Synthesis of Photochromic Oligophenylenimines: Optical and Computational Studies
Abstract
:1. Introduction
2. Results and Discussion
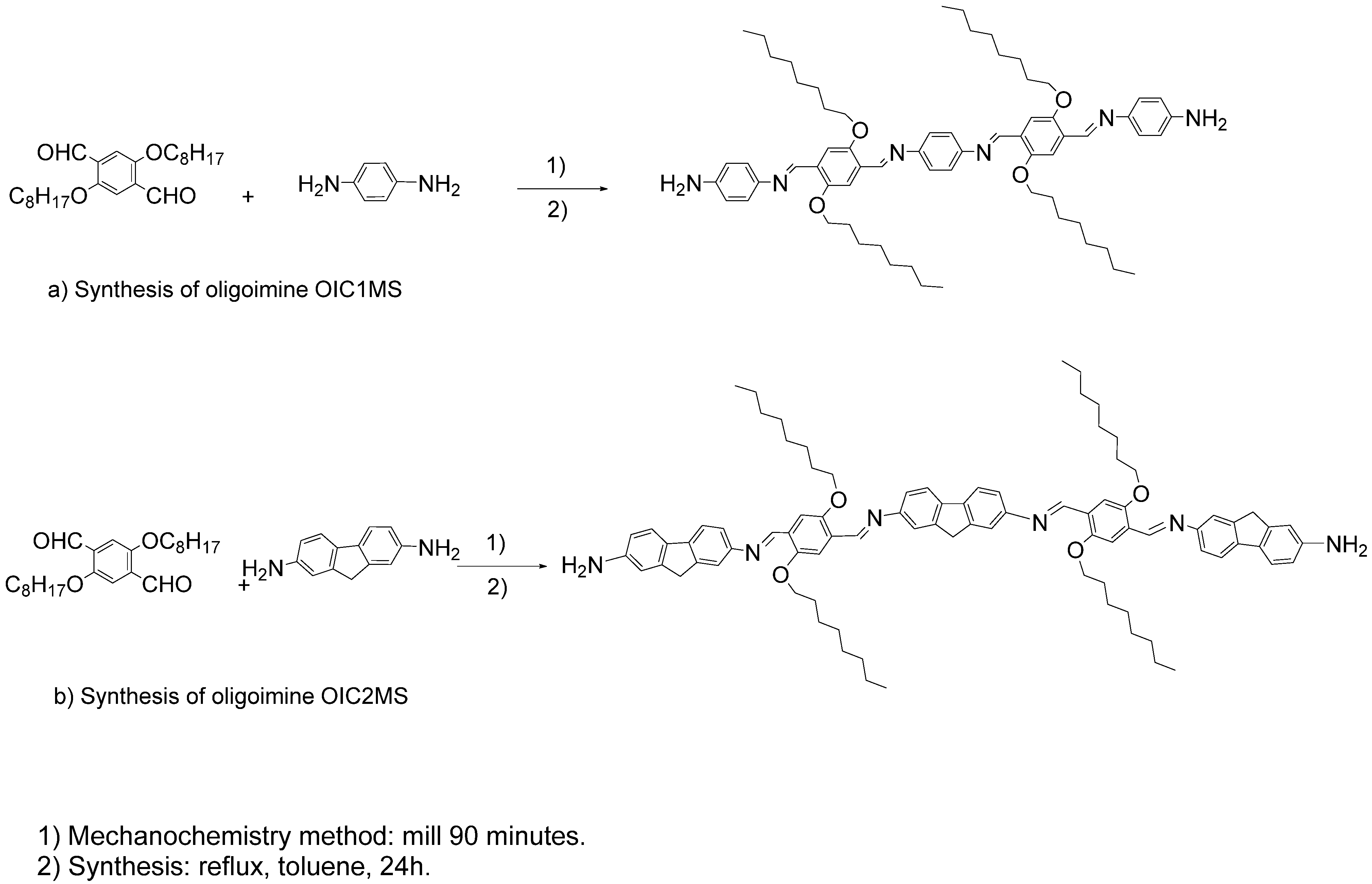
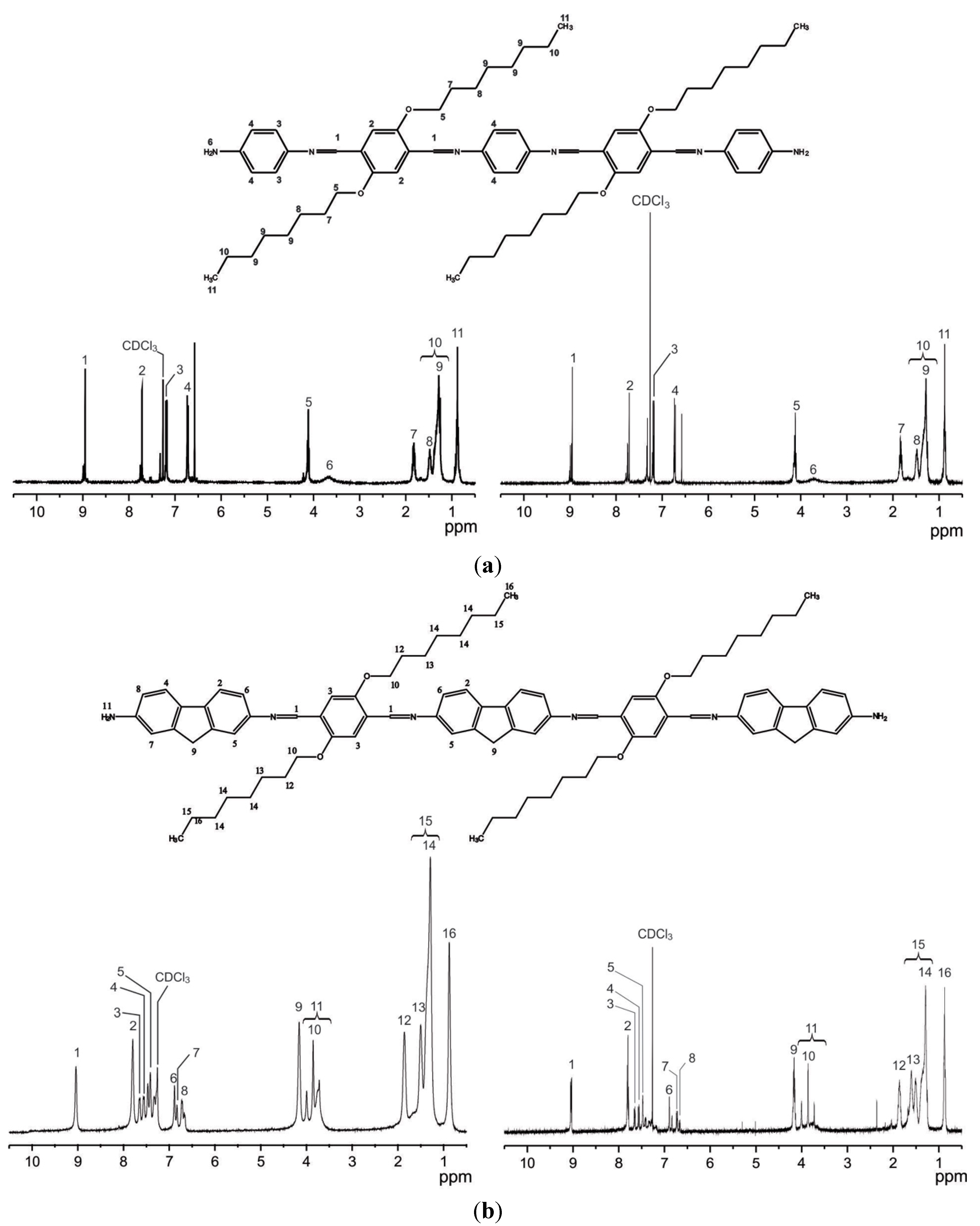
2.1. Synthesis
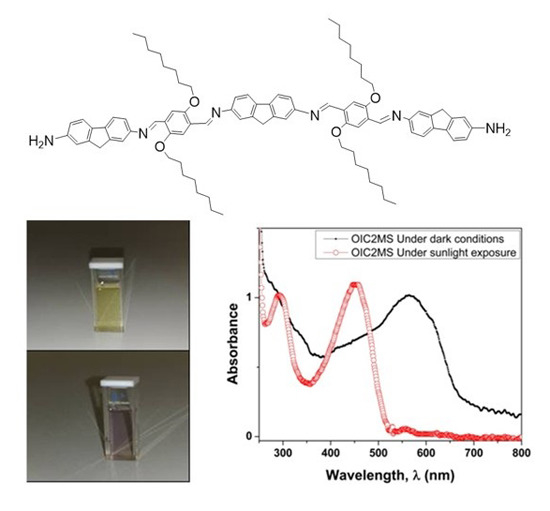
2.2. Optical Studies
2.2.1. Absorption Spectra in Different Solvents
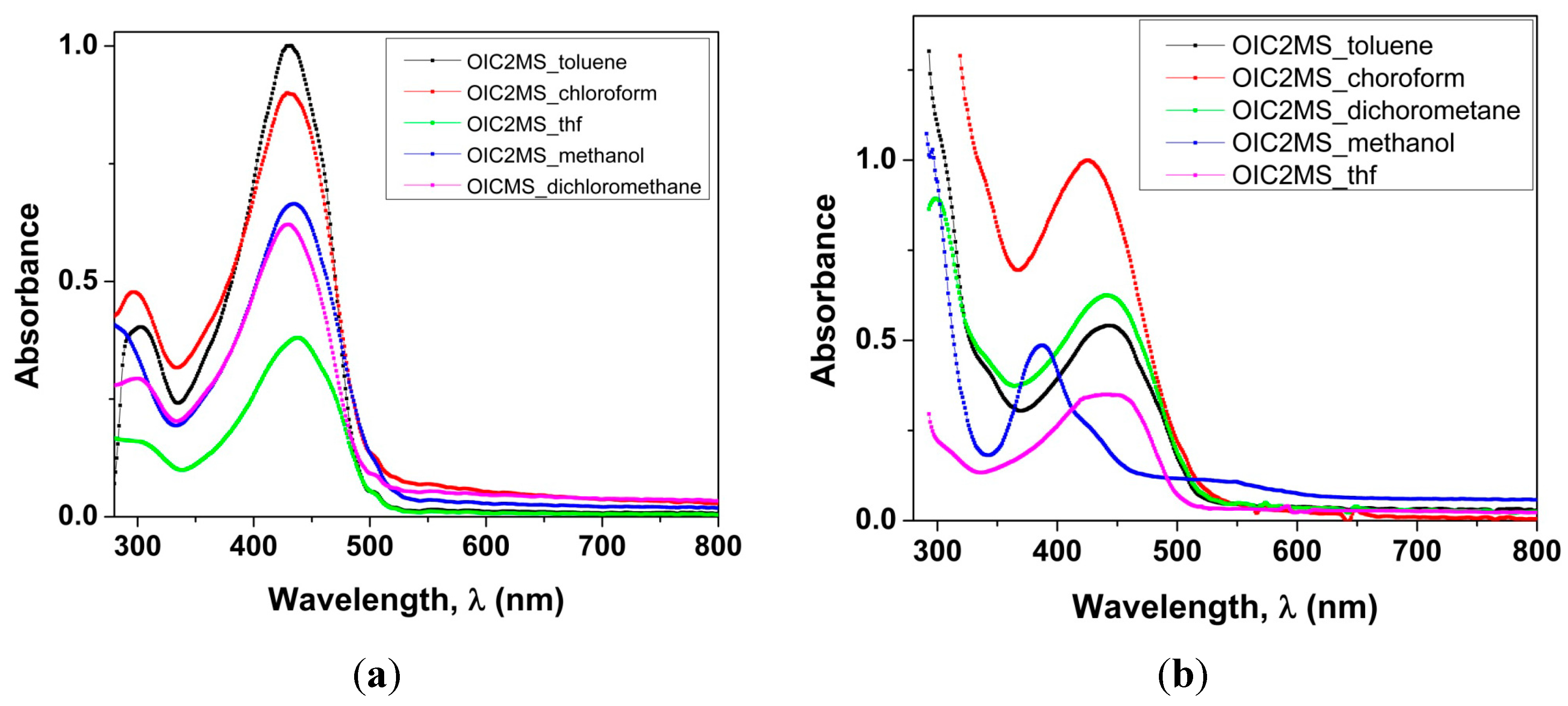
| SOLVENT | λmax/nm OIC1MS | ε × 104 (M−1 cm−1) OIC1MS | λmax/nm OIC2MS | ε × 104 (M−1 cm−1) OIC2MS |
|---|---|---|---|---|
| Toluene | 430 | 2.4 | 449 | 1.7 |
| Chloroform | 430 | 2.3 | 425 | 3.2 |
| Methanol | 434 | 1.7 | 425 | 1.5 |
| Dichloromethane | 430 | 1.6 | 449 | 2 |
| Tetrahydrofuran | 437 | 0.0982 | 440 | 1.1 |
2.2.2. Photochromic Properties


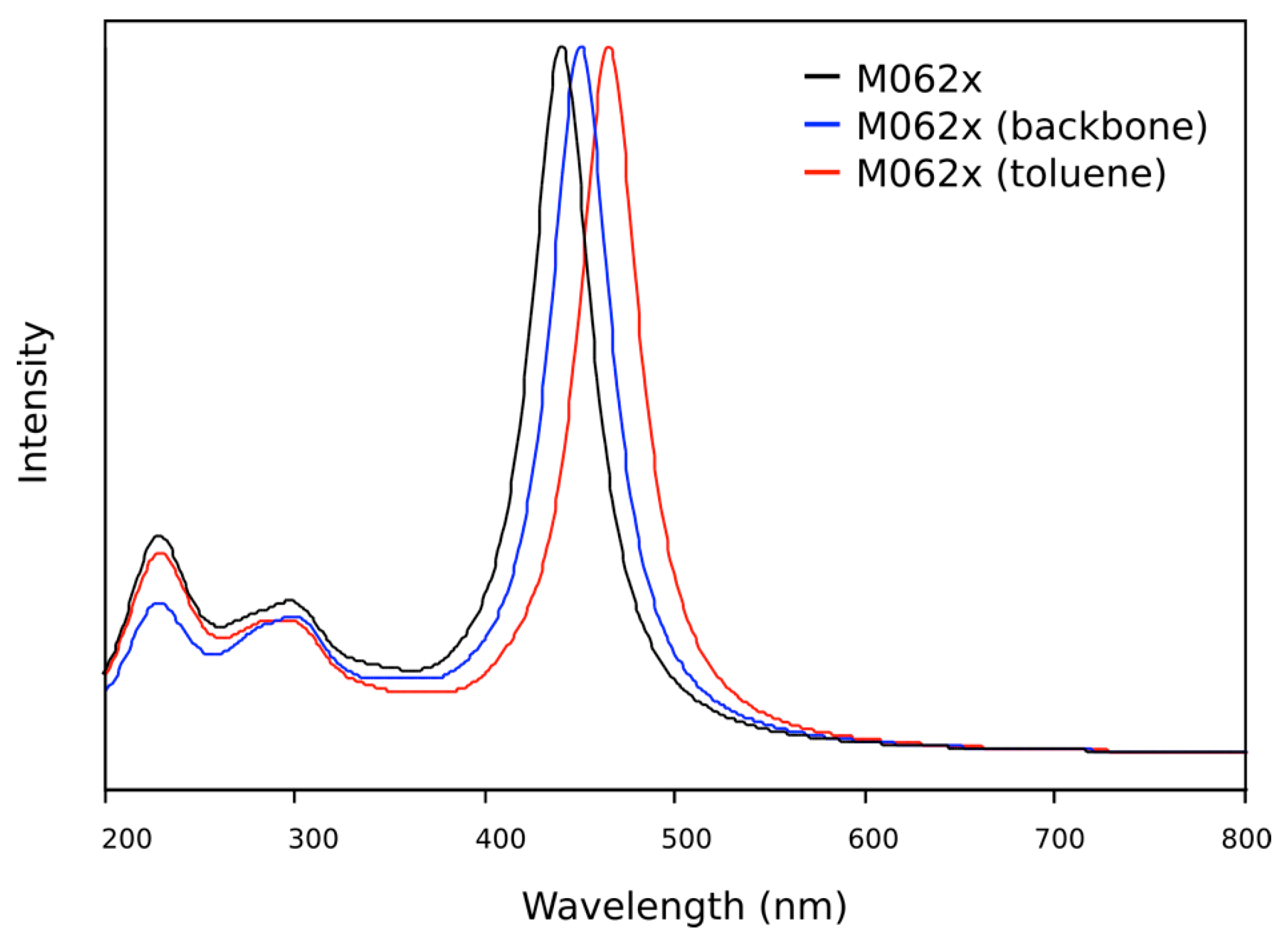
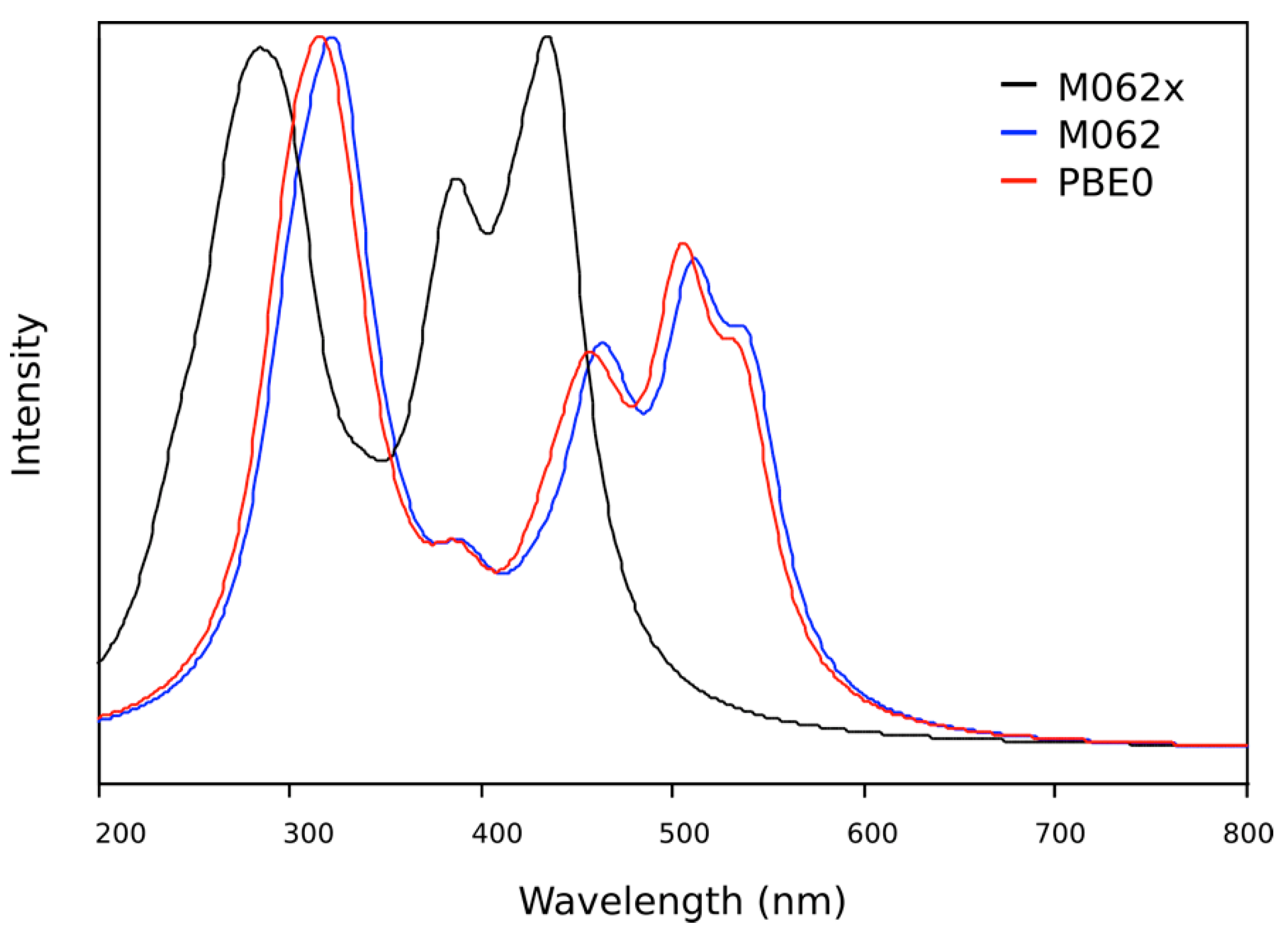
