A Facile and Mild Synthesis of Trisubstituted Allylic Sulfones from Morita-Baylis-Hillman Carbonates
Abstract
:1. Introduction
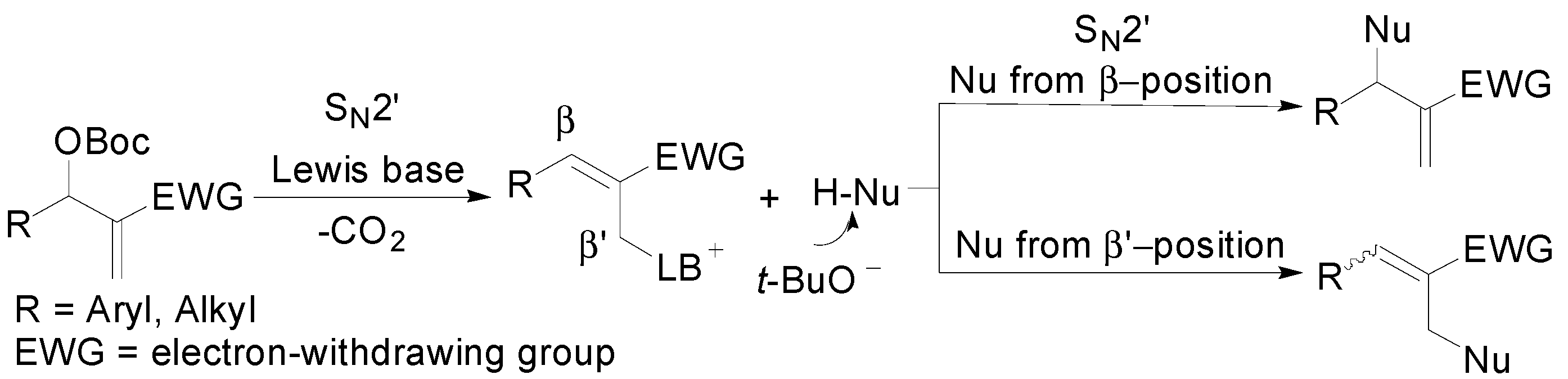
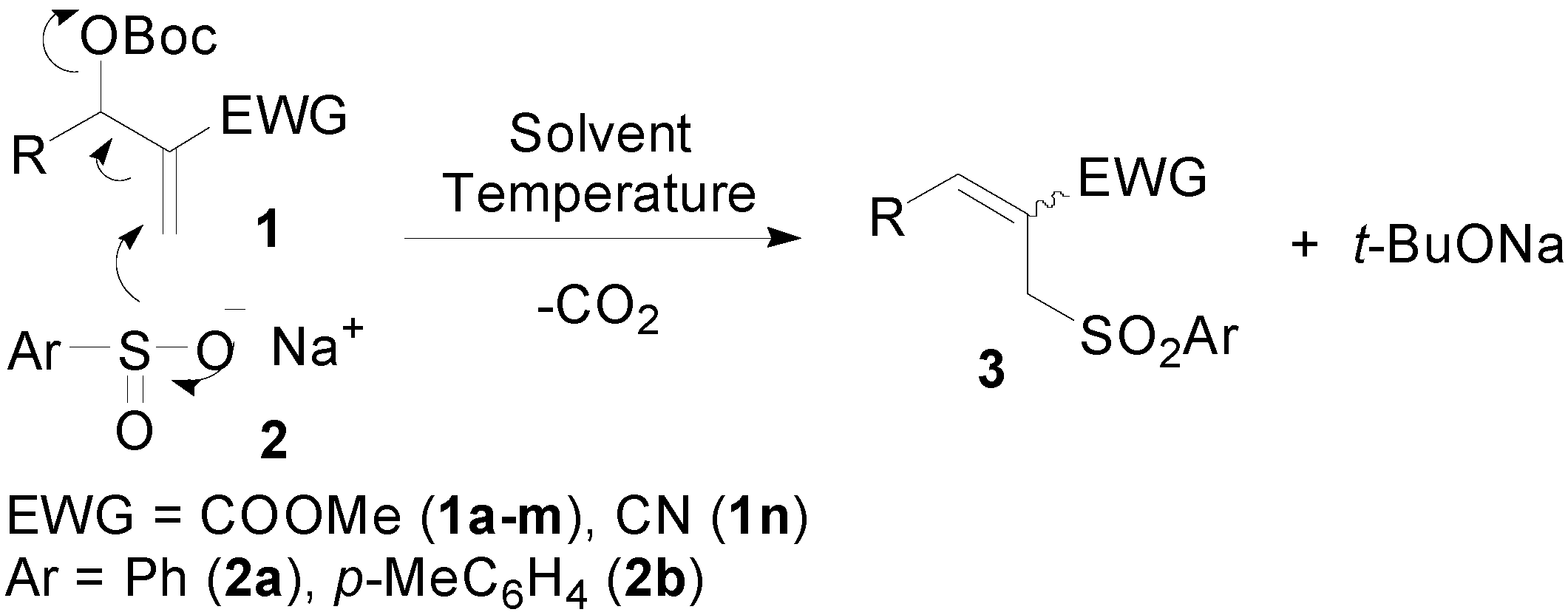
2. Results and Discussion
2.1. Optimization Studies
| Entry | Solvent | T (°C) | Time (h) | Yield (%) b,c |
|---|---|---|---|---|
| 1 | toluene | 25 | 36 | 37 |
| 2 | CHCl3 | 25 | 48 | 17 |
| 3 | PhCF3 | 25 | 72 | trace |
| 4 | DCE | 25 | 36 | 82 |
| 5 | THF | 25 | 36 | 87 |
| 6 | MeCN | 25 | 36 | 92 |
| 7 | MeCN | 40 | 2 | 96 d |
| 8 e | MeCN | 40 | 5 | 96 |
2.2. Synthesis of Trisubstituted Allylic Sulfones 3a–o
| Entry | R | EWG | Ar | Yield (%) b | Z/E c,d |
|---|---|---|---|---|---|
| 1 | Ph (1a) | CO2Me | Ph | 96 (3a) | 96:4 |
| 2 | o-ClC6H4 (1b) | CO2Me | Ph | 88 (3b) | 95:5 |
| 3 | p-ClC6H4 (1c) | CO2Me | Ph | 96 (3c) | 85:15 |
| 4 | p-NO2C6H4 (1d) | CO2Me | Ph | 83 (3d) | 79:21 |
| 5 | m-BrC6H4 (1e) | CO2Me | Ph | 93 (3e) | 94:6 |
| 6 | p-MeOC6H4 (1f) | CO2Me | Ph | 78 (3f) | 88:12 |
| 7 |  (1g) (1g) | CO2Me | Ph | 98 (3g) | 90:10 |
| 8 | m-MeC6H4 (1h) | CO2Me | Ph | 96 (3h) | 88:12 |
| 9 | p-MeC6H4 (1i) | CO2Me | Ph | 92 (3i) | 96:4 |
| 10 | 1-naphthyl (1j) | CO2Me | Ph | 71 (3j) | 96:4 |
| 11 | 2-furyl (1k) | CO2Me | Ph | 85 (3k) | >99:1 |
| 12 | 2-thienyl (1l) | CO2Me | Ph | 96 (3l) | 81:19 |
| 13 | n-propyl (1m) | CO2Me | Ph | 91 (3m) | 82:18 |
| 14 | Ph (1n) | CN | Ph | 99 (3n) | <1:99 |
| 15 | Ph (1a) | CO2Me | p-MeC6H4 | 95 (3o) | 84:16 |
3. Experimental Section
3.1. General Information
3.2. General Procedure for Preparation of Trisubstituted Allylic Sulfones 3a–o
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References and Notes
- Basavaiah, D.; Reddy, B.S.; Badsara, S.S. Recent contributions from the Baylis-Hillman reaction to organic chemistry. Chem. Rev. 2010, 110, 5447–5674. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.-N.; Jiang, J.-J.; Shi, M.; Wei, Y. Recent extensions of the Morita-Baylis-Hillman reaction. Chem. Commun. 2009, 5496–5514. [Google Scholar] [CrossRef]
