New 1-(3-Nitrophenyl)-5,6-dihydro-4H-[1,2,4]triazolo[4,3-a][1,5]benzodiazepines: Synthesis and Computational Study
Abstract
:1. Introduction
2. Results and Discussion
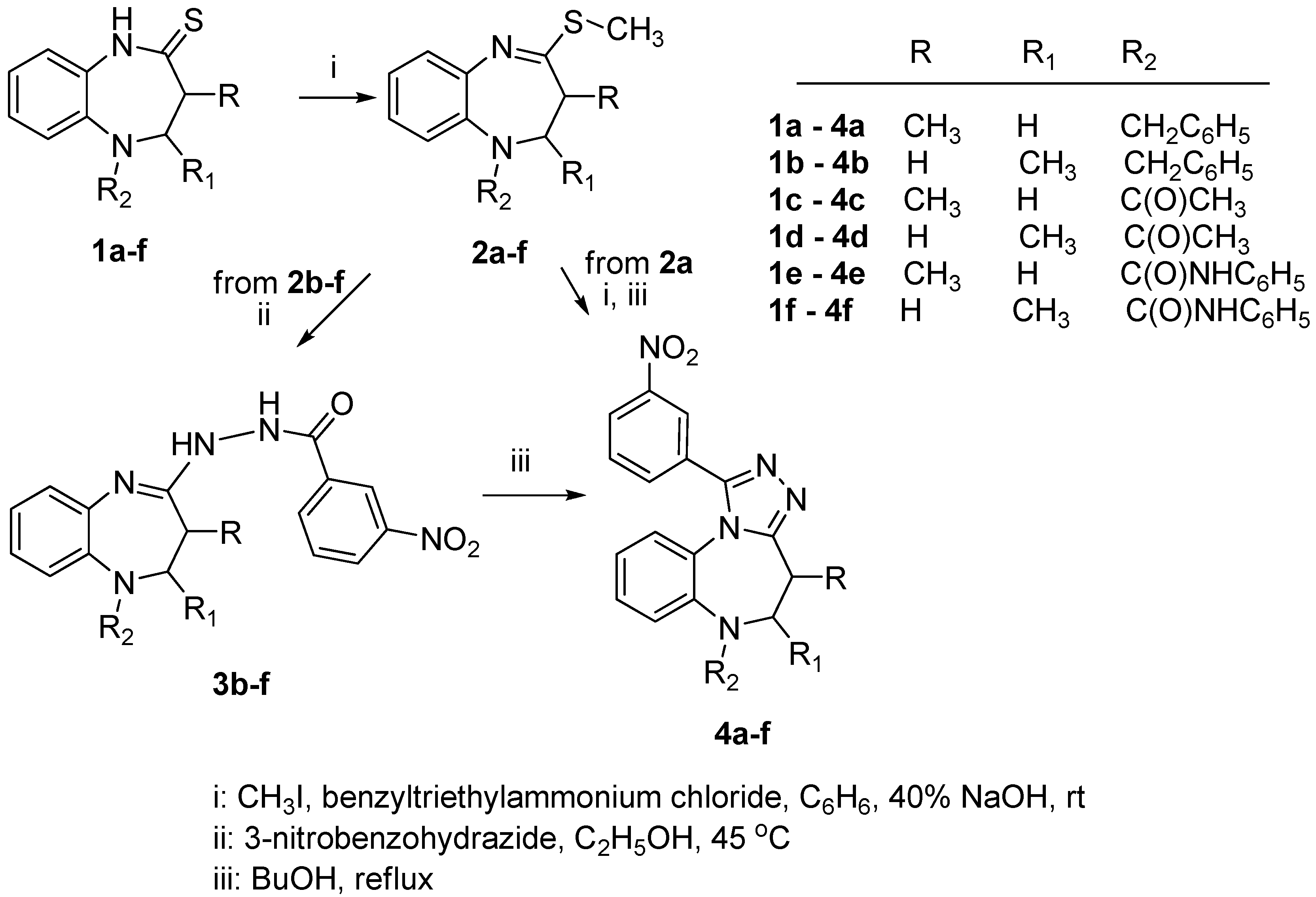
2.1. Chemistry
| Compound | Ethanol, Time (h) | Toluene, Time (h) | 1-Butanol, Time (h) |
|---|---|---|---|
| 4a | 36 | 19 | 9 |
| 4b | 43 | 21 | 11 |
| 4c | 41 | 20 | 14 |
| 4d | 48 | 25 | 16 |
| 4e | – | ‒ | 21 |
| 4f | 47 | 22 | 15 |
| Compound | Solvent | Temp (°C ) | t (h) | Yield (%) |
|---|---|---|---|---|
| N'-(1-acetyl-2-methyl-2,3-dihydro-1H-1,5-benzodiazepin-4-yl)benzohydrazide | ethanol | room | 16 | 91 |
| N'-(1-acetyl-2-methyl-2,3-dihydro-1H-1,5-benzodiazepin-4-yl)-3-nitrobenzohydrazide | ethanol | 45 | 18 | 65 |
| 6-acetyl-5-methyl-1-phenyl-5,6-dihydro-4 H-[1,2,4]triazolo[4,3-a][1,5]benzodiazepine | ethanol | 78 | 8 | 77 |
| 6-acetyl-5-methyl-1-(3-nitrophenyl)-5,6-dihydro-4 H-[1,2,4]triazolo[4,3-a][1,5]benzodiazepine | 1-butanol | 118 | 20 | 85 |
2.2. Computational Study
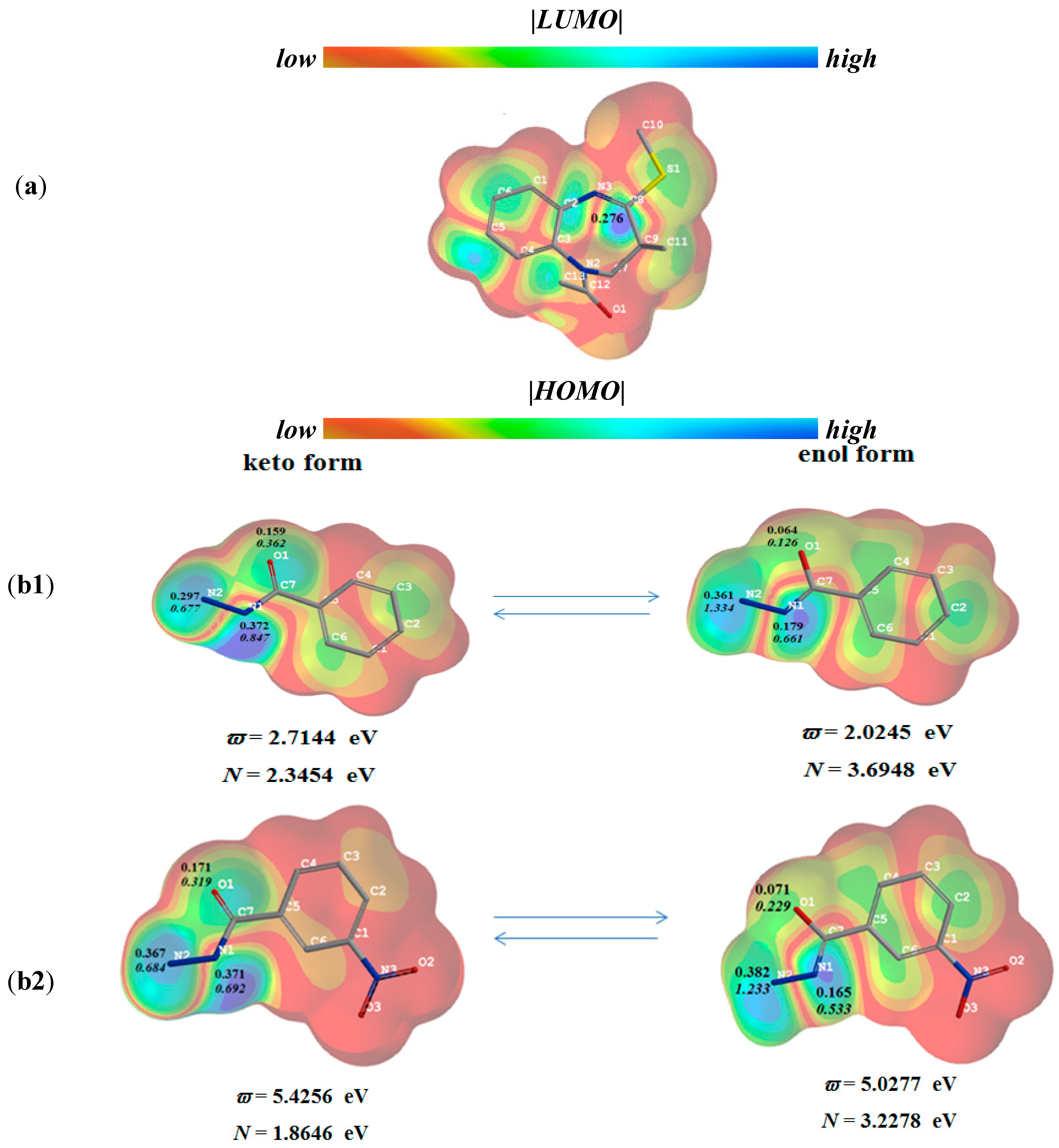
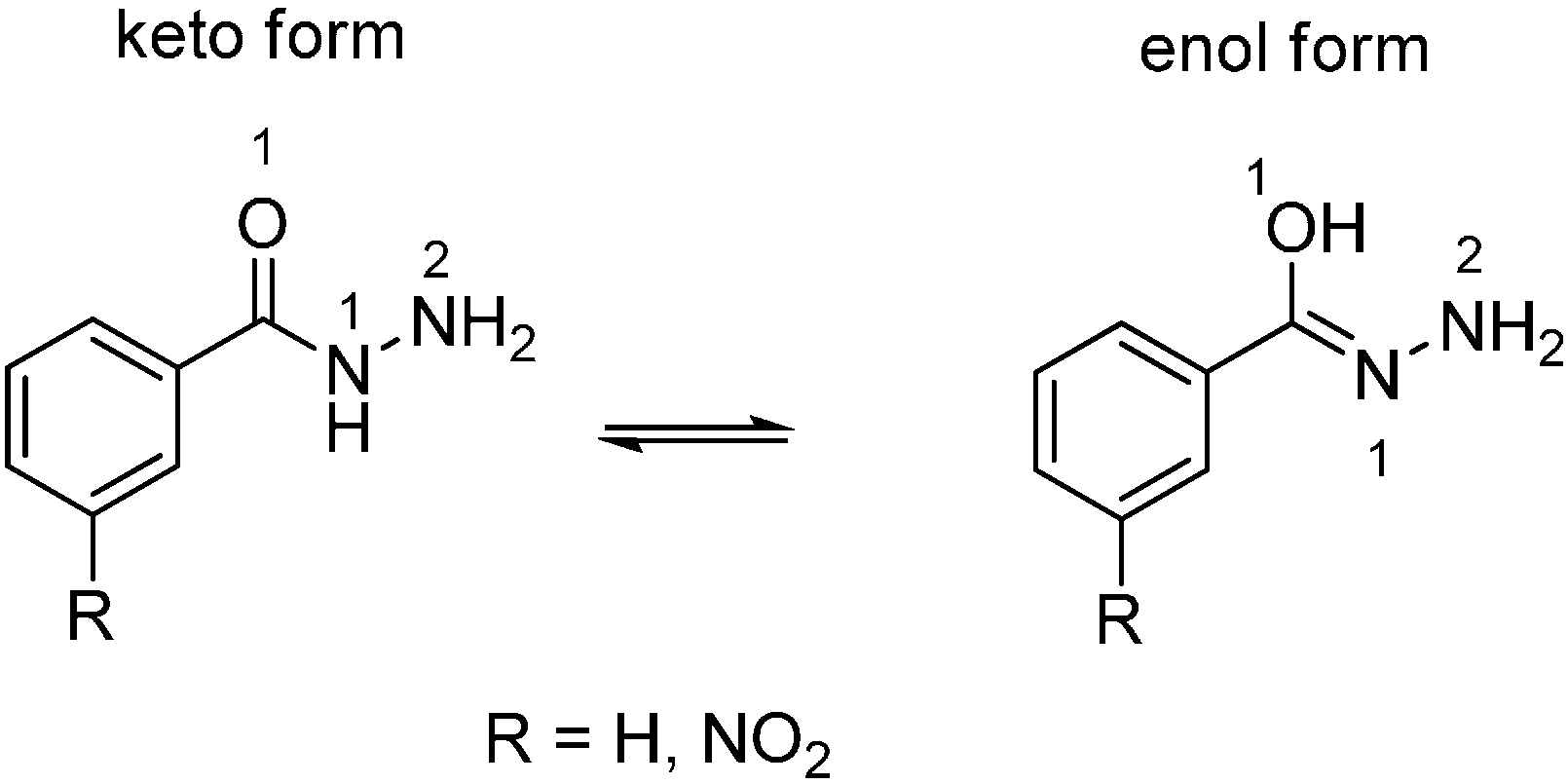

3. Experimental Section
3.1. General Information
3.1.1. General Procedure for the Synthesis of 4-(Methylsulfanyl)-2,3-dihydro-1H-1,5-benzodiazepines 2c,e,f
3.1.2. General Procedure for the Synthesis of N'-(2,3-Dihydro-1H-1,5-benzodiazepin-4-yl)-3-nitro-benzohydrazides 3b–f
3.1.3. Synthesis of 6-Benzyl-4-methyl-1-(3-nitrophenyl-5,6-dihydro-4H-[1,2,4]triazolo[4,3-a][1,5] benzodiazepine (4a)
3.1.4. General Procedure for the Synthesis of 1-(3-Nitrophenyl)-5,6-dihydro-4H-[1,2,4]triazolo[4,3-a][1,5]benzodiazepines 4b–f
3.2. Quantum Mechanical Computations
3.2.1. General
3.2.2. The Assessment of DFT-Based Global and Local Reactivity Indices