2.3. DFT Theoretical Studies
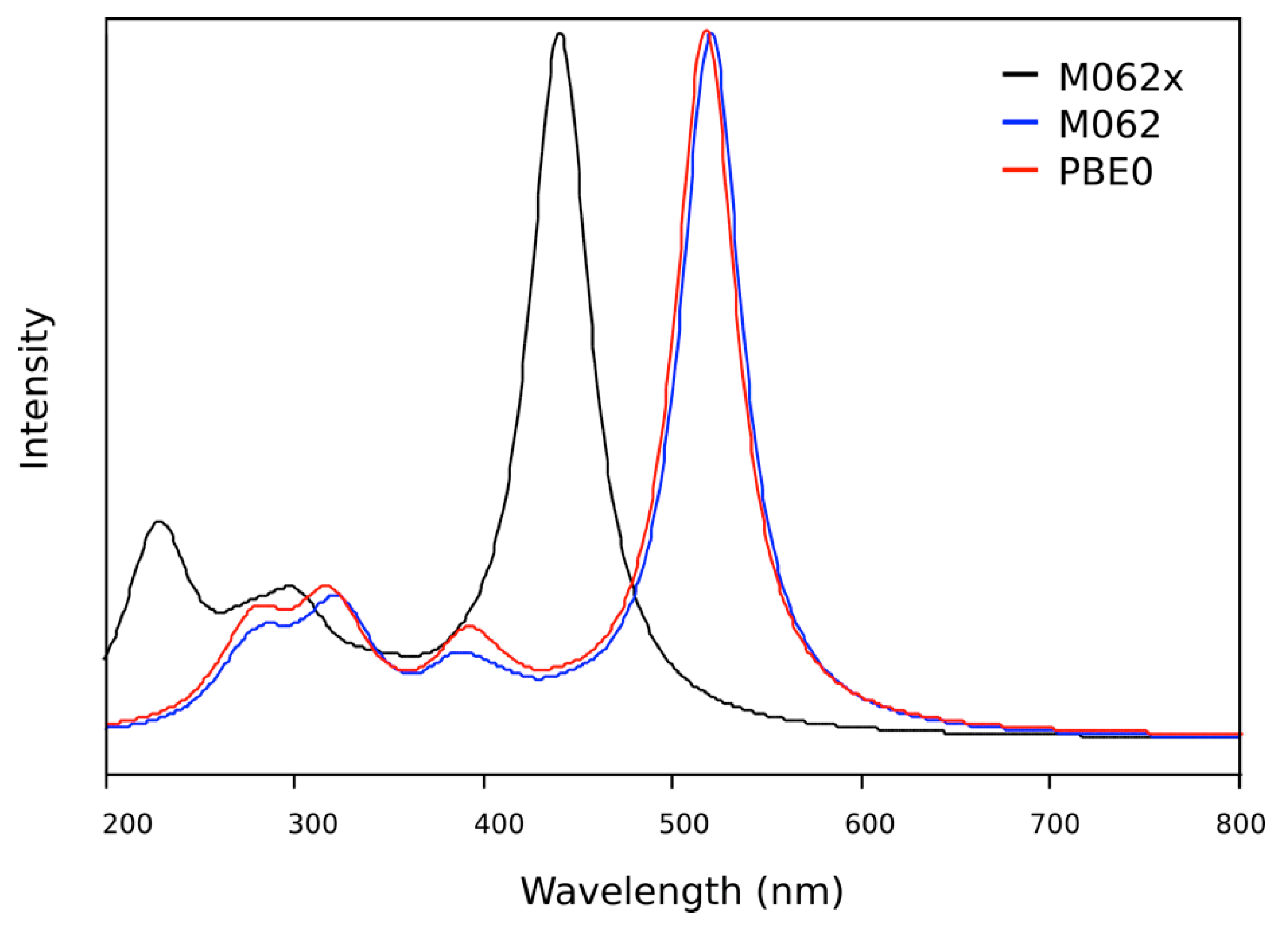
| Wavelength (nm) | Oscillator Strength | Major Contributions |
|---|---|---|
| 435.6 | 3.1 | H-1→L+1 (13%), HOMO→LUMO (73%) |
| 348.7 | 0.1 | H-2→LUMO (42%), H-1→L+1 (19%) |
| 298.5 | 0.3 | H-7→LUMO (16%) |
| 275.8 | 0.2 | H-2→LUMO (30%) |
| 221.1 | 0.2 | H-6→L+1 (16%), H-2→L+2 (22%) |
| 218.3 | 0.1 | H-6→L+1 (52%), H-2→L+2 (11%) |
| 215.2 | 0.1 | H-1→L+7 (25%), HOMO→L+7 (18%), HOMO→L+8 (14%) |
| 212.9 | 0.1 | H-3→L+2 (20%), HOMO→L+7 (16%) |
| Wavelength (nm) | Oscillator Strength | Major Contributions |
|---|---|---|
| 426.4 | 1.13 | H-2→LUMO (13%), H-1→L+1 (13%), HOMO→LUMO (44%) |
| 409.2 | 0.43 | H-2→LUMO (17%), H-1→LUMO (23%), HOMO→L+1 (26%) |
| 380.2 | 0.47 | H-2→LUMO (20%), H-1→L+1 (22%) |
| 371.0 | 0.53 | H-2→LUMO (16%), H-2→L+1 (40%) |
