.R.R.; Naik, H.S.B.; Raghavendra, M.; Naik, S.G.K. Synthesis of thieno[2,3-b]benzo[1,8]naphthyridine-2-carboxylic acids under microwave irradiation and interaction with DNA studies. ARKIVOC 2006, 15, 84–94. [Google Scholar] [CrossRef]
- Ahn, S.H.; Jang, S.S.; Kim, Y.H.; Lee, K.J.; Baylis, H. Morita-Baylis-Hillman Route to 8,9,9a,10-Tetrahydrobenzo[b][1,8]naphthyridine-6(7H)-ones and 3,4,4a,5-Tetrahydrodibenzo[b,g][1,8]naphthyridine-1(2H)-ones. Bull. Korean Chem. Soc. 2011, 32, 3145–3148. [Google Scholar] [CrossRef]
- Manoj, M.; Prasad, K.J.R. Synthesis of linear dibenzo[1,8]naphthyridines using 2-chloro-4-methylquinolines. ARKIVOC 2011, 9, 289–307. [Google Scholar] [CrossRef]
- Yamuna, E.; Zeller, M.; Prasad, K.J.R. Microwave assisted synthesis of indolo[2,3-b]dibenzo[b,g][1,8]naphthyridines. Tetrahedron Lett. 2012, 53, 1514–1517. [Google Scholar] [CrossRef]
- Chen, K.; Kuo, S.C.; Hsieh, M.C.; Mauger, A.; Lin, C.M.; Hamel, E.; Lee, K.H. Antitumor agents. 178. Synthesis and biological evaluation of substituted 2-aryl-1,8-naphthyridin-4(1H)-ones as antitumor agents that inhibit tubulin polymerization. J. Med. Chem. 1997, 40, 3049–3056. [Google Scholar] [CrossRef] [PubMed]
- Mekheimer, R.A.; Hameed, A.M.A.; Sadek, K.U. 1,8-Naphthyridines II: Synthesis of novel polyfunctionally substituted 1,8-naphthyridinones and their degradation to 6 aminopyridones. ARKIVOC 2007, 20, 269–281. [Google Scholar] [CrossRef]
- Mohamed, E.A.; Abdel-Rahman, R.M.; El-Gendy, Z.; Ismail, M.M. Synthesis and reactions of 3-halo-1,8-naphthyridin-2,4-diones. J. Indian Chem. Soc. 1994, 71, 765–768. [Google Scholar]
- Ferrarini, P.L.; Mori, C.; Primofiore, G.; Gazlolari, L. One step synthesis of pyrimido[1,2-a][1,8]naphthyridinones, pyrido[1,2-a]pyrimidinones and 1,8-naphthyridinones. J. Heterocycl. Chem. 1990, 27, 881–886. [Google Scholar] [CrossRef]
- Strauss, C.R.; Varma, R.S. Microwaves in green and sustainable chemistry. Top. Curr. Chem. 2006, 266, 199–231. [Google Scholar]
- Kappe, C.O. Controlled microwave heating in modern organic synthesis. Angew. Chem. Int. Ed. 2004, 43, 6250–6284. [Google Scholar] [CrossRef]
- Quiroga, J.; Sanchez, N.; Acosta, P.; Insuasty, B.; Abonia, R. Microwave-assisted synthesis of fused pyrazolo[3,4-b]pyrazines by the reaction of ortho-aminonitrosopyrazoles and cyclic β-diketones. Tetrahedron Lett. 2012, 53, 3181–3187. [Google Scholar] [CrossRef]
- Martins, M.; Frizzo, C.; Moreira, D.; Buriol, L.; Machado, P. Solvent-free heterocyclic synthesis. Chem. Rev. 2009, 109, 4140–4182. [Google Scholar] [CrossRef] [PubMed]
- Quiroga, J.; Portilla, J.; Abonia, R.; Insuasty, B.; Nogueras, M.; Cobo, J. Regioselective synthesis of novel substituted pyrazolo[1,5-a]pyrimidines under solvent-free conditions. Tetrahedron Lett. 2008, 49, 6254–6256. [Google Scholar] [CrossRef]
- Quiroga, J.; Trilleras, J.; Insuasty, B.; Abonia, R.; Nogueras, M.; Cobo, J. Microwave-assisted synthesis of pyrazolo[3,4-d]pyrimidines from 2-amino-4,6-dichloropyrimidine-5-carbaldehyde under solvent-free conditions. Tetrahedron Lett. 2008, 49, 3257–3259. [Google Scholar] [CrossRef]
- Quiroga, J.; Portilla, J.; Abonia, R.; Insuasty, B.; Nogueras, M.; Cobo, J. Regioselective synthesis of novel polyfunctionally substituted pyrazolo[1,5-a]pyrimidines under solvent-free conditions. Tetrahedron Lett. 2007, 48, 6352–6355. [Google Scholar] [CrossRef]