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Colanceska-Ragenovic, K.; Dimova, V.; Kakurinov, V.; Molnar, D.G.; Buzarovska, A. Synthesis, antibacterial and antifungal activity of 4-substituted-5-aryl-1,2,4-triazoles. Molecules 2001, 6, 815–824. [Google Scholar] [CrossRef]
- Kucukguzel, I.; Kucukguzel, S.G.; Rollas, S.; Kiraz, M. Some 3-thioxo/alkylthio-1,2,4-triazoles with a substituted thiourea moiety as possible antimycobacterials. Bioorg. Med. Chem. Lett. 2001, 11, 1703–1707. [Google Scholar] [CrossRef] [PubMed]
- Amir, M.; Shikha, K. Synthesis and anti-inflammatory, analgesic, ulcerogenic and lipid peroxi-dation activities of some new 2-[(2,6-dichloroanilino)phenyl]acetic acid derivatives. Eur. J. Med. Chem. 2004, 39, 535–545. [Google Scholar] [CrossRef] [PubMed]
- Labanauskas, L.; Udrenaite, E.; Gaidelis, P.; Brukstus, A. Synthesis of 5-(2-, 3- and 4-methoxyphenyl)-4H-1,2,4-triazole-3-thiol derivatives exhibiting anti-inflammatory activity. Farmaco 2004, 59, 255–259. [Google Scholar] [CrossRef] [PubMed]
- Tozkoparan, B.; Kupeli, E.; Yesilada, E.; Ertan, M. Preparation of 5-aryl-3-alkylthio-l,2,4-triazoles and corresponding sulfones with antiinflammatory–analgesic activity. Bioorg. Med. Chem. 2007, 15, 1808–1814. [Google Scholar] [CrossRef] [PubMed]
- Hasan, A.; Thomas, N.F.; Gapil, S. Synthesis, Characterization and Antifungal Evaluation of 5-Substituted-4-Amino-1,2,4-Triazole-3-Thioesters. Molecules 2011, 16, 1297–1309. [Google Scholar] [CrossRef]
