Determination of Phenolic Compounds and Antioxidant Activity in Leaves from Wild Rubus L. Species
Abstract
:1. Introduction
2. Results and Discussion
2.1. Peak Identification and Assignment
| Compounds | Rt (min) | λmax (nm) | [MS]− | [MS-MS]− |
|---|---|---|---|---|
| p-Coumaric acid derivative | 1.35 | 314 | 787.9050 | 420.9105/347.9189/163.0380 |
| Neochlorogenic acid | 2.27 | 323 | 353.0866 | 235.9249/191.0553/146.9378 |
| Chlorogenic acid | 2.35 | 323 | 353.0866 | 235.9249/191.0553/146.9378 |
| Caffeoyl hexoside | 2.99 | 320 | 341.0849 | 179.0349/135.0464 |
| p-Coumaroylquinic acid | 3.14 | 314 | 337.0937 | 191.0553/163.0380 |
| Caffeoyl hexoside | 3.45 | 320 | 341.0849 | 179.0349/135.0464 |
| p-Coumaric acid | 4.22 | 312 | 163.0380 | |
| Sanguiin H-6 | 4.79 | 245 | 1869.0851 | 935.0760/633.075/300.9999 |
| Ellagitannins Lambertianin C | 5.03 | 244 | 1401.3730 | 633.075/300.9999 |
| Ellagitannins hexoside (casuarinin) | 5.53 | 244 | 935.0760 | 633.075/300.9999 |
| Ellagic acid pentoside | 6.28 | 360 | 433.0777 | 300.9999 |
| Quercetin-3-methoxyhexoside | 6.38 | 360 | 493.1001 | 463.3010 |
| Ellagic acid | 6.51 | 364 | 300.9999 | |
| Ellagic acid rhamnoside | 6.64 | 360 | 447.0527 | 300.9999 |
| Kaempferol-3-O-glucoside-rhamnoside-7-O-rhamnoside | 6.73 | 346 | 739.1930 | 593.1559/285.0187 |
| Quercetin-3-O-rutinoside | 6.90 | 352 | 609.1080 | 463.0397/301.0277/151.0034 |
| Quercetin-3-O-galactoside | 7.04 | 353 | 463.0843 | 301.0277/151.0034 |
| Quercetin-3-O-glucuronