Formylation of Electron-Rich Aromatic Rings Mediated by Dichloromethyl Methyl Ether and TiCl4: Scope and Limitations
Abstract
:1. Introduction
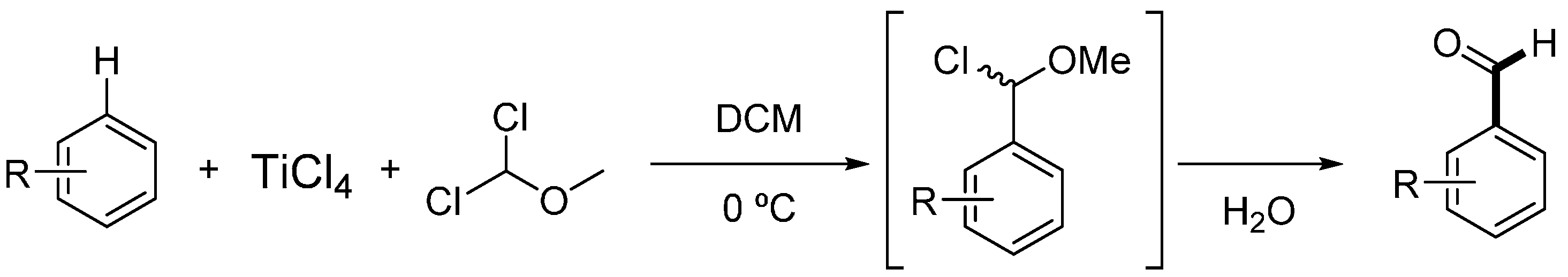
2. Results and Discussion
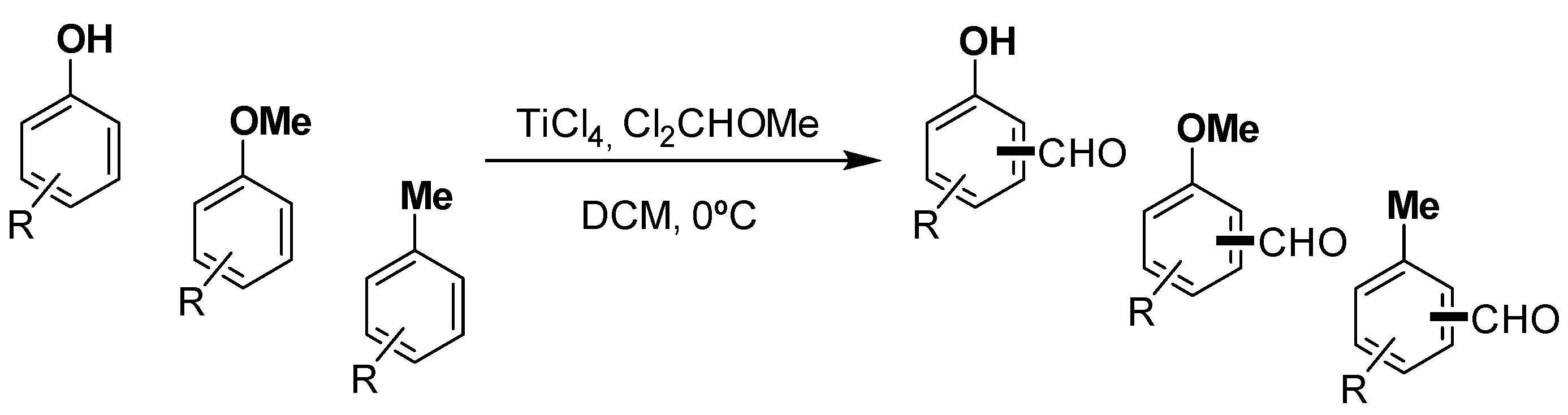
| Entry | Reactant | Formylation Conversion [a] %, [%] | Main Product | Regioisomeric Ratio [b] [mp:  :▲] :▲] | ||
|---|---|---|---|---|---|---|
| Aldehyde | Yield (%) | |||||
| Phenols | ||||||
| 1 |  | 68 [22] |  | ▬ [c] | [2.8:1] | |
| 2 |  | 94 [6] |  | 40 [d] | [3:1.6:1] | |
| 3 |  | 97 |  | 44 [d] | [3.7:1.3:1] | |
| 4 |  | 98 [2] |  | 65 | [5:1] | |
| 5 |  | 80 [11] |  | 63 | [30:1] | |
| 6 |  | 64 [25] |  | 56 | ▬ | |
| Methoxybenzenes | ||||||
| 7 |  | >99 |  | 97 [d] | [1.1:1] | |
| 8 |  | >99 |  | 61 | [3:1] | |
| 9 |  | 62 [38] |  | 44 | ▬ | |
| 10 |  | >99 |  | 15 | [3.5:1] | |
| Methylbenzenes | ||||||
| 11 |  | 89 {4} |  | 70 [d] | [3.2:1] | |
| 12 |  | >99 |  | 62 [d] | [32:1] | |
| 13 |  | 95% {3%} |  | 97 | ▬ | |
| 14 |  | 97 |  | 96 | ▬ | |
 :▲ indicates the ratio for the formylation of the main product and the other regioisomers. [c] Degradation during the purification. [d] The final products were isolated as mixture of regioisomers.
