Predicting the Uncertain Future of Aptamer-Based Diagnostics and Therapeutics
Abstract
:1. Introduction
| Advantage | References |
|---|---|
| Facile in vitro development which obviates host animals. | [7] |
| Ability to develop aptamers against native toxins without toxoid production. | [7] |
| Greater reproducibility of aptamers from batch-to-batch due to chemical synthesis | [7] |
| More rapid ability to develop neutralizing agents by robotic means against multi-drug resistant “doomsday bug” bacteria or emerging lethal viruses (e.g., Ebola, influenzas, MERS, SARS, etc.) for prophylaxis or a “bridge to life” prior to host-mounted immune response (i.e., prior to seroconversion). | [8,9,10,11,12] |
| Ability to develop aptamers onboard spacecraft, other planets, or in remote locations on Earth where neutralizing aptamers may be needed. | [13,14,15] |
| Unlimited inexpensive production of DNA aptamers at the gram or greater scale by PCR or asymmetric PCR (predominately single-stranded PCR products). | [16,17] |
| Ability to store lyophilized aptamers indefinitely and obviate cold storage. | [18] |
| Reusability; aptamers can be heat-denatured, cooled to reconform and used for many rounds of analyte binding and detection. | [19,20,21] |
| Little or no immunogenicity.* Even humanized mAbs can be immunogenic. | [22,23,24] |
2. New Diagnostic Aptamer Reagent Innovations and Marketing Strategies
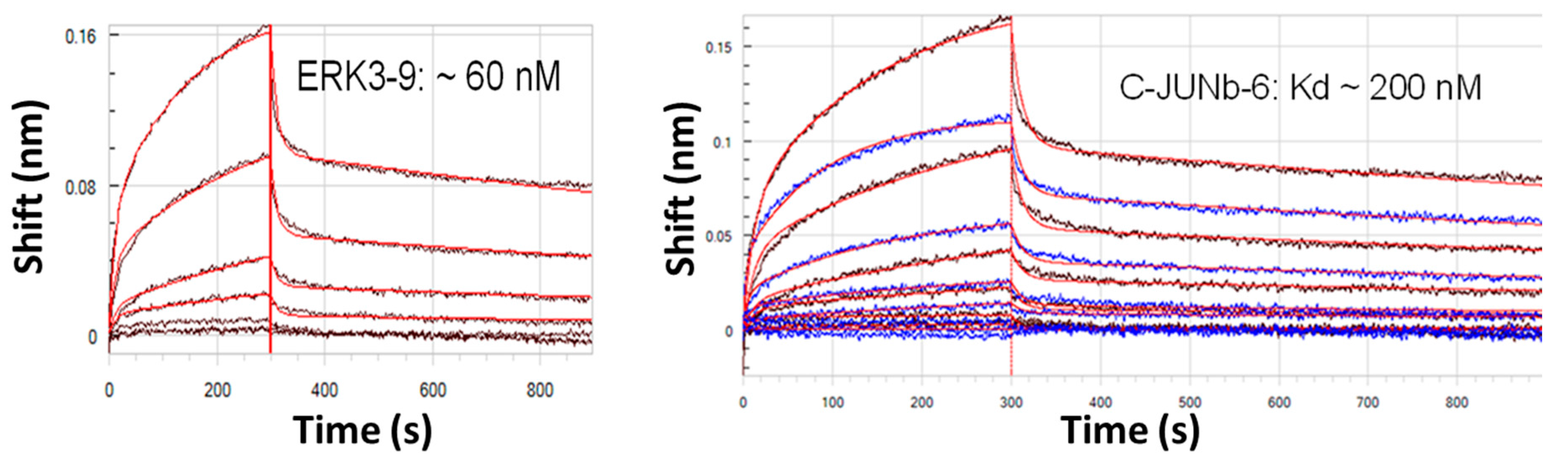
2.1. Longer Multivalent or Multidentate Aptamers
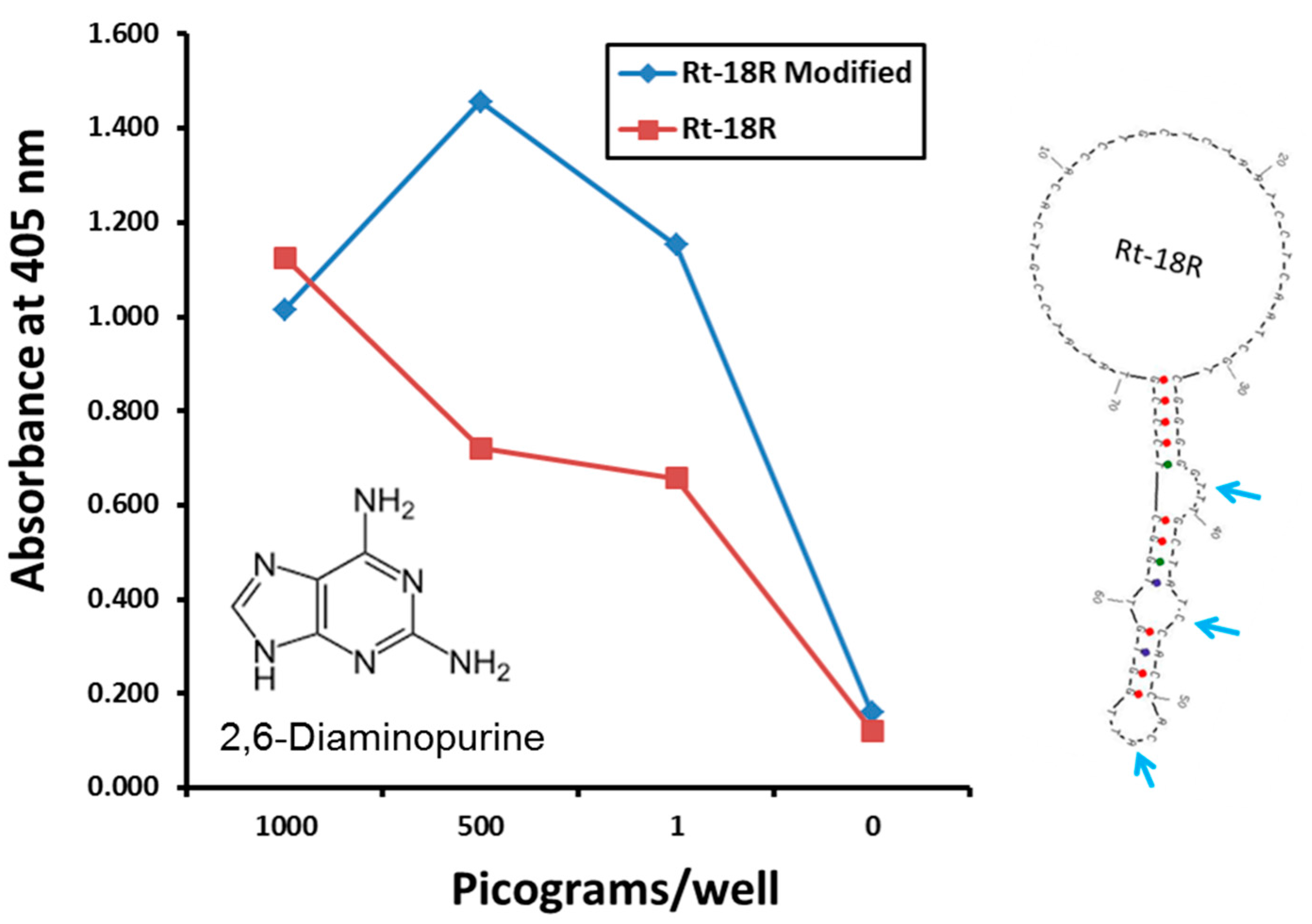
2.2. The Use of Exotic, Modified or Unnatural Bases
2.3. Modifications to Aptamer Backbone and Sugar Moieties
2.4. One Step and In Silico Aptamer Development
2.5. Environmental, Food Safety and Other Niche Market Aptamer Assays
3. New Therapeutic Innovations and Applications
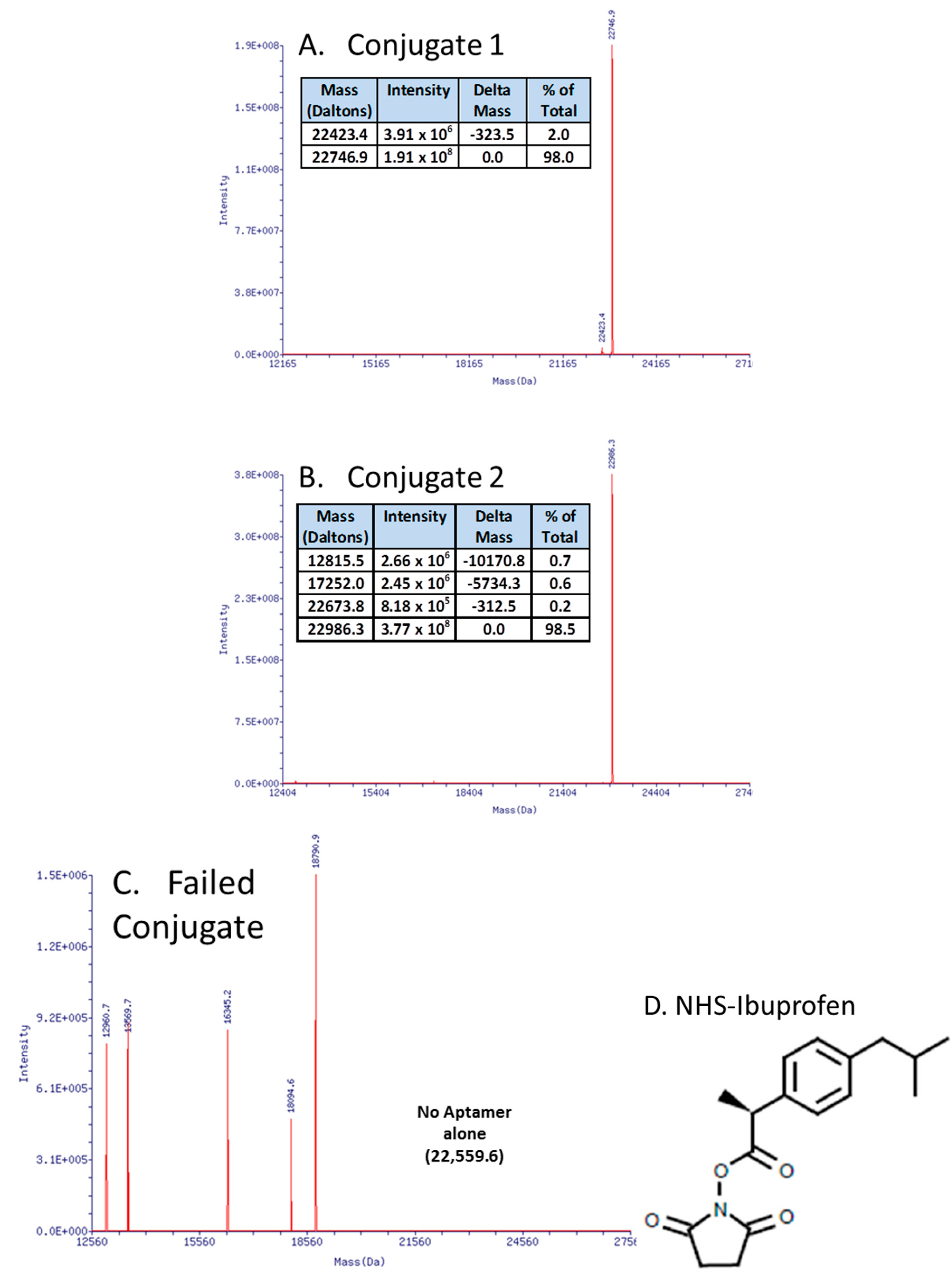
3.1. Update on Aptamer Conjugates
3.2. Aptamers to Neutralize Lethal Viruses
3.3. Aptamers or Aptamer Conjugates to Kill Drug-Resistant Pathogenic Bacteria
3.4. Aptamers to Neutralize Toxins and Venoms
3.5. Robotic Aptamer Development and Production for Rapid Medical Countermeasures
3.6. Aptamers to Potentially Induce Stem Cell Differentiation and Transdifferentiation
3.7. Aptamers to Induce Rapid Blood Clotting
3.8. Mass Production Methods
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Penner, G. Commercialization of an aptamer-based diagnostic test. IVD Technol. 2012, 18, 31–37. [Google Scholar]
- Webber, J.; Stone, T.C.; Katilius, E.; Smith, B.C.; Gordon, B.; Mason, M.D.; Tabi, Z.; Brewis, I.A.; Clayton, A. Proteomics analysis of cancer exosomes using a novel modified aptamer-based array (SOMAscan™) platform. Mol. Cell. Proteomics 2014, 13, 1050–1064. [Google Scholar]
- Lollo, B.; Steele, F.; Gold, L. Beyond antibodies: New affinity reagents to unlock the proteome. Proteomics 2014, 14, 638–644. [Google Scholar]
- Vance, S.A.; Sandros, M.G. Zeptomole detection of C-reactive protein in serum by a nanoparticle amplified surface plasmon resonance imaging aptasensor. Sci. Rep. 2014, 4, 5129. [Google Scholar]
- Bruno, J.G.; Richarte, A.M.; Phillips, T. Preliminary development of a DNA aptamer-magnetic bead capture electrochemiluminescence sandwich assay for Brain Natriuretic Peptide. Microchem. J. 2014, 115, 32–38. [Google Scholar]
- Bruno, J.G. A review of therapeutic aptamer conjugates with emphasis on new approaches. Pharmaceuticals 2013, 6, 340–357. [Google Scholar]
- Jayasena, S.D. Aptamers: An emerging class of molecules that rival antibodies in diagnostics. Clin. Chem. 1999, 45, 1628–1650. [Google Scholar]
- Lee, J.F.; Cox, J.C.; Collett, J.R.; Ellington, A.D. Exploring sequence space through automated aptamer selection. J. Lab. Autom. 2005, 10, 213–218. [Google Scholar]
- Wochner, A.; Cech, B.; Menger, M.; Erdmann, V.A.; Glökler, J. Semi-automated selection of DNA aptamers using magnetic particle handling. BioTechniques 2007, 43, 344–353. [Google Scholar]