- Guirado, A.; Lopez Sanchz, J.I.; Bautista, D.; Galvez, J. Synthesis and biological evaluation of 4-alkoxy-6,9-dichloro[1,2,4]triazole[4,3-a] quinoxalines as inhibitors of TNF-α and IL-6. Eur. J. Med. Chem. 2012, 54, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Swamy, S.N.; Priya, B.B.S.; Prabhuswamy, B.; Doreswamy, B.H.; Prasad, J.S.; Kanchugarakoppal, S.; Rangappa, K.S. Synthesis of pharmaceutically important condensed heterocyclic 4,6-disubstituted-1,2,4-triazolo-1,3,4-thiadiazole derivatives as antimicrobials. Eur. J. Med. Chem. 2006, 41, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Bhat, K.S.; Poojary, B.; Prasad, D.J.; Naik, P.; Holla, B.S. Synthesis and antitumor activity studies of some new fused 1,2,4-triazole derivatives carrying 2,4-dichloro-5-fluorophenyl moiety. Eur. J. Med. Chem. 2009, 44, 5066–5070. [Google Scholar] [CrossRef] [PubMed]
- Johnston, G.A.R. Medicinal chemistry and molecular pharmacology of GABA-C receptors. Curr. Top. Med. Chem. 2002, 2, 903–913. [Google Scholar] [CrossRef] [PubMed]
- Clemons, M.; Coleman, R.E.; Verma, S. Aromatase inhibitors in the adjuvant setting: Bringing the Gold to the standards? Cancer Treat. Rev. 2004, 30, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Al-Soud, Y.A.; Al-Dweri, M.N.; Al-Masoudi, N.A. Synthesis, antitumor and antiviral properties of some 1,2,4-triazole derivatives. Farmaco 2004, 59, 775–783. [Google Scholar] [CrossRef] [PubMed]
- Hester, J.B., Jr.; von Voigtlander, P. 6-Aryl-4H-s-triazolo[4,3-a][1,4]benzodiazepines. Influence of 1-substitution on pharmacological activity. J. Med. Chem. 1979, 22, 489–496. [Google Scholar] [CrossRef]
- Bayrak, H.; Demirbas, A.; Bektas, H.; Alpay, K.S.; Demirbasm, N. Synthesis and antimicrobial activities of some new 1,2,4-triazole derivatives. Turk. J. Chem. 2010, 34, 835–846. [Google Scholar] [CrossRef]