:▲ indicates the ratio for the formylation of the main product and the other regioisomers. [c] Degradation during the purification. [d] The final products were isolated as mixture of regioisomers.2.1. Phenols
2.2. Methoxybenzenes
2.3. Methylbenzenes
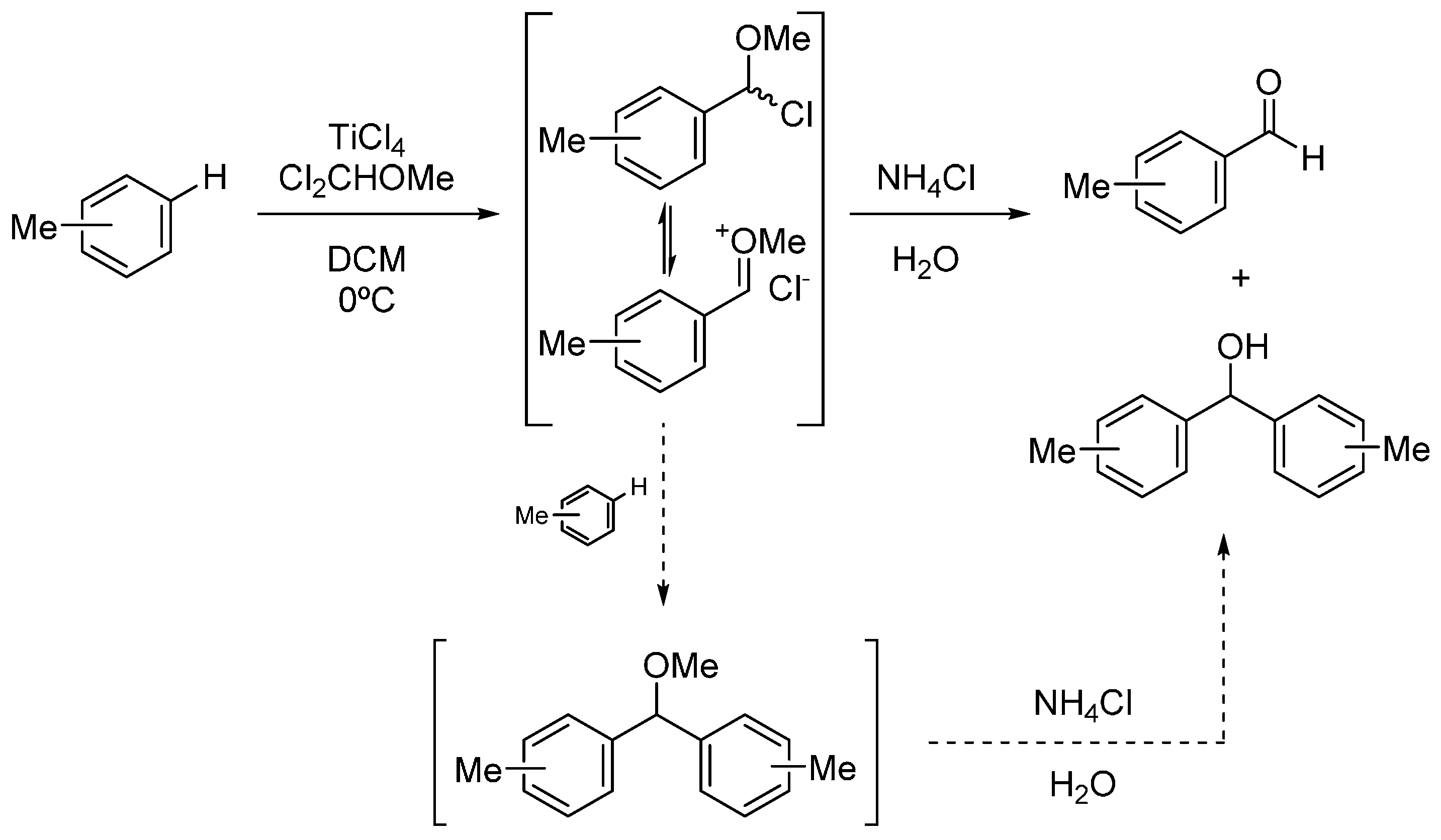
3. Experimental Section
3.1. General Information
3.2. General Formylation Procedure
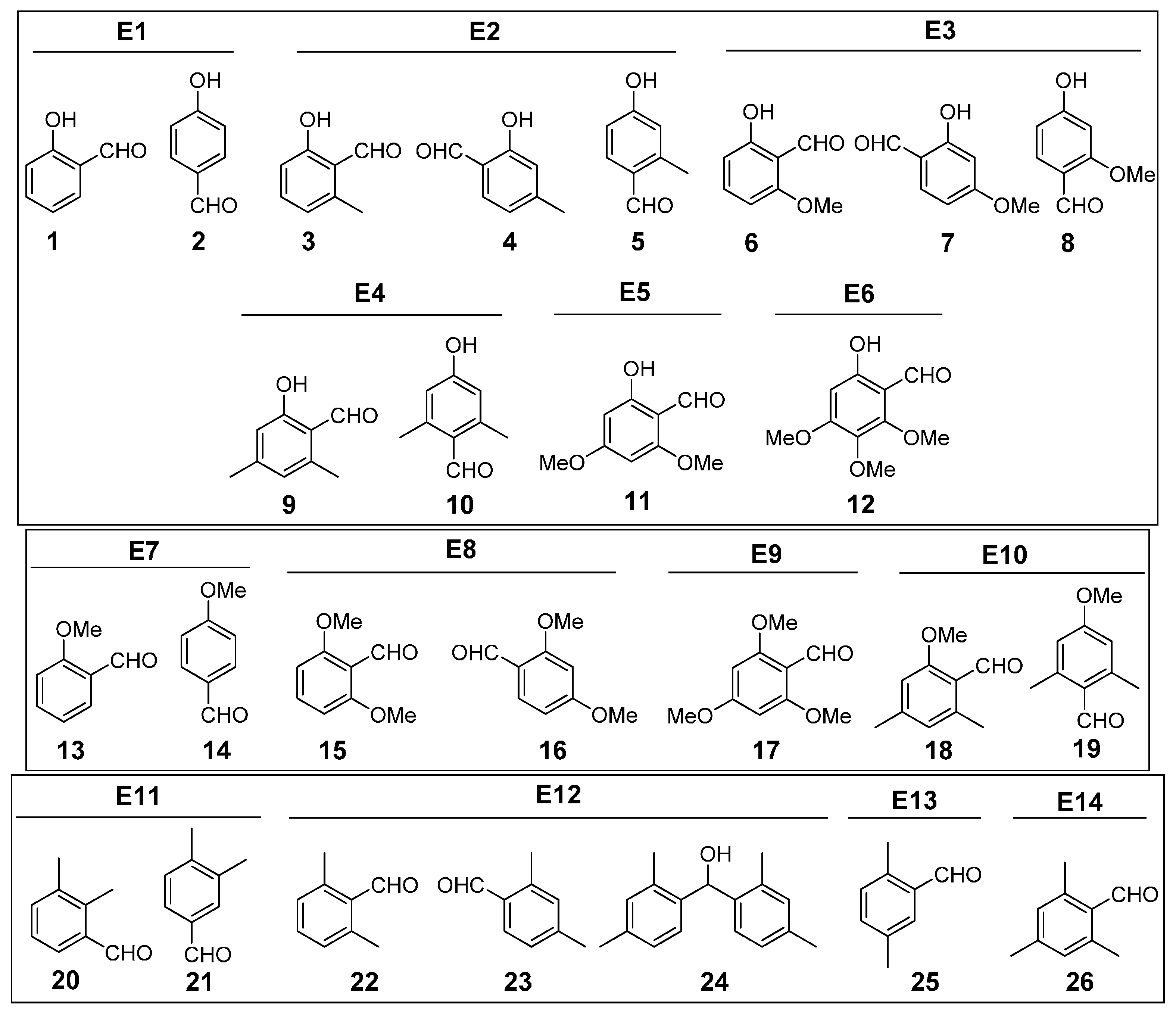
3.3. Phenols
3.3.1. Entry 1
3.3.2. Entry 2
3.3.3. Entry 3
3.3.4. Entry 4
3.3.5. Entry 5
3.3.6. Entry 6
3.4. Methoxybenzenes
3.4.1. Entry 7
3.4.2. Entry 8
3.4.3. Entry 9
3.4.4. Entry 10
3.5. Methylbenzenes
3.5.1. Entry 11
3.5.2. Entry 12
3.5.3. Entry 13
3.5.4. Entry 14
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lawrence, N.J. Aldehydes and ketones. J. Chem. Soc. Perkin Trans. 1 1998, 10, 1739–1750. [Google Scholar] [CrossRef]
- Tenson, T.; Mankin, A. Antibiotics and the ribosome. Mol. Microbiol. 2006, 59, 1664–1677. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, L.N. The synthesis of aromatic aldehydes. Chem. Rev. 1946, 38, 227–254. [Google Scholar] [CrossRef] [PubMed]
- Casnati, G.; Crisafulli, M.; Ricca, A. A new method for the selective ortho-formylation of phenols. Tetrahedron Lett. 1965, 6, 243–245. [Google Scholar] [CrossRef]
- Hofsløkken, N.U.; Skattebøl, L. Convenient method for the ortho-formylation of phenols. Acta Chem. Scand. 1999, 53, 258–262. [Google Scholar] [CrossRef]
- Kantlehner, W. New Methods for the preparation of aromatic aldehydes. Eur. J. Org. Chem. 2003, 14, 2530–2546. [Google Scholar] [CrossRef]
- Hoover, J.M.; Stahl, S.S. Highly practical copper(I)/TEMPO catalyst system for chemoselective aerobic oxidation of primary alcohols. J. Am. Chem. Soc. 2011, 133, 16901–16910. [Google Scholar] [CrossRef] [PubMed]