| 329.7 | 0.13 | H-4→LUMO (38%), H-3→L+1 (23%) |
| 300.0 | 0.23 | H-14→L+1 (10%), H-1→LUMO (14%) |
| 295.1 | 0.23 | H-17→LUMO (11%), H-15→LUMO (10%), H-1→LUMO (11%), H-1→L+1 (10%), HOMO→L+1 (12%) |
| 291.1 | 0.18 | H-2→LUMO (11%), H-1→LUMO (13%), H-1→L+1 (12%), HOMO→LUMO (18%) |
| 283.1 | 0.10 | H-1→L+1 (20%), HOMO→L+1 (25%) |
| 281.6 | 0.26 | H-1→L+6 (10%), HOMO→L+6 (15%) |
| 276.2 | 0.23 | H-1→L+6 (17%), HOMO→L+6 (14%) |
| 266.7 | 0.22 | H-1→L+3 (10%), HOMO→L+2 (15%), HOMO→L+3 (10%) |
| 261.7 | 0.28 | H-2→L+2 (25%) |
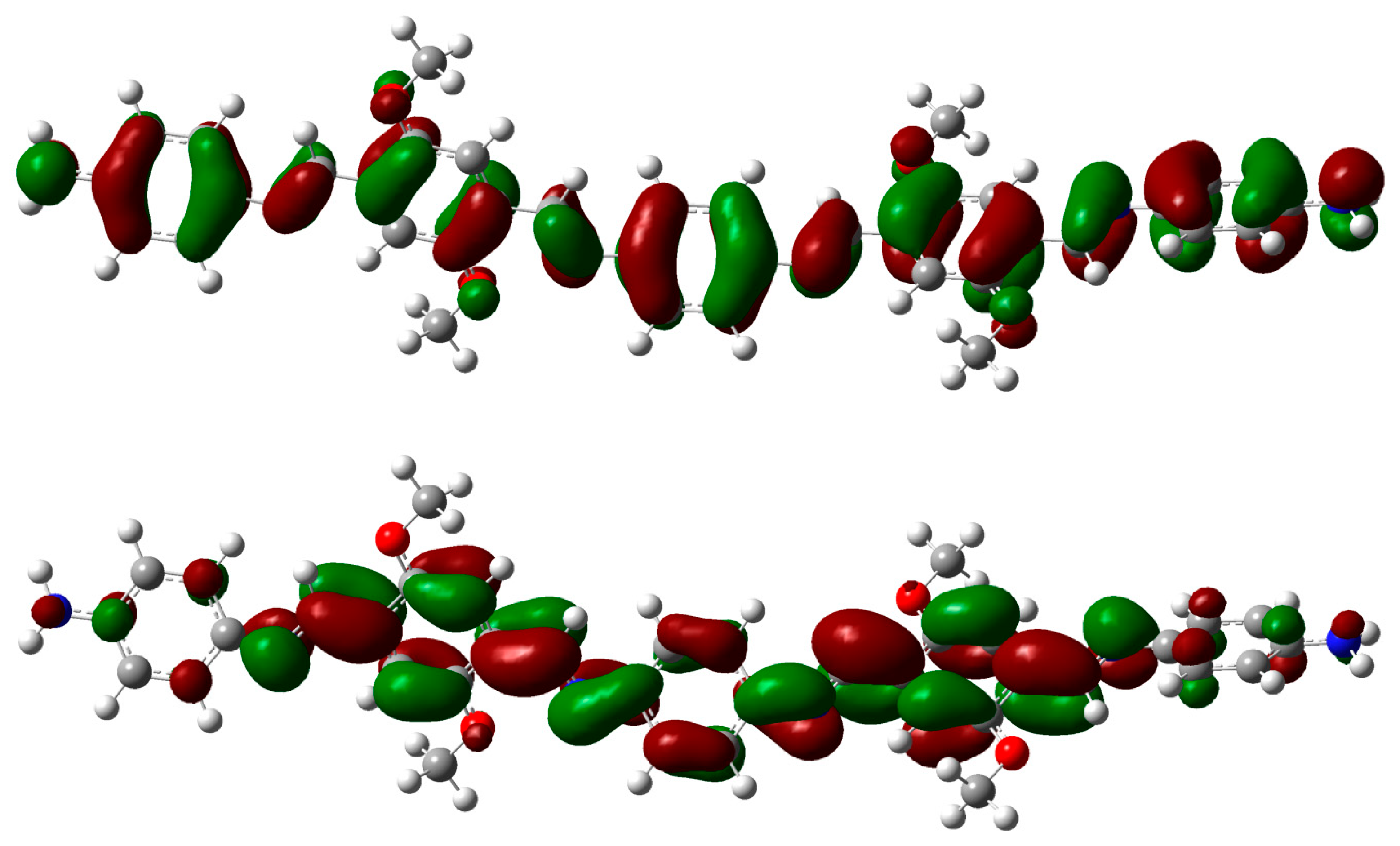
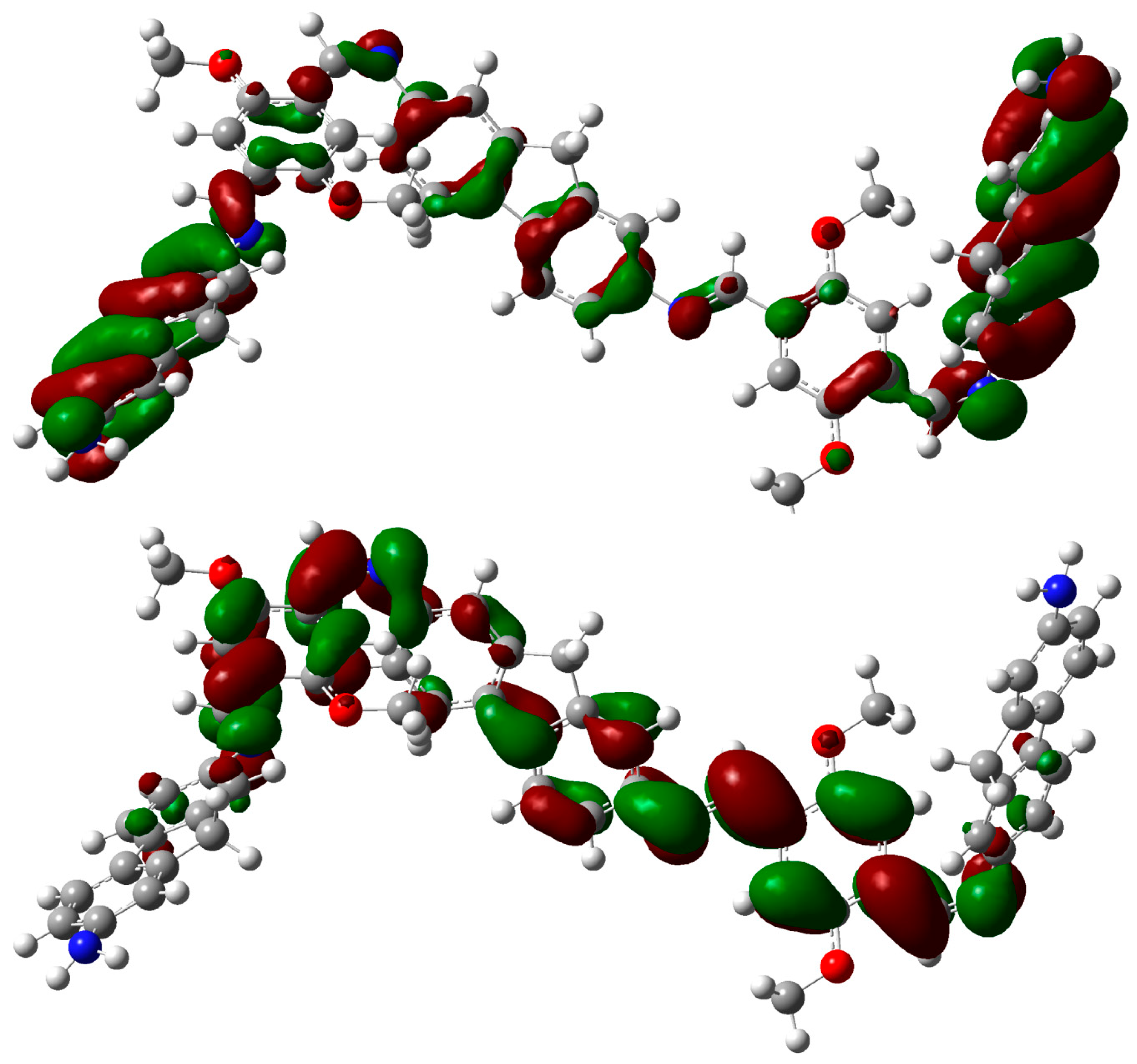
3. Experimental
3.1. General
3.2. Theoretical Calculations
3.3. Mechanosynthesis
Experimental Procedure
3.4. Conventional Synthesis
Experimental procedure