- Varalakshmi, C.; Appa Rao, B.V. Inhibition of corrosion of copper by 5-mercapto-3-p-nitrophenyl-1,2,4-triazole in aqueous environment. Anti-Corros. Methods Mater. 2001, 48, 171–180. [Google Scholar] [CrossRef]
- Kommu, N.; Ghule, V.D.; Kumar, A.S.; Sahoo, A.K. Triazole- substituted nitroarene derivatives: Synthesis, Characterization and Energetic studies. Chem.-Asian J. 2014, 9, 166–178. [Google Scholar] [CrossRef] [PubMed]
- Puodžiūnaitė, B.; Jančienė, R.; Kosychova, L.; Stumbrevičiūtė, Z. On the synthetic way to novel peri-annelated imidazo[1,5]benzodiazepinones as the potent non-nucleoside reverse transcriptase inhibitors. ARKIVOC 2000, iv, 512–522. [Google Scholar] [CrossRef]
- Janciene, R.; Stumbreviciute, Z.; Vektariene, A.; Kosychova, L.; Klimavicius, A.; Palaima, A.; Puodziunaite, B. Synthesis of novel annelated systems based on the interaction and reactivity estimation of amino-1,5-benzodiazepin-2-ones with dimethyl-2-oxogluconate. J. Heterocycl. Chem. 2009, 46, 1339–1345. [Google Scholar] [CrossRef]
- Jančienė, R.; Stumbrevičiutė, Z.; Vektariene, A.; Kosychova, L.; Sirutkaitis, R.; Palaima, A.; Staniulytė, Z.; Puodziunaitė, B.D. Researches on thiazolobenzodiazepines: Behavior of tetrahydro-1,5-benzodiazepine-thiones with aromatic alpha-haloketones. Heteroatom. Chem. 2008, 19, 72–81. [Google Scholar] [CrossRef]
- Kosychova, L.; Stumbrevičiūtė, Z.; Plečkaitienė, L.; Jančienė, R.; Puodžiūnaitė, B.D. Synthesis of substituted 5,6-dihydro-4H-[1,2,4]triazolo[4,3-a][1,5]benzodiazepines. Khim. Geterotsikl. Soedin. 2004, 6, 943–948. [Google Scholar]
- Kosychova, L.; Stumbrevičiūtė, Z.; Jančienė, R.; Staniulytė, Z.; Puodžiūnaitė, B.D. A convenient synthesis of novel [1,2,4]oxadiazolo[4,3-a][1,5]benzodiazepine derivatives. ARKIVOC 2011, xi, 82–91. [Google Scholar] [CrossRef]