- Wang, M.; Wu, A.; Pan, X.; Yang, H. Total synthesis of two naturally occurring bicyclo[3.2.1]octanoid neolignans. J. Org. Chem. 2002, 67, 5405–5409. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, K.; Nakamura, S.; Yamamoto, H. The first enantioselective biomimetic cyclization of polyprenoids. J. Am. Chem. Soc. 1999, 121, 4906–5001. [Google Scholar] [CrossRef]
- Quiroga, J.; Trilleras, J.; Abonia, R.; Insuasty, B.; Nogueras, M.; Cobo, J.; de la Torre, J.M. 4-Aminopyrimidine-5-carbaldehydes as intermediates in a Friedländer type synthesis of 7-arylpyrido[2,3-d]pyrimidines. ARKIVOC 2009, 14, 9–27. [Google Scholar]
- Groundwater, P.W.; Munawar, M.A. Synthesis of pyrido[2,3-c]acridines. J. Chem. Soc. Perkin Trans.1 1997, 22, 3381–3386. [Google Scholar] [CrossRef]
- Tabarrini, O.; Cecchetti, V.; Temperini, A.; Filipponi, E.; Lamperti, M.G.; Fravolini, A. Synthesis and application of a novel, crystalline phosphoramidite monomer with thiol terminus, suitable for the synthesis of DNA conjugates. Bioorg. Med. Chem. 2001, 9, 2921–2929. [Google Scholar] [CrossRef] [PubMed]
- Munawar, M.A.; Groundwater, P.W. Synthesis of thieno[2,3-c]acridines. J Chem. Soc. Pak. 2004, 26, 264–269. [Google Scholar]
- Khalilzadeh, M.A.; Hosseini, A.; Tajbakhsh, M. Synthesis of tacrine derivatives under solventless conditions. J. Heterocycl. Chem. 2007, 44, 535–542. [Google Scholar] [CrossRef]
- Marco, J.L.; De los Rios, C.; Carreiras, M.C.; Banos, J.E.; Badia, A.; Vivas, N.M. Synthesis and acetylcholinesterase/butyrylcholinesterase inhibition activity of new tacrine-like analogues. Bioorg. Med. Chem. 2001, 9, 727–738. [Google Scholar] [CrossRef] [PubMed]
- Marco, J.L.; De los Rios, C.; Garcia, A.G.; Villarroya, M.; Carreiras, M.C.; Martins, C.; Eleuterio, A.; Morreale, A.; Orozco, M.; Luque, F. Synthesis, biological evaluation and molecular modelling of diversely functionalized heterocyclic derivatives as inhibitors of acetylcholinesterase/butyrylcholinesterase and modulators of Ca2+ channels and nicotinic receptors. Bioorg. Med. Chem. 2004, 12, 2199–2209. [Google Scholar] [CrossRef] [PubMed]
- Leon, R.; Contelles, J.; Garcia, A.G.; Villarroya, M. Synthesis, acetylcholinesterase inhibition and neuroprotective activity of new tacrine analogues. Bioorg. Med. Chem. 2005, 13, 1167–1176. [Google Scholar] [CrossRef] [PubMed]
- Barreiro, E.J.; Camara, C.A.; Verli, H.; Brazil-Mas, L.; Castro, N.G.; Cintra, W.M.; Aracava, Y.; Rodrigues, C.R.; Fraga, C.A.M. Design, synthesis, and pharmacological profile of novel fused pyrazolo[4,3-d]pyridine and pyrazolo[3,4-b][1,8]naphthyridine isosteres: A new class of potent and selective acetylcholinesterase inhibitors. J. Med. Chem. 2003, 46, 1144–1149. [Google Scholar] [CrossRef] [PubMed]
- Luo, F.T.; Ravi, V.K.; Xue, C.H. The novel reaction of ketones with o-oxazoline-substituted anilines. Tetrahedron 2006, 62, 9365–9371. [Google Scholar] [CrossRef]
- Lawen, A. Apoptosis—An introduction. Bioessays 2003, 25, 888–896. [Google Scholar] [CrossRef] [PubMed]