- Lai, H.; Wang, C.; Weng, C.; Liou, T.; Lee, G. An integrated SELEX microfluidic system for rapid screening of influenza virus specific aptamers. In Proceedings of the 16th International Conference on Miniaturized Systems for Chemistry and Life Sciences, Okinawa, Japan, 28 October–1 November 2012; pp. 1402–1404.
- Cheng, C.; Dong, J.; Yao, L.; Chen, A.; Jia, R.; Huan, L.; Guo, J.; Shu, Y.; Zhang, Z. Potent inhibition of human influenza H5N1 virus by oligonucleotides derived by SELEX. Biochem. Biophys. Res. Commun. 2008, 366, 670–674. [Google Scholar]
- Binning, J.M.; Wang, T.; Luthra, P.; Shabman, R.S.; Borek, D.M.; Liu, G.; Xu, W.; Leung, D.W.; Basler, C.F.; Amarasinghe, G.K. Development of RNA aptamers targeting Ebola virus VP35. Biochemistry 2013, 52, 8406–8419. [Google Scholar]
- Dobler, R.K.; Maki, W.C. Mars health care delivery systems: Aptamers provide critical technology. In Proceedings of the 12th NASA Symposium of VLSA Design, Coeur d'Alene, ID, USA, 4–5 October 2005.
- Schmidt, M.A.; Goodwin, T.J. Personalized medicine in human space flight: Using omics based analyses to develop individualized countermeasures that enhance astronaut safety and performance. Metabolomics 2013, 9, 1134–1156. [Google Scholar]
- Sommer, G.J.; Hecht, A.H.; Durland, R.H.; Yang, X.; Singh, A.K.; Hatch, A.V. A fully automated aptamer-based affinity assay platform for monitoring astronaut health in space. In Proceedings of the 14th International Conference on Miniaturized Systems in the Life Sciences, Groningen, The Netherlands, 3–7 October 2010; pp. 1463–1465.
- Murray, E.; Norton, M.L.; Towler, W.I. Method for a continuous rapid thermal cycle system. U.S. Patent 8,163,489, 24 April 2012. [Google Scholar]
- Dutton, G. DNA vaccines inch toward human use. Gen. Eng. Biotechnol. News 2009, 29, 1–4. [Google Scholar]
- Quaak, S.G.; Haanen, J.B.; Beijnen, J.H.; Nuijen, B. Naked plasmid DNA formulation: Effect of different disaccharides on stability after lyophilisation. AAPS PharmSciTech 2010, 11, 344–350. [Google Scholar]
- Park, J.H.; Byun, J.Y.; Mun, H.; Shim, W.B.; Shin, Y.B.; Li, T.; Kim, M.G. A regeneratable, label-free, localized surface plasmon resonance (LSPR) aptasensor for the detection of ochratoxin A. Biosens. Bioelectron. 2014, 59, 321–327. [Google Scholar]
- Shen, T.; Yue, Q.; Jiang, X.; Wang, L.; Xu, S.; Li, H.; Gu, X.; Zhang, S.; Liu, J. A reusable and sensitive biosensor for total mercury in canned fish based on fluorescence polarization. Talanta 2013, 117, 81–86. [Google Scholar]
- Xu, S.; Zhang, X.; Liu, W.; Sun, Y.; Zhang, H. Reusable light-emitting-diode induced chemiluminescence aptasensor for highly sensitive and selective detection of riboflavin. Biosens. Bioelectron. 2013, 43, 160–164. [Google Scholar]
- Cload, S.T.; McCauley, T.G.; Keefe, A.D.; Healy, J.M.; Wilson, C. Chapter 17: Properties of therapeutic aptamers. In The Aptamer Handbook; Klussmann, S., Ed.; Wiley-VCH, Verlag GmBH & Co.: Weinheim, Germany, 2006; pp. 363–416. [Google Scholar]
- Avci-Adali, M.; Steinle, H.; Michel, T.; Schlensak, C.; Wendel, H.P. Potential capacity of aptamers to trigger immune activation in human blood. PLoS ONE 2013, 8, e68810. [Google Scholar]
- Harding, F.A.; Stickler, M.M.; Razo, J.; DuBridge, R.B. The immunogenicity of humanized and fully human antibodies: Residual immunogenicity resides in the CDR regions. MAbs 2010, 2, 256–265. [Google Scholar]
- Barhona, F.; Bardliving, C.; Phifer, A.; Bruno, J.G.; Batt, C. New aptasensor based on polymer-gold nanoparticles composite microspheres for the detection of malathion using surface-enhanced Raman spectroscopy. Ind. Biotechnol. 2013, 9, 42–49. [Google Scholar]
- Najafabadi, M.E.; Khayamian, T.; Hashemian, Z. Aptamer-conjugated magnetic nanoparticles for extraction of adenosine from urine followed by electrospray ion mobility spectrometry. J. Pharm. Biomed. Anal. 2015, 107, 244–250. [Google Scholar]
- Weiss, R.L. The long and winding regulatory road for laboratory-developed tests. Am. J. Clin. Pathol. 2012, 138, 20–26. [Google Scholar]
- Anonymous. FDA announces plans to regulate LDTs. Cancer Discov. 2014, 4, 1250. [Google Scholar]