- Neves, Â.C.B.; Calvete, M.J.F.; Pinho e Melo, T.M.V.D.; Pereira, M.M. Immobilized catalysts for hydroformylation reactions: A versatile tool for aldehyde synthesis. Eur. J. Org. Chem. 2012, 2012, 6309–6320. [Google Scholar] [CrossRef]
- Cheng, C.; Brookhart, M. Efficient reduction of esters to aldehydes through iridium-catalyzed hydrosilylation. Angew. Chem. Int. Ed. Engl. 2012, 51, 9422–9424. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Misal Castro, L.C.; Zheng, J.; Roisnel, T.; Dorcet, V.; Sortais, J.-B.; Darcel, C. Selective reduction of esters to aldehydes under the catalysis of well-defined NHC-iron complexes. Angew. Chem. Int. Ed. Engl. 2013, 52, 8045–8049. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.; Song, Q. Aldehydes and ketones formation: Copper-catalyzed aerobic oxidative decarboxylation of phenylacetic acids and α-hydroxyphenylacetic acids. J. Org. Chem. 2014, 79, 1867–1871. [Google Scholar] [CrossRef] [PubMed]
- Tamura, N.; Aoyama, T.; Takido, T.; Kodomari, M. Novel [4-Hydroxy-TEMPO + NaCl]/SiO2 as a reusable catalyst for aerobic oxidation of alcohols to carbonyls. Synlett 2012, 23, 1397–1401. [Google Scholar] [CrossRef]
- De M. Muñoz, J.; Alcázar, J.; de la Hoz, A.; Díaz-Ortiz, A. Application of flow chemistry to the selective reduction of esters to aldehydes. Eur. J. Org. Chem. 2012, 2, 260–263. [Google Scholar] [CrossRef]
- Nicolaou, K.C.; Adsool, V.A.; Hale, C.R.H. An expedient procedure for the oxidative cleavage of olefinic bonds with PhI(OAc)2, NMO, and catalytic OsO4. Org. Lett. 2010, 12, 1552–1555. [Google Scholar] [CrossRef] [PubMed]
- Isidro-Llobet, A.; Alvarez, M.; Albericio, F. Amino acid-protecting groups. Chem. Rev. 2009, 109, 2455–2504. [Google Scholar] [PubMed]