- Declerck, V.; Martinez, J.; Lamaty, F. Aza-Baylis-Hillman reaction. Chem. Rev. 2009, 109, 1–48. [Google Scholar] [CrossRef]
- Gowrisankar, S.; Lee, H.S.; Kim, S.H.; Lee, K.Y.; Kim, J.N. Recent advances in the Pd-catalyzed chemical transformations of Baylis-Hillman adducts. Tetrahedron 2009, 65, 8769–8780. [Google Scholar] [CrossRef]
- Wei, Y.; Shi, M. Multifunctional chiral phosphine organocatalysts in catalytic asymmetric Morita-Baylis-Hillman and related reactions. Acc. Chem. Res. 2010, 43, 1005–1018. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Han, X.; Lu, X. Alkaloids-catalyzed regio- and enantioselective allylic nucleophilic substitution of tert-butyl carbonate of the Morita-Baylis-Hillman products. Tetrahedron Lett. 2004, 45, 4967–4971. [Google Scholar] [CrossRef]
- Liu, T.-Y.; Xie, M.; Chen, Y.-C. Organocatalytic asymmetric transformations of modied Morita-Baylis-Hillman adducts. Chem. Soc. Rev. 2012, 41, 4101–4112. [Google Scholar] [CrossRef] [PubMed]
- Rios, R. Organocatalytic enantioselective methodologies using Morita-Baylis-Hillman carbonates and acetates. Catal. Sci. Technol. 2012, 2, 267–278. [Google Scholar] [CrossRef]
- Wei, Y.; Shi, M. Recent advances in organocatalytic asymmetric Morita-Baylis-Hillman/aza-Morita-Baylis-Hillman reactions. Chem. Rev. 2013, 113, 6659–6690. [Google Scholar] [CrossRef] [PubMed]
- Zhan, G.; Zhou, Q.-Q.; Du, W.; Chen, Y.-C. Enantioselective N-allylic alkylation of N-propargylsulfonamides with Morita-Baylis-Hillman carbonates and sequential electrophilic cyclization. Synthesis 2014, 46, 3383–3393. [Google Scholar] [CrossRef]
- Yang, H.-B.; Zhao, Y.-Z.; Sang, R.; Shi, M. (DHQ)2AQN-Catalyzed asymmetric substitution of isatin-derived hydrazones with O-BoC-protected Morita-Baylis-Hillman adducts: A strategy for synthesizing enantioenriched Azo compounds incorporating an oxindole scaffold. J. Org. Chem. 2014, 79, 3519–3528. [Google Scholar] [CrossRef] [PubMed]
- Companyó, X.; Geant, P.-Y.; Mazzanti, A.; Moyano, A.; Rios, R. Catalytic asymmetric one-pot synthesis of α-methylene-γ-lactams. Tetrahedron 2014, 70, 75–82. [Google Scholar] [CrossRef]