- Mertz, A. Duplicative and unnecessary regulation of LDTs will hamper diagnostic innovation. Med. Lab. Opt. 2014, 46, 44. [Google Scholar]
- Shi, H.; Lis, J.T. Multivalent RNA Aptamers and Their Expression in Multicellular Organisms. U.S. Patent 6,458,559, 1 October 2002. [Google Scholar]
- Ahmad, K.M.; Xiao, Y.; Soh, H.T. Selection is more intelligent than design: Improving the affinity of a bivalent ligand through directed evolution. Nucleic Acids Res. 2012, 40, 11777–11783. [Google Scholar]
- Zhao, X.; Lis, J.T.; Shi, H. A systematic study of the features critical for designing a high avidity multivalent aptamer. Nucleic Acid Ther. 2013, 23, 238–242. [Google Scholar]
- Mallikaratchy, P.R.; Ruggiero, A.; Gardner, J.R.; Kuryavyi, V.; Maguire, W.F.; Heaney, M.L.; McDevitt, M.R.; Patel, D.J.; Scheinberg, D.A. A multivalent DNA aptamer specific for the B-cell receptor on human lymphoma and leukemia. Nucleic Acids Res. 2011, 39, 2458–2469. [Google Scholar]
- Stovall, G.M.; Bedenbaugh, R.S.; Singh, S.; Meyer, A.J.; Hatala, P.J.; Ellington, A.D.; Hall, B. In vitro selection using modified or unnatural nucleotides. Curr. Protoc. Nucleic Acid Chem. 2014, 56. [Google Scholar] [CrossRef]
- Sefah, K.; Yang, Z.; Bradley, K.M.; Hoshika, S.; Jiménez, E.; Zhang, L.; Zhu, G.; Shanker, S.; Yu, F.; Turek, D.; et al. In vitro selection with artificial expanded genetic information systems. Proc. Natl. Acad. Sci. USA 2014, 111, 1449–1454. [Google Scholar]
- Vallone, P.M.; Devaney, J.M.; Marino, M.A.; Butler, J.M. A strategy for examining complex mixtures of deoxyoligonucleotides using ion-pair-reverse-phase high-performance liquid chromatography, matrix-assisted laser desorption ionization time-of-flight mass spectrometry, and informatics. Anal. Biochem. 2002, 304, 257–265. [Google Scholar]
- He, W.; Elizondo-Riojas, M.A.; Li, X.; Lokesh, G.L.; Somasunderam, A.; Thiviyanathan, V.; Volk, D.E.; Durland, R.H.; Englehardt, J.; Cavasotto, C.N.; et al. X-aptamers: A bead-based selection method for random incorporation of druglike moieties onto next-generation aptamers for enhanced binding. Biochemistry 2012, 51, 8321–8323. [Google Scholar]
- Fujita, S.; Arinaga, K.; Fujihara, T.; Aki, M.; Kichise, T. Novel protein detection system using DNA as a constituent material. Fujitsu Sci. Tech. J. 2012, 48, 237–243. [Google Scholar]
- Shigdar, S.; MacDonald, J.; O’Connor, M.; Wang, T.; Xiang, D.; Al Shamaileh, H.; Qiao, L.; Wei, M.; Zhou, S.; Zhu, Y.; et al. Aptamers as theranostic agents: Modifications, serum stability and functionalisation. Sensors 2013, 13, 13624–13637. [Google Scholar]
- Pinheiro, V.B.; Taylor, A.I.; Cozens, C.; Abramov, M.; Renders, M.; Zhang, S.; Chaput, J.C.; Wengel, J.; Peak-Chew, S.Y.; McLaughlin, S.H.; et al. Synthetic genetic polymers capable of heredity and evolution. Science 2012, 336, 341–344. [Google Scholar]
- Taylor, A.I.; Arangundy-Franklin, S.; Holliger, P. Towards applications of synthetic genetic polymers in diagnosis and therapy. Curr. Opin. Chem. Biol. 2014, 22, 79–84. [Google Scholar]
- Pinheiro, V.B.; Holliger, P. Towards XNA nanotechnology: New materials from synthetic genetic polymers. Trends Biotechnol. 2014, 32, 321–328. [Google Scholar]
- Darmostuk, M.; Rimpelová, S.; Gbelcová, H.; Ruml, T. Current approaches in SELEX: An update to aptamer selection technology. Biotechnol. Adv. 2015, in press. [Google Scholar]
- Kong, H.Y.; Byun, J. Nucleic acid aptamers: New methods for selection, stabilization, and application in biomedical science. Biomol. Ther. (Seoul) 2013, 21, 423–434. [Google Scholar]
- Fan, M.; Roper-McBurnett, S.; Andrews, C.J.; Allman, A.M.; Bruno, J.G.; Kiel, J.L. Aptamer selection express (ASExp): A novel method for rapid single step selection and sensing of aptamers. J. Biomol. Tech. 2008, 19, 311–321. [Google Scholar]
- Nitsche, A.; Kurth, A.; Dunkhorst, A.; Pänke, O.; Sielaff, H.; Junge, W.; Muth, D.; Scheller, F.; Stöcklein, W.; Dahmen, C.; et al. One-step selection of Vaccinia virus-binding DNA aptamers by MonoLEX. BMC Biotechnol. 2007, 7, 48. [Google Scholar]