- Góngora-Benítez, M.; Tulla-Puche, J.; Albericio, F. Multifaceted roles of disulfide bonds. peptides as therapeutics. Chem. Rev. 2013, 114, 901–926. [Google Scholar]
- Postma, T.; Albericio, F. Cysteine pseudoprolines for thiol protection and peptide macrocyclization enhancement in fmoc-based solid-phase peptide synthesis. Org. Lett. 2014, 16, 1772–1775. [Google Scholar] [PubMed]
- Postma, T.M.; Giraud, M.; Albericio, F. Trimethoxyphenylthio as a highly labile replacement for tert-butylthio cysteine protection in Fmoc solid phase synthesis. Org. Lett. 2012, 14, 5468–5471. [Google Scholar] [CrossRef] [PubMed]
- Góngora-Benítez, M.; Mendive-Tapia, L.; Ramos-Tomillero, I.; Breman, A.C.; Tulla-Puche, J.; Albericio, F. Acid-labile cys-protecting groups for the Fmoc/tBu strategy: Filling the gap. Org. Lett. 2012, 14, 5472–5475. [Google Scholar] [CrossRef] [PubMed]
- Garcı́a, O.; Nicolás, E.; Albericio, F. Solid-phase synthesis: A linker for side-chain anchoring of arginine. Tetrahedron Lett. 2003, 44, 5319–5321. [Google Scholar]
- Garcia, O.; Bofill, J.M.; Nicolas, E.; Albericio, F. 2,2,4,6,7-Pentamethyl-2,3-dihydrobenzofuran-5-methyl (Pbfm) as an alternative to the trityl group for the side-chain protection of cysteine and asparagine/glutamine. Eur. J. Org. Chem. 2010, 19, 3631–3640. [Google Scholar] [CrossRef]
- Ramos-Tomillero, I.; Mendive-Tapia, L.; Góngora-Benítez, M.; Nicolás, E.; Tulla-Puche, J.; Albericio, F. Understanding acid lability of cysteine protecting groups. Molecules 2013, 18, 5155–5162. [Google Scholar] [CrossRef] [PubMed]
- Ohsawa, K.; Yoshida, M.; Doi, T. A direct and mild formylation method for substituted benzenes utilizing dichloromethyl methyl ether-silver trifluoromethanesulfonate. J. Org. Chem. 2013, 78, 3438–3444. [Google Scholar] [CrossRef] [PubMed]