- Yan, Y.; En, D.; Zhuang, Z.; Guo, Y.; Liao, W.-W. Synthesis of densely functionalized α-methylene γ-butyrolactones via an organ Catalytic one-pot allylic-alkylation-cyclization reaction. Tetrahedron Lett. 2014, 55, 479–482. [Google Scholar] [CrossRef]
- Mao, H.; Lin, A.; Shi, Y.; Mao, Z.; Zhu, X.; Li, W.; Hu, H.; Cheng, Y.; Zhu, C. Construction of enantiomerically enriched diazo compounds using diazo esters as nucleophiles: Chiral Lewis base catalysis. Angew. Chem. Int. Ed. 2013, 52, 6288–6292. [Google Scholar] [CrossRef]
- Wang, Q.-G.; Zhou, Q.-Q.; Deng, J.-G.; Chen, Y.-C. An asymmetric allylic alkylation-smiles rearrangement sulfinate addition sequence to construct chiral cyclic sulfones. Org. Lett. 2013, 15, 4786–4789. [Google Scholar] [CrossRef] [PubMed]
- Companyó, X.; Mazzanti, A.; Moyano, A.; Janecka, A.; Rios, R. First one-pot organocatalytic synthesis of α-methylene-γ-lactones. Chem. Commun. 2013, 49, 1184–1186. [Google Scholar] [CrossRef]
- Drewes, S.E.; Roos, G.H.P. Synthetic potential of the tertiary-amine-catalysed reaction of activated vinyl carbanions with aldehydes. Tetrahedron 1988, 44, 4653–4670. [Google Scholar] [CrossRef]
- Basavaiah, D.; Rao, P.D.; Hyma, R.S. The Baylis-Hillman reaction: A novel carbon-carbon bond forming reaction. Tetrahedron 1996, 52, 8001–8062. [Google Scholar] [CrossRef]
- Li, G.; Wei, H.-X.; Gao, J.J.; Caputo, T.D. TiCl4-Mediated Baylis-Hillman and aldol reactions without the direct use of a Lewis base. Tetrahedron Lett. 2000, 41, 1–5. [Google Scholar] [CrossRef]
- Basavaiah, D.; Pandiaraju, S.; Padmaja, K. The Friedel-Crafts chemistry: Acetates of the Baylis-Hillman adducts as novel stereodefined β-electrophiles. Synlett 1996, 393–395. [Google Scholar] [CrossRef]
- Quiclet-Sire, B.; Seguin, S.; Zard, S.Z. A new radical allylation reaction of dithiocarbonates. Angew. Chem. Int. Ed. 1998, 37, 2864–2866. [Google Scholar] [CrossRef]
- Kim, S.; Lim, C.J. Tin-free radical-mediated C-C-bond formations with alkyl allyl sulfones as radical precursors. Angew. Chem. Int. Ed. 2002, 41, 3265–3267. [Google Scholar] [CrossRef]