- Jančienė, R.; Vektorienė, A.; Stumbrevičiūtė, Z.; Kosychova, L.; Klimavičius, A.; Puodžiūnaitė, B. A Straightforward Synthesis or Novel 4H-Thiazolo-[3,2-d][1,5]benzodiazepine Derivatives. Heteroatom. Chem. 2004, 15, 363–368. [Google Scholar] [CrossRef]
- Puodžiūnaitė, B.; Kosychova, L.; Jančienė, R.; Stumbrevičiūtė, Z. 2,3-Dihydro-1H-1,5-benzodiazepines: A Conversion of Thiolactams to Amidines. Monatsh. Chem. 1997, 128, 1275–1281. [Google Scholar] [CrossRef]
- Janciene, R.; Stumbreviciute, Z.; Podeniene, D.; Puodziunaite, B.D.; Black, S.; Husbands, S.M. Synthesis of novel 1-substituted [1,3]thiazolo[3,2-a]–[1,5]benzodiazepine derivatives from 1,5–benzodiazepine-2-thiones and α-halogen carbonyl coumpounds. J. Heterocycl. Chem. 2009, 43, 979–984. [Google Scholar] [CrossRef]
- Puodžiūnaitė, B.; Kosychova, L.; Jančienė, R.; Stumbrevičiūtė, Z. Synthesis and mild conversion of 1,5-benzodiazepine iminothioethers into hydrazides. Khim. Geterotsikl. Soedin. 1998, 3, 368–372. [Google Scholar]
- Kosychova, L.; Stumbrevičiūtė, Z.; Jančienė, R.; Klimavičius, A.; Staniulytė, Z.; Palaima, A.; Puodžiūnaitė, B.D. Synthesis of new [1,2,4]triazolo[4,3-a][1,5]benzodiazepine derivatives. Chemija (Vilnius) 2011, 22, 60–64. [Google Scholar]
- Pearson, R.G. Absolute electronegativity and hardness correlated with molecular orbital theory. Proc. Natl. Acad. Sci. USA. 1986, 83, pp. 8440–8441. Available online: http://www.pnas.org/content/83/22/8440.full.pdf (accessed on 7 August 2014). [CrossRef] [PubMed]
- Parr, R.G.; Szentpaly, L.; Liu, S. Electrophilicity Index. J. Am. Chem. Soc. 1999, 121, 1922–1924. [Google Scholar] [CrossRef]
- Pearson, R.G. Chemical hardness and density functional theory. J. Chem. Sci. 2005, 117, pp. 369–377. Available online: http://www.ias.ac.in/chemsci/Pdf-sep2005/369.pdf (accessed on 8 August 2014). [CrossRef]