- Bruno, J.G.; Carrillo, M.P.; Phillips, T.; Hanson, D.; Bohmann, J.A. DNA aptamer beacon assay for C-telopeptide and handheld fluorometer to monitor bone resorption. J. Fluoresc. 2011, 21, 2021–2033. [Google Scholar]
- Chushak, Y.; Stone, M.O. In silico selection of RNA aptamers. Nucleic Acids Res. 2009, 37, e87. [Google Scholar]
- Ashrafuzzaman, M.; Tseng, C.Y.; Kapty, J.; Mercer, J.R.; Tuszynski, J.A. A computationally designed DNA aptamer template with specific binding to phosphatidylserine. Nucleic Acid Ther. 2013, 23, 418–426. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Luque, F.J.; Stich, M.; Manrubia, S.; Briones, C.; Berzal-Herranz, A. Efficient HIV-1 inhibition by a 16 nt-long RNA aptamer designed by combining in vitro selection and in silico optimisation strategies. Sci. Rep. 2014, 4, 6242–6251. [Google Scholar] [CrossRef] [PubMed]
- Savory, N.; Takahashi, Y.; Tsukakoshi, K.; Hasegawa, H.; Takase, M.; Abe, K.; Yoshida, W.; Ferri, S.; Kumazawa, S.; Sode, K.; et al. Simultaneous improvement of specificity and affinity of aptamers against Streptococcus mutans by in silico maturation for biosensor development. Biotechnol. Bioeng. 2014, 111, 454–461. [Google Scholar]
- Savory, N.; Lednor, D.; Tsukakoshi, K.; Abe, K.; Yoshida, W.; Ferri, S.; Jones, B.V.; Ikebukuro, K. In silico maturation of binding-specificity of DNA aptamers against Proteus mirabilis. Biotechnol. Bioeng. 2013, 110, 2573–2580. [Google Scholar] [CrossRef] [PubMed]
- Bruno, J.G.; Carrillo, M.P.; Phillips, T.; Vail, N.K.; Hanson, D. Competitive FRET-aptamer-based detection of methylphosphonic acid: A common nerve agent metabolite. J. Fluoresc. 2008, 18, 867–876. [Google Scholar] [CrossRef] [PubMed]
- Bruno, J.G.; Carrillo, M.P.; Cadieux, C.L.; Lenz, D.L.; Cerasoli, D.M.; Phillips, T. DNA aptamers developed against a soman derivative cross-react with methylphosphonic acid but not with the flanking hydrophobic groups. J. Mol. Recognit. 2009, 22, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Bruno, J.G.; Carrillo, M.P. Development of aptamer beacons for rapid presumptive detection of Bacillus spores. J. Fluoresc. 2012, 22, 915–924. [Google Scholar] [CrossRef] [PubMed]
- Bruno, J.G.; Richarte, A.M.; Carrillo, M.P.; Edge, A. An aptamer beacon responsive to botulinum toxins. Biosens. Bioelectron. 2012, 31, 240–243. [Google Scholar] [CrossRef] [PubMed]
- Lamont, E.A.; He, L.; Warriner, K.; Labuza, T.P.; Sreevatsan, S. A single DNA aptamer functions as a biosensor for ricin. Analyst 2011, 136, 3884–3895. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, X. Aptamer-based technology for food analysis. Appl. Biochem. Biotechnol. 2015, 175, 603–624. [Google Scholar] [CrossRef] [PubMed]
- Bruno, J.G.; Phillips, T.; Carrillo, M.P.; Crowell, R. Plastic-adherent DNA aptamer-magnetic bead and quantum dot sandwich assay for Campylobacter detection. J. Fluoresc. 2009, 19, 427–435. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, H.P.; Smiley, R.D.; Jaykus, L.A. Selection and characterization of DNA aptamers with binding selectivity to Campylobacter jejuni using whole-cell SELEX. Appl. Microbiol. Biotechnol. 2010, 87, 2323–2334. [Google Scholar] [CrossRef] [PubMed]
- Bruno, J.G.; Carrillo, M.P.; Phillips, T.; Andrews, C.J. A novel screening method for competitive FRET-aptamers applied to E. coli assay development. J. Fluoresc. 2010, 20, 1211–1223. [Google Scholar] [CrossRef]
- Queirós, R.B.; Gouveia, C.; Fernandes, J.R.; Jorge, P.A. Evanescent wave DNA-aptamer biosensor based on long period gratings for the specific recognition of E. coli outer membrane proteins. Biosens. Bioelectron. 2014, 62, 227–233. [Google Scholar] [CrossRef]
- Queirós, R.B.; de-los-Santos-Álvarez, N.; Noronhae, J.P.; Sales, M.G.F. A label-free DNA aptamer-based impedance biosensor for the detection of E. coli outer membrane proteins. Sens. Actuators B Chem. 2013, 181, 766–772. [Google Scholar] [CrossRef]
- Bruno, J.G.; Phillips, T.; Montez, T.; Garcia, A.; Sivils, J.C.; Mayo, M.W.; Greis, A. Metrix360 Laboratories. Development of a fluorescent enzyme-linked DNA aptamer-magnetic bead sandwich assay and portable fluorometer for ultrasensitive and rapid Listeria detection. J. Fluoresc. 2015, 25, 173–183. [Google Scholar]