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- He, Z.; Zhong, C.; Su, S.; Xu, M.; Wu, H.; Cao, Y. Enhanced power-conversion efficiency in polymer solar cells using an inverted device structure. Nat. Photonics 2012, 6, 591–595. [Google Scholar]
- Flores, R.; Vázquez, R.; Arias, E.; Moggio, I.; Rodríguez, M.; Ziolo, R.; Rodríguez, O.; Evans, E.; Liebig, C. Synthesis and optoelectronic properties of phenylenevinylenequinoline macromolecules. New. J. Chem. 2014, 38, 974–984. [Google Scholar] [CrossRef]
- Mashiro, I.; Thorsten, L.; Seiba, K.; Nobuo, K. Photochromism of 1,2-bis(2-methyl-5-phenyl-3-thienyl)perfluorocyclopenteneina Single-Crystalline Phase. J. Am. Chem. Soc. 2000, 122, 4871–4876. [Google Scholar] [CrossRef]
- Ziyong, L.; Jun, Y.; Xianghua, W.; Yan, L.; Qingbin, Z.; Fangyng, F.; Sheng, H.L. Diarylethene based imines and amines: Synthesis, photochromic properties and effects of substitution. J. Photochem. Photobiol. A Chem. 2011, 218, 192–198. [Google Scholar] [CrossRef]