- Mathew, B.; Nath, M. Recent approaches to antifungal therapy for invasive mycoses. ChemMedChem 2009, 4, 310–323. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Playford, E.; Sorrell, T. Antifungal therapy in invasive fungal infections. Curr. Opin. Pharmacol. 2010, 10, 522–530. [Google Scholar] [CrossRef] [PubMed]
- Brown, E.; Wright, G. New targets and screening approaches in antimicrobial drug discovery. Chem. Rev. 2005, 105, 759–774. [Google Scholar] [CrossRef] [PubMed]
- Vicente, M.F.; Basilio, A.; Cabello, A.; Peláez, F. Microbial natural products as a source of antifungals. Clin. Microbiol. Infect. 2003, 9, 15–32. [Google Scholar] [CrossRef] [PubMed]
- Park, B.; Wannemuehler, K.; Marston, B.; Govender, N.; Pappas, P. Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. Aids 2009, 23, 525–530. [Google Scholar] [CrossRef] [PubMed]
- Butts, A.; Krysan, D. Antifungal drug discovery: Something old and something new. PLoS Pathog. 2012, 8, e1002870. [Google Scholar] [CrossRef] [PubMed]
- Tang, I.; Li, J.; Zhang, L.; Ma, S.; Shi, D.; Zhang, O.; Yang, L.; Wang, X.; Liu, X.; Liu, C. The divergent transformations of aromatic o-aminonitrile with carbonyl compound. J. Heterocycl. Chem. 2012, 49, 533–542. [Google Scholar] [CrossRef]
- Silva, D.; Chioua, M.; Samadi, A.; Carreiras, M.; Jimeno, M.; Mendes, E.; De los Ríos, C.; Romero, A.; Villarroya, M.; López Contellesb, M.J. Synthesis and pharmacological assessment of diversely substituted pyrazolo[3,4-b]quinoline, and benzo[b]pyrazolo[4,3-g][1,8]naphthyridine derivatives. Eur. J. Med. Chem. 2011, 46, 4676–4681. [Google Scholar] [CrossRef] [PubMed]
- Pfaller, M.A.; Diekema, D.J. Epidemiology of invasive candidiasis: A persistent public health problem. Clin. Microbiol. Rev. 2007, 20, 133–163. [Google Scholar] [CrossRef] [PubMed]
- Trpkovic, M.; Pekmezovic, A.; Barac, L.; Crncevic, R.; Arsic Arsenijevic, V. In vitro antifungal activities of amphotericin B, 5-fluorocytosine, fluconazole and itraconazole against Cryptococcus neoformans isolated from cerebrospinal fluid and blood from patients in Serbia. J. Mycol. Med. 2012, 22, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute (CLSI). Reference Method for Broth Dilution Antifungal Susceptibility Testing for YeastsCLSI Document M27A3, Approved Standard—Third Edition; CLSI: Wayne, PA, USA, 2008; Volume 28, pp. 1–25. [Google Scholar]
- Ernst, E.J.; Roling, E.E.; Petzold, R.; Keele, D.J.; Klepser, M.E. In vitro activity of micafungin (fk-463) against Candida spp.: Microdilution, time-kill, and postantifungal-effect studies. Antimicrob. Agents Chemother. 2002, 46, 3846–3853. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Dong, Y.; Wang, L.; Han, S. Acute toxicity of substituted phenols to Rana japonica tadpoles and mechanism-based quantitative structure-activity relationship (QSAR) study. Chemosphere 2001, 44, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Leal, P.C.; Mascarello, A.; Derita, M.; Zuljan, F.; Nunes, R.J.; Zacchino, S.; Yunes, R.A. Relation between lipophilicity of alkyl gallates and antifungal activity against yeasts and filamentous fungi. Bioorg. Med. Chem. Lett. 2009, 19, 1793–1796. [Google Scholar] [CrossRef] [PubMed]
- C.S. Chem, Office; Version 9.0; Cambridge Soft Corporation: 100 Cambridge Park Drive, Cambridge, MA, USA, 2005.