- Wróbel, Z. Silane-mediated direct condensation of nitroarenes with cinnamyl-type sulfones. The way to 2-aryl-4-X-quinolines and their hetero analogs. Tetrahedron 1998, 54, 2607–2618. [Google Scholar] [CrossRef]
- Jiang, L.; Lei, Q.; Huang, X.; Cui, H.-L.; Zhou, X.; Chen, Y.-C. Lewis base assisted Brønsted base catalysis: Direct regioselective asymmetric vinylogous alkylation of allylic sulfones. Chem. Eur. J. 2011, 17, 9489–9493. [Google Scholar] [CrossRef] [PubMed]
- Neamati, N.; Kabalka, G.W.; Venkataiah, B.; Dayam, R. Small Molecules for Treating Cancer and Abnormal Cell Proliferation Disorders. U.S. Patent WO 2,007,081,966 A3, 9 January 2007. [Google Scholar]
- Wang, Q.; Sheng, S.-R.; Lin, S.-Y.; Guo, L.; Wei, M.-H.; Huang, X. Liquid-phase synthesis of methyl (2Z)-2-arylsulfonylmethyl-2-alkenoates from PEG-supported α-phenylselenopropionate. Chin. J. Chem. 2007, 25, 1027–1030. [Google Scholar] [CrossRef]
- Karnakar, K.; Shankar, J.; Murthy, S.N.; Nageswar, Y.V.D. Synthesis of allyl aryl sulfone derivatives from Baylis-Hillman acetates in water. Helv. Chim. Acta 2011, 94, 875–880. [Google Scholar] [CrossRef]
- Kabalka, G.W.; Venkataiah, B.; Dong, G. Preparation of substituted allyl acetates and sulfones from Baylis-Hillman adducts in ionic liquid media. Tetrahedron Lett. 2003, 44, 4673–4675. [Google Scholar] [CrossRef]
- Chandrasekhar, S.; Saritha, B.; Jagadeshwer, V.; Narsihmulu, C.; Vijay, D.; Sarma, G.D.; Jagadeesh, B. Hydroxy-assisted catalyst-free Michael addition-dehydroxylation of Baylis-Hillman adducts in poly(ethylene glycol). Tetrahedron Lett. 2006, 47, 2981–2984. [Google Scholar] [CrossRef]
- Srivastava, V.P.; Yadav, L.D.S. Direct sulfonylation of Baylis-Hillman alcohols and diarylmethanols with TosMIC in ionic liquid-[Hmim]HSO4: An unexpected reaction. Tetrahedron Lett. 2011, 52, 4622–4626. [Google Scholar] [CrossRef]
- Reddy, L.R.; Hu, B.; Prashad, M.; Prasad, K. An unexpected reaction of arenesulfonyl cyanides with allylic alcohols: Preparation of trisubstituted allyl sulfones. Angew. Chem. Int. Ed. 2009, 48, 172–174. [Google Scholar] [CrossRef]