- Lee, S.H.; Ahn, J.Y.; Lee, K.A.; Um, H.J.; Sekhon, S.S.; Sun Park, T.; Min, J.; Kim, Y.H. Analytical bioconjugates, aptamers, enable specific quantitative detection of Listeria monocytogenes. Biosens. Bioelectron. 2015, 68, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Bruno, J.G. Application of DNA aptamers and quantum dots to lateral flow test strips for detection of foodborne pathogens with improved sensitivity versus colloidal gold. Pathogens 2014, 3, 341–355. [Google Scholar] [CrossRef] [PubMed]
- Park, H.C.; Baig, I.A.; Lee, S.C.; Moon, J.Y.; Yoon, M.Y. Development of ssDNA aptamers for the sensitive detection of Salmonella typhimurium and Salmonella enteritidis. Appl. Biochem. Biotechnol. 2014, 174, 793–802. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.; Kim, G.; Lee, S.; Park, S. Identification of Salmonella Typhimurium-specific DNA aptamers developed using whole-cell SELEX and FACS analysis. J. Microbiol. Methods 2013, 95, 162–166. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, H.P.; Smiley, R.D.; Jaykus, L.A. Selection of DNA aptamers for capture and detection of Salmonella Typhimurium using a whole-cell SELEX approach in conjunction with cell sorting. Appl. Microbiol. Biotechnol. 2013, 97, 3677–3686. [Google Scholar] [CrossRef] [PubMed]
- Duan, N.; Wu, S.; Chen, X.; Huang, Y.; Xia, Y.; Ma, X.; Wang, Z. Selection and characterization of aptamers against Salmonella typhimurium using whole-bacterium Systemic Evolution of Ligands by Exponential Enrichment (SELEX). J. Agric. Food Chem. 2013, 61, 3229–3234. [Google Scholar] [CrossRef] [PubMed]
- Joshi, R.; Janagama, H.; Dwivedi, H.P.; Senthil Kumar, T.M.; Jaykus, L.A.; Schefers, J.; Sreevatsan, S. Selection, characterization, and application of DNA aptamers for the capture and detection of Salmonella enterica serovars. Mol. Cell. Probes 2009, 23, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Escudero-Abarca, B.I.; Suh, S.H.; Moore, M.D.; Dwivedi, H.P.; Jaykus, L.A. Selection, characterization and application of nucleic acid aptamers for the capture and detection of human norovirus strains. PLoS ONE 2014, 9, e106805. [Google Scholar] [CrossRef] [PubMed]
- Beier, R.; Pahlke, C.; Quenzel, P.; Henseleit, A.; Boschke, E.; Cuniberti, G.; Labudde, D. Selection of a DNA aptamer against norovirus capsid protein VP1. FEMS Microbiol. Lett. 2014, 351, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Giamberardino, A.; Labib, M.; Hassan, E.M.; Tetro, J.A.; Springthorpe, S.; Sattar, S.A.; Berezovski, M.V.; DeRosa, M.C. Ultrasensitive norovirus detection using DNA aptasensor technology. PLoS ONE 2013, 8, e79087. [Google Scholar] [CrossRef] [PubMed]
- DeGrasse, J.A. A single-stranded DNA aptamer that selectively binds to Staphylococcus aureus enterotoxin B. PLoS ONE 2012, 7, e33410. [Google Scholar] [CrossRef] [PubMed]
- Nikolaus, N.; Strehlitz, B. DNA-aptamers binding aminoglycoside antibiotics. Sensors 2014, 14, 3737–3755. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Liu, J.; Yong, W.; Chen, Q.; Zhang, L.; Dong, Y.; Su, H.; Tan, T. A direct competitive assay-based aptasensor for sensitive determination of tetracycline residue in honey. Talanta 2015, 131, 562–569. [Google Scholar] [CrossRef] [PubMed]
- Pang, S.; Labuza, T.P.; He, L. Development of a single aptamer-based surface enhanced Raman scattering method for rapid detection of multiple pesticides. Analyst 2014, 139, 1895–1901. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liu, X.; Zhang, Q.; Zhang, C.; Liu, Y.; Tu, K.; Tu, J. Selection of DNA aptamers that bind to four organophosphorus pesticides. Biotechnol. Lett. 2012, 34, 869–874. [Google Scholar] [CrossRef] [PubMed]