- Vilsmeier, A.; Haack, A. Action of phosphorus halides on alkylformanilides. A new method for the preparation of secondary and tertiary p-alkylaminonobenzaldehydes. Ber. Dtsch. Chem. Ges. A/B 1927, 60, 119–122. [Google Scholar] [CrossRef]
- Duff, J.C.; Bills, E.J. Reactions between hexamethylenetetramine and phenolic compounds. Part I. A new method for the preparation of 3- and 5-aldehydosalicylic acids. J. Chem. Soc. 1932, 2861–2862. [Google Scholar]
- Hansen, T.V.; Skattebøl, L. Ortho-Formylation of phenols; Preparation of 3-bromosalicylaldehyde. Org. Synth. 2005, 82, 64–68. [Google Scholar] [CrossRef]
- Garcıa, O.; Nicolás, E.; Albericio, F. o-Formylation of electron-rich phenols with dichloromethyl methyl ether and TiCl4. Tetrahedron Lett. 2003, 44, 4961–4963. [Google Scholar] [CrossRef]
- Heras, C.; Ramos-Tomillero, I.; Caballero, M.; Paradís-Bas, M.; Nicolás, E.; Albericio, F.; de P. R. Moreira, I.; Bofill, J.M. On the mechanism of phenolic formylation mediated by ticl4 complexes: Existence of diradical intermediates induced by valence tautomerism. Eur. J. Org. Chem. 2015. [Google Scholar] [CrossRef]
- Gross, H.; Rieche, A.; Matthey, G. Über α-Halogenäther, XIII. Neue verfahren zur darstellung von phenolaldehyden. Chem. Ber. 1963, 96, 308–313. [Google Scholar]
- Cresp, T.M.; Sargent, M.V.; Elix, J.A.; Murphy, D.P.H. Formylation and bromination ortho to the hydroxy-group of 2-carbonylsubstituted phenols in the presence of titanium(VI)chloride. J. Chem. Soc. Perkin Trans. 1 1973, 340–345. [Google Scholar] [CrossRef]