- Boyd, M.R.; Paull, K.D. Some practical considerations and applications of the National Cancer Institute in vitro anticancer drug discovery screen. Drug Dev. Res. 1995, 34, 91–109. [Google Scholar] [CrossRef]
- Hubbard, W.C.; Alley, M.C.; Gray, G.N.; Green, K.C.; McLemore, T.L.; Boyd, M.R. Evidence for prostanoid biosynthesis as a biochemical feature of certain subclasses of non-small cell carcinomas of the lung as determined in established cell lines derived from human lung tumors. Cancer Res. 1989, 49, 826–832. [Google Scholar] [PubMed]
- Monks, A.; Scudiero, D.; Skehan, P.; Shoemaker, R.; Paull, K.; Vistica, D.; Hose, C.; Langley, J.; Cronise, P.; Vaigro-Wolff, A.; et al. Feasibility of a heigh-flux anticancer drug screen using a diverse panel of cultured human tumor cell lines. J. Natl. Cancer Inst. 1991, 83, 757–766. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, J.N.; Myers, T.G.; O’Connor, P.M.; Friend, S.H.; Fornace, A.J.; Kohn, K.W.; Fojo, T.; Bates, S.E.; Rubinstein, L.V.; Anderson, N.L.; et al. An information-intensive approach to the molecular pharmacology of cancer. Science 1997, 275, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Grever, M.R.; Sehepartz, S.A.; Chabners, B.A. The national cancer institute: Cancer drug discovery and development program. Semin. Oncol. 1992, 19, 622–638. [Google Scholar] [PubMed]
- Collins, J.M. Developmental Therapeutics Program NCI/NIH. Available online: http://dtp.cancer.gov/branches/btb/ivclsp.html (accessed on 12 March 2015).
- Sample Availability: Samples of the compounds 3a–i are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Acosta, P.; Butassi, E.; Insuasty, B.; Ortiz, A.; Abonia, R.; Zacchino, S.A.; Quiroga, J. Microwave-Assisted Synthesis of Novel Pyrazolo[3,4-g][1,8]naphthyridin-5-amine with Potential Antifungal and Antitumor Activity. Molecules 2015, 20, 8499-8520. https://doi.org/10.3390/molecules20058499
Acosta P, Butassi E, Insuasty B, Ortiz A, Abonia R, Zacchino SA, Quiroga J. Microwave-Assisted Synthesis of Novel Pyrazolo[3,4-g][1,8]naphthyridin-5-amine with Potential Antifungal and Antitumor Activity. Molecules. 2015; 20(5):8499-8520. https://doi.org/10.3390/molecules20058499
Chicago/Turabian StyleAcosta, Paola, Estefanía Butassi, Braulio Insuasty, Alejandro Ortiz, Rodrigo Abonia, Susana A. Zacchino, and Jairo Quiroga. 2015. "Microwave-Assisted Synthesis of Novel Pyrazolo[3,4-g][1,8]naphthyridin-5-amine with Potential Antifungal and Antitumor Activity" Molecules 20, no. 5: 8499-8520. https://doi.org/10.3390/molecules20058499