- Li, H.-H.; Dong, D.-J.; Jin, Y.-H.; Tian, S.-K. An expeditious entry to benzylic and allylic sulfones through byproduct-catalyzed reaction of alcohols with sulfinyl chlorides. J. Org. Chem. 2009, 74, 9501–9504. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xu, X.; Tang, Y. Tetrabutylammonium iodide catalyzed allylic sulfonylation of Baylis-Hillman acetates with sulfonylhydrazides in water. Org. Biomol. Chem. 2013, 11, 1739–1742. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-R.; Li, M.-B.; Cheng, D.-J.; Yang, C.-F.; Tian, S.-K. Catalyst-free alkylation of sulfinic acids with sulfonamides via sp3 C-N bond cleavage at room temperature. Org. Lett. 2009, 11, 2543–2545. [Google Scholar] [CrossRef] [PubMed]
- The NMR characteristic data of 3a are consistent with that of (Z)-methyl 3-phenyl-2-((phenylsulfonyl)methyl)acrylate, which were reported in the ESI section of Ref. [33].
- Feng, J.; Lu, X.; Kong, A.; Han, X. A highly regio- and stereo-selective [3+2] annulation of allylic compounds and 2-substituted 1,1-dicyanoalkenes through a catalytic carbon-phosphorus ylide reaction. Tetrahedron 2007, 63, 6035–6041. [Google Scholar] [CrossRef]
- Samples Availability: Samples of the compounds 3a–o are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, L.; Li, Y.-G.; Zhou, J.-F.; Chuan, Y.-M.; Li, H.-L.; Yuan, M.-L. A Facile and Mild Synthesis of Trisubstituted Allylic Sulfones from Morita-Baylis-Hillman Carbonates. Molecules 2015, 20, 8213-8222. https://doi.org/10.3390/molecules20058213
Jiang L, Li Y-G, Zhou J-F, Chuan Y-M, Li H-L, Yuan M-L. A Facile and Mild Synthesis of Trisubstituted Allylic Sulfones from Morita-Baylis-Hillman Carbonates. Molecules. 2015; 20(5):8213-8222. https://doi.org/10.3390/molecules20058213
Chicago/Turabian StyleJiang, Lin, Yong-Gen Li, Jiang-Feng Zhou, Yong-Ming Chuan, Hong-Li Li, and Ming-Long Yuan. 2015. "A Facile and Mild Synthesis of Trisubstituted Allylic Sulfones from Morita-Baylis-Hillman Carbonates" Molecules 20, no. 5: 8213-8222. https://doi.org/10.3390/molecules20058213