- Jyoti, A.; Vajpayee, P.; Singh, G.; Patel, C.B.; Gupta, K.C.; Shanker, R. Identification of environmental reservoirs of nontyphoidal salmonellosis: Aptamer-assisted bioconcentration and subsequent detection of Salmonella typhimurium by quantitative polymerase chain reaction. Environ. Sci. Technol. 2011, 45, 8996–9002. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Vajpayee, P.; Rani, N.; Jyoti, A.; Gupta, K.C.; Shanker, R. Bio-capture of S. Typhimurium from surface water by aptamer for culture-free quantification. Ecotoxicol. Environ. Saf. 2012, 78, 320–326. [Google Scholar]
- Suh, S.H.; Jaykus, L.A. Nucleic acid aptamers for capture and detection of Listeria spp. J. Biotechnol. 2013, 167, 454–461. [Google Scholar] [CrossRef] [PubMed]
- Biagi, C.; Conti, V.; Montanaro, N.; Melis, M.; Buccellato, E.; Donati, M.; Covezzoli, A.; Amato, R.; Pazzi, L.; Venegoni, M.; et al. Comparative safety profiles of intravitreal bevacizumab, ranibizumab and pegaptanib: The analysis of the WHO database of adverse drug reactions. Eur. J. Clin. Pharmacol. 2014, 70, 1505–1512. [Google Scholar]
- Ni, X.; Castanares, M.; Mukherjee, A.; Lupold, S.E. Nucleic acid aptamers: Clinical applications and promising new horizons. Curr. Med. Chem. 2011, 18, 4206–4214. [Google Scholar] [CrossRef] [PubMed]
- Sundaram, P.; Kurniawan, H.; Byrne, M.E.; Wower, J. Therapeutic RNA aptamers in clinical trials. Eur. J. Pharm. Sci. 2013, 48, 259–271. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Rossi, J.J. Cell-type-specific, aptamer-functionalized agents for targeted disease therapy. Mol. Ther. Nucleic Acids 2014, 3, e169. [Google Scholar] [CrossRef] [PubMed]
- Manoharan, M.; Inamati, G.B.; Lesnik, E.A.; Sioufi, N.B.; Freier, S.M. Improving antisense oligonucleotide binding to human serum albumin: Dramatic effect of ibuprofen conjugation. ChemBioChem 2002, 3, 1257–1260. [Google Scholar] [CrossRef] [PubMed]
- Dougan, H.; Lyster, D.M.; Vo, C.V.; Stafford, A.; Weitz, J.I.; Hobbs, J.B. Extending the lifetime of anticoagulant oligodeoxynucleotide aptamers in blood. Nucl. Med. Biol. 2000, 27, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Keefe, A.D.; Pai, S.; Ellington, A. Aptamers as therapeutics. Nat. Rev. Drug Discov. 2010, 9, 537–550. [Google Scholar] [CrossRef] [PubMed]
- Healy, J.M.; Lewis, S.D.; Kurz, M.; Boomer, R.M.; Thompson, K.M.; Wilson, C.; McCauley, T.G. Pharmacokinetics and biodistribution of novel aptamer compositions. Pharm. Res. 2004, 21, 2234–2246. [Google Scholar] [CrossRef] [PubMed]
- Duclair, S.; Gautam, A.; Ellington, A.; Prasad, V.R. High-affinity RNA aptamers against the HIV-1 protease inhibit both in vitro protease activity and late events of viral replication. Mol. Ther. Nucleic Acids 2015, 4, e228. [Google Scholar] [CrossRef] [PubMed]
- Wandtke, T.; Woźniak, J.; Kopiński, P. Aptamers in diagnostics and treatment of viral infections. Viruses 2015, 7, 751–780. [Google Scholar] [CrossRef] [PubMed]
- Shum, K.T.; Zhou, J.; Rossi, J.J. Aptamer-based therapeutics: New approaches to combat human viral diseases. Pharmaceuticals 2013, 6, 1507–1542. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.M.; Lee, K.H.; Han, B.W.; Han, M.R.; Kim, D.H.; Kim, D.E. An RNA aptamer that specifically binds to the glycosylated hemagglutinin of avian influenza virus and suppresses viral infection in cells. PLoS ONE 2014, 9, e97574. [Google Scholar] [CrossRef] [PubMed]
- Suenaga, E.; Kumar, P.K. An aptamer that binds efficiently to the hemagglutinins of highly pathogenic avian influenza viruses (H5N1 and H7N7) and inhibits hemagglutinin-glycan interactions. Acta Biomater. 2014, 10, 1314–1323. [Google Scholar] [CrossRef] [PubMed]
- Shiratori, I.; Akitomi, J.; Boltz, D.A.; Horii, K.; Furuichi, M.; Waga, I. Selection of DNA aptamers that bind to influenza A viruses with high affinity and broad subtype specificity. Biochem. Biophys. Res. Commun. 2014, 443, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Musafia, B.; Oren-Banaroya, R.; Noiman, S. Designing anti-influenza aptamers: Novel quantitative structure activity relationship approach gives insights into aptamer-virus interaction. PLoS ONE 2014, 9, e97696. [Google Scholar] [CrossRef] [PubMed]
- Gopinath, S.C.; Kumar, P.K. Aptamers that bind to the hemagglutinin of the recent pandemic influenza virus H1N1 and efficiently inhibit agglutination. Acta Biomater. 2013, 9, 8932–8941. [Google Scholar] [CrossRef] [PubMed]