- Geerlings, P.; de Proft, F.; Langeneker, W. Conceptual density functional theory. Chem. Rev. 2003, 103, 1793–1873. [Google Scholar] [CrossRef] [PubMed]
- Domingo, L.R.; Perez, P.; Seez, J. Understanding C-C bond formation in polar reactions. An ELF analysis of the Friedel-Crafts reaction between indoles and nitroolefins. RCS Adv. 2013, 3, 7520–7528. [Google Scholar] [CrossRef]
- Perez, P.; Domingo, L.R.; Duque-Norena, M.; Chamorro, E. A condensed-to-atom nucleophilicity index. An application to the direct effects on the electrophilic aromatic substitutions. J. Mol. Struct.-Theochem. 2009, 895, 86–91. [Google Scholar]
- Contreras, R.; Andres, J.; Safont, V.S.; Campodonico, P.; Santos, J.G. A theoretical study on the relationship between nucleophilicity and ionization potentials in solution phase. J. Phys. Chem. A 2003, 107, 5588–5593. [Google Scholar] [CrossRef]
- Domingo, L.R.; Chamorro, E.; Perez, P. Understanding the reactivity of captodative ethylenes in polar cycloaddition reactions. A theoretical study. J. Org. Chem. 2008, 73, 4615–4624. [Google Scholar] [CrossRef] [PubMed]
- Domingo, L.R.; Perez, P. The nucleophilicity N index in organic chemistry. Org. Biomol. Chem. 2011, 9, 7168–7175. [Google Scholar] [CrossRef]
- Contreras, R.R.; Fuentealba, P.; Galvin, M.; Perez, P. A direct evaluation of regional Fukui functions in molecules. Chem. Phys. Lett. 1999, 304, 405–413. [Google Scholar] [CrossRef]
- Fuentealba, P.; Perez, P.; Contreras, R. On the condensed Fukui function. J. Chem. Phys. 2000, 113, 2544–2551. [Google Scholar] [CrossRef]