- Yunus, B.; KürGat, E. Microwave Assisted Solvent-Free Synthesis of Some Imine Derivatives. Org. Chem. Int. 2014, 2014, 1–5. [Google Scholar]
- Bradley, A.Z.; Thompson, J.S.; Thorn, D.L. Composition Comprising Amino-Imine Compounds. U.S. Patent 7186835 B2, 6 March 2007. [Google Scholar]
- Gutiérrez, A.R.; Vázquez, R.A.; Moggio, I.; Arias, E.; Coreño, O.; Maldonado, J.L.; Ramos, G.; Rodríguez, O.; Jiménez, R.M. Mechanosythesis of a phenylenedivinylidenebisquinoline. Optical, morphological and electroluminescence properties. J. Mol. Struct. 2015, 1086, 138–145. [Google Scholar] [CrossRef]
- Dachille, F.; Roy, R. High-pressure phase transformations in laboratory mechanical mixers and mortars. Nature 1960, 186, 34. [Google Scholar] [CrossRef]
- Lamba, J.J.S.; Tour, J.M. Imine-Bridge planar poly(p-phenylene) Derivatives fro maximization of extended π-Conjugation. The Common intermediate approach. J. Am. Chem. Soc. 1994, 116, 11723–11736. [Google Scholar] [CrossRef]
- Guzmán, E.; Alvarado, J.G.; Cruz, J.; Andrade, N.; Vázquez, R.A.; Nava, R.N.; Pandiyan, T. Subtle C-H…Hal (Hal = Cl, Br) Bonding as Predominant Synthon in the Assembly of Supramolecular Architectures Based on Luminiscent Tin(IV) Complexes. Crystallography, Hirshfeld surfaces, DFT Calculations, and Fluorescence. Cryst. Growth Des. 2014, 14, 3742–3757. [Google Scholar] [CrossRef]
- Guo, H.B.; He, F.; Gu, B.; Liang, L.; Smith, J.C. Time-dependent density functional theory assessment of UV absorption of benzoic acid derivatives. J. Phys. Chem. A 2012, 116, 11870–11879. [Google Scholar] [CrossRef] [PubMed]