- Hamilton, P.M.; McBeth, R.; Bekebrede, W.; Sisler, H.H. Molecular addition compounds of titanium tetrachloride with several ethers. J. Am. Chem. Soc. 1953, 75, 2881–2883. [Google Scholar] [CrossRef]
- Calderazzo, F.; Ferri, I.; Pampaloni, G.; Troyanov, S. η6-Arene derivatives of titanium(IV), zirconium(IV) and hafnium(IV). J. Organomet. Chem. 1996, 518, 189–196. [Google Scholar] [CrossRef]
- Martín, R.; Murruzzu, C.; Pericàs, M.A.; Riera, A. General approach to glycosidase inhibitors. Enantioselective synthesis of deoxymannojirimycin and swainsonine. J. Org. Chem. 2005, 70, 2325–2328. [Google Scholar]
- Andrus, M.B.; Liu, J.; Ye, Z.; Cannon, J.F. Asymmetric glycolate aldol reactions using cinchonium phase-transfer catalysts. Org. Lett. 2005, 7, 3861–3864. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Not available.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramos-Tomillero, I.; Paradís-Bas, M.; De Pinho Ribeiro Moreira, I.; Bofill, J.M.; Nicolás, E.; Albericio, F. Formylation of Electron-Rich Aromatic Rings Mediated by Dichloromethyl Methyl Ether and TiCl4: Scope and Limitations. Molecules 2015, 20, 5409-5422. https://doi.org/10.3390/molecules20045409
Ramos-Tomillero I, Paradís-Bas M, De Pinho Ribeiro Moreira I, Bofill JM, Nicolás E, Albericio F. Formylation of Electron-Rich Aromatic Rings Mediated by Dichloromethyl Methyl Ether and TiCl4: Scope and Limitations. Molecules. 2015; 20(4):5409-5422. https://doi.org/10.3390/molecules20045409
Chicago/Turabian StyleRamos-Tomillero, Iván, Marta Paradís-Bas, Ibério De Pinho Ribeiro Moreira, Josep María Bofill, Ernesto Nicolás, and Fernando Albericio. 2015. "Formylation of Electron-Rich Aromatic Rings Mediated by Dichloromethyl Methyl Ether and TiCl4: Scope and Limitations" Molecules 20, no. 4: 5409-5422. https://doi.org/10.3390/molecules20045409