- Campodonico, P.R.; Aliaga, M.E.; Santos, J.G.; Castro, E.A.; Contreras, R. Reactivity of benzohydrazide derivatives towards acetylation reaction. Experimental and theoretical studies. Chem. Phys. Lett. 2010, 488, 86–89. [Google Scholar] [CrossRef]
- Spartan’10, Version 1.1.0. Wavefunction, Inc.: Irvine, CA, USA, 2010. Available online: http://www.wavefun.com (accessed on 22 March 2011).
- Sample Availability: Samples of the compounds 4a–f are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kosychova, L.; Karalius, A.; Staniulytė, Z.; Sirutkaitis, R.A.; Palaima, A.; Laurynėnas, A.; Anusevičius, Ž. New 1-(3-Nitrophenyl)-5,6-dihydro-4H-[1,2,4]triazolo[4,3-a][1,5]benzodiazepines: Synthesis and Computational Study. Molecules 2015, 20, 5392-5408. https://doi.org/10.3390/molecules20045392
Kosychova L, Karalius A, Staniulytė Z, Sirutkaitis RA, Palaima A, Laurynėnas A, Anusevičius Ž. New 1-(3-Nitrophenyl)-5,6-dihydro-4H-[1,2,4]triazolo[4,3-a][1,5]benzodiazepines: Synthesis and Computational Study. Molecules. 2015; 20(4):5392-5408. https://doi.org/10.3390/molecules20045392
Chicago/Turabian StyleKosychova, Lidija, Antanas Karalius, Zita Staniulytė, Romualdas Aleksas Sirutkaitis, Algirdas Palaima, Audrius Laurynėnas, and Žilvinas Anusevičius. 2015. "New 1-(3-Nitrophenyl)-5,6-dihydro-4H-[1,2,4]triazolo[4,3-a][1,5]benzodiazepines: Synthesis and Computational Study" Molecules 20, no. 4: 5392-5408. https://doi.org/10.3390/molecules20045392
APA StyleKosychova, L., Karalius, A., Staniulytė, Z., Sirutkaitis, R. A., Palaima, A., Laurynėnas, A., & Anusevičius, Ž. (2015). New 1-(3-Nitrophenyl)-5,6-dihydro-4H-[1,2,4]triazolo[4,3-a][1,5]benzodiazepines: Synthesis and Computational Study. Molecules, 20(4), 5392-5408. https://doi.org/10.3390/molecules20045392