- Bruno, J.G.; Carrillo, M.P.; Richarte, A.M.; Phillips, T.; Andrews, C.; Lee, J.S. Development, screening, and analysis of a small DNA aptamer library potentially useful for diagnosis and passive immunity of arboviruses. BMC Res. Notes 2012, 5, 633. [Google Scholar] [CrossRef] [PubMed]
- DiGiandomenico, A.; Keller, A.E.; Gao, C.; Rainey, G.J.; Warrener, P.; Camara, M.M.; Bonnell, J.; Fleming, R.; Bezabeh, B.; Dimasi, N.; et al. A multifunctional bispecific antibody protects against Pseudomonas aeruginosa. Sci. Transl. Med. 2014, 6. [Google Scholar] [CrossRef]
- Oleksiewicz, M.B.; Nagy, G.; Nagy, E. Anti-bacterial monoclonal antibodies: Back to the future? Arch. Biochem. Biophys. 2012, 526, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Saylor, C.; Dadachova, E.; Casadevall, A. Monoclonal antibody-based therapies for microbial diseases. Vaccine 2009, 56 (Suppl. 6), G38–G46. [Google Scholar] [CrossRef]
- Kolovskaya, O.S.; Savitskaya, A.G.; Zamay, T.N.; Reshetneva, I.T.; Zamay, G.S.; Erkaev, E.N.; Wang, X.; Wehbe, M.; Salmina, A.B.; Perianova, O.V.; et al. Development of bacteriostatic DNA aptamers for salmonella. J. Med. Chem. 2013, 56, 1564–1572. [Google Scholar]
- Schlesinger, S.R.; Lahousse, M.J.; Foster, T.O.; Kim, S. Metallo-β-lactamase and aptamer-based inhibition. Pharmaceuticals 2011, 4, 419–428. [Google Scholar] [CrossRef]
- Bruno, J.G.; Carrillo, M.P.; Phillips, T. In vitro antibacterial effects of anti-lipopolysaccharide DNA aptamer-C1qrs complexes. Folia Microbiol. 2008, 53, 295–302. [Google Scholar] [CrossRef]
- Bruno, J.G. Aptamer-biotin-streptavidin-C1q complexes can trigger the classical complement pathway to kill cancer cells. In Vitro Cell. Dev. Biol. 2010, 46, 107–113. [Google Scholar] [CrossRef]
- Stecker, J.R.; Savage, A.; Bruno, J.G.; Garcia, D.M.; Koke, J.R. Dynamics and visualization of MCF7 adenocarcinoma cell death by aptamer-C1q-mediated membrane attack. Nucleic Acid Ther. 2012, 22, 275–282. [Google Scholar] [PubMed]
- Mallik, P.K.; Nishikawa, K.; Millis, A.J.; Shi, H. Commandeering a biological pathway using aptamer-derived molecular adaptors. Nucleic Acids Res. 2010, 38, e93. [Google Scholar] [CrossRef] [PubMed]
- Bruno, J.G.; Carrillo, M.P.; Crowell, R. Preliminary development of DNA aptamer-Fc conjugate opsonins. J. Biomed. Mater. Res. A 2009, 90, 1152–1161. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Gan, L.; Jiang, L.; Zhang, X.; Yang, X.; Chen, M.; Lan, X. Neutralization of Staphylococcal enterotoxin B by aptamer antagonist. Antimicrob. Agents Chemother. 2015, in press. [Google Scholar]
- Vivekananda, J.; Salgado, C.; Millenbaugh, N.J. DNA aptamers as a novel approach to neutralize Staphylococcus aureus α-toxin. Biochem. Biophys. Res. Commun. 2014, 444, 433–438. [Google Scholar] [CrossRef] [PubMed]
- Ye, F.; Zheng, Y.; Wang, X.; Tan, X.; Zhang, T.; Xin, W.; Wang, J.; Huang, Y.; Fan, Q.; Wang, J. Recognition of Bungarus multicinctus venom by a DNA aptamer against β-bungarotoxin. PLoS ONE 2014, 9, e105404. [Google Scholar] [CrossRef] [PubMed]
- Lauridsen, L.H.; Shamaileh, H.A.; Edwards, S.L.; Taran, E.; Veedu, R.N. Rapid one-step selection method for generating nucleic acid aptamers: Development of a DNA aptamer against α-bungarotoxin. PLoS ONE 2012, 7, e41702. [Google Scholar] [CrossRef] [PubMed]
- Sapag, A.; Salinas-Luypaert, C.; Constenla-Muñoz, C. First report of in vitro selection of RNA aptamers targeted to recombinant Loxosceles laeta spider toxins. Biol. Res. 2014, 47, 2. [Google Scholar] [PubMed]
- Bruno, J.G. In vitro selection of DNA to chloroaromatics using magnetic microbead-based affinity separation and fluorescence detection. Biochem. Biophys. Res. Commun. 1997, 234, 117–120. [Google Scholar] [CrossRef] [PubMed]
- Bruno, J.G.; Kiel, J.L. Use of magnetic beads in selection and detection of biotoxin aptamers by ECL and enzymatic methods. BioTechniques 2002, 32, 178–183. [Google Scholar] [PubMed]