- Aradi, B.; Hourahine, B.; Frauenheim, T. DFTB+, a sparse matrix-based implementation of the DFTB method. J. Phys. Chem. 2007, 111, 5678. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W. Gaussian 09; Gaussian Inc.: Wallingford, CT, USA, 2010. [Google Scholar]
- Sample Availability: Available.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez, A.I.M.; Alonso, O.C.; Borbolla, J.C.; Vásquez-Pérez, J.M.; Alonso, J.C.; Ayala, K.A.; Luna-Bárcenas, G.; Pandiyan, T.; García, R.A.V. Synthesis of Photochromic Oligophenylenimines: Optical and Computational Studies. Molecules 2015, 20, 5440-5455. https://doi.org/10.3390/molecules20045440
Pérez AIM, Alonso OC, Borbolla JC, Vásquez-Pérez JM, Alonso JC, Ayala KA, Luna-Bárcenas G, Pandiyan T, García RAV. Synthesis of Photochromic Oligophenylenimines: Optical and Computational Studies. Molecules. 2015; 20(4):5440-5455. https://doi.org/10.3390/molecules20045440
Chicago/Turabian StylePérez, Armando I. Martínez, Oscar Coreño Alonso, Julián Cruz Borbolla, José M. Vásquez-Pérez, Juan Coreño Alonso, Karina Alemán Ayala, Gabriel Luna-Bárcenas, Thangarasu Pandiyan, and Rosa A. Vázquez García. 2015. "Synthesis of Photochromic Oligophenylenimines: Optical and Computational Studies" Molecules 20, no. 4: 5440-5455. https://doi.org/10.3390/molecules20045440
APA StylePérez, A. I. M., Alonso, O. C., Borbolla, J. C., Vásquez-Pérez, J. M., Alonso, J. C., Ayala, K. A., Luna-Bárcenas, G., Pandiyan, T., & García, R. A. V. (2015). Synthesis of Photochromic Oligophenylenimines: Optical and Computational Studies. Molecules, 20(4), 5440-5455. https://doi.org/10.3390/molecules20045440