- Bruno, J.G.; Kiel, J.L. In vitro selection of DNA aptamers to anthrax spores with electrochemiluminescence detection. Biosens. Bioelectron. 1999, 14, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Zhang, W.; Jia, S.; Guan, Z.; Yang, C.J.; Zhu, Z. Microfluidic approaches to rapid and efficient aptamer selection. Biomicrofluidics 2014, 8, 041501. [Google Scholar] [CrossRef] [PubMed]
- Lai, H.C.; Wang, C.H.; Liou, T.M.; Lee, G.B. Influenza A virus-specific aptamers screened by using an integrated microfluidic system. Lab Chip 2014, 14, 2002–2013. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.S.; Ahmad, K.M.; Cho, M.; Kim, S.; Xiao, Y.; Soh, H.T. Improving aptamer selection efficiency through volume dilution, magnetic concentration, and continuous washing in microfluidic channels. Anal. Chem. 2011, 83, 6883–6889. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, K.M.; Oh, S.S.; Kim, S.; McClellen, F.M.; Xiao, Y.; Soh, H.T. Probing the limits of aptamer affinity with a microfluidic SELEX platform. PLoS ONE 2011, 6, e27051. [Google Scholar] [CrossRef] [PubMed]
- Haller, C.; Sobolewska, B.; Schibilsky, D.; Avci-Adali, M.; Schlensak, C.; Wendel, H.P.; Walker, T. One-staged aptamer-based isolation and application of endothelial progenitor cells in a porcine myocardial infarction model. Nucleic Acid Ther. 2015, 25, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Qi, P.; Yan, W.; Yang, Y.; Li, Y.; Fan, Y.; Chen, J.; Yang, Z.; Tu, Q.; Huang, N. Immobilization of DNA aptamers via plasma polymerized allylamine film to construct an endothelial progenitor cell-capture surface. Colloids Surf. B Biointerfaces 2015, 126, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, J.; Paul, A.; Harwardt, M.; Groll, J.; Reeswinkel, T.; Klee, D.; Moeller, M.; Fischer, H.; Walker, T.; Greiner, T.; et al. Immobilized DNA aptamers used as potent attractors for porcine endothelial precursor cells. J. Biomed. Mater. Res. A 2008, 84, 614–621. [Google Scholar]
- Guo, K.T.; Schafer, R.; Paul, A.; Gerber, A.; Ziemer, G.; Wendel, H.P. A new technique for the isolation and surface immobilization of mesenchymal stem cells from whole bone marrow using high-specific DNA aptamers. Stem Cells 2006, 24, 2220–2231. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Khaing, Z.Z.; Li, N.; Hall, B.; Schmidt, C.E.; Ellington, A.D. Aptamer antagonists of myelin-derived inhibitors promote axon growth. PLoS ONE 2010, 5, e9726. [Google Scholar] [CrossRef] [PubMed]
- Dalziel, M.; Crispin, M.; Dwek, R.A. Directing stem cell differentiation with antibodies. Proc. Natl. Acad. Sci. USA 2013, 110, 17608–17609. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Zhang, H.; Yea, K.; Lerner, R.A. Autocrine signaling based selection of combinatorial antibodies that transdifferentiate human stem cells. Proc. Natl. Acad. Sci. USA 2013, 110, 8099–8104. [Google Scholar] [CrossRef] [PubMed]
- Yea, K.; Zhang, H.; Xie, J.; Jones, T.M.; Yang, G.; Song, B.D.; Lerner, R.A. Converting stem cells to dendritic cells by agonist antibodies from unbiased morphogenic selections. Proc. Natl. Acad. Sci. USA 2013, 110, 14966–14971. [Google Scholar] [CrossRef] [PubMed]
- Shetty, S.; Ghosh, K. Novel therapeutic approaches to haemophilia. Haemophlilia 2015, 21, 152–161. [Google Scholar] [CrossRef]
- Altman, M.O.; Chang, Y.M.; Xiong, X.; Tan, W. Modifying cellular properties using artificial aptamer-lipid receptors. Sci. Rep. 2013, 3, 3343. [Google Scholar] [CrossRef] [PubMed]
- Baird, G.S. Where are all the aptamers? Am. J. Clin. Pathol. 2010, 134, 529–531. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bruno, J.G. Predicting the Uncertain Future of Aptamer-Based Diagnostics and Therapeutics. Molecules 2015, 20, 6866-6887. https://doi.org/10.3390/molecules20046866
Bruno JG. Predicting the Uncertain Future of Aptamer-Based Diagnostics and Therapeutics. Molecules. 2015; 20(4):6866-6887. https://doi.org/10.3390/molecules20046866
Chicago/Turabian StyleBruno, John G. 2015. "Predicting the Uncertain Future of Aptamer-Based Diagnostics and Therapeutics" Molecules 20, no. 4: 6866-6887. https://doi.org/10.3390/molecules20046866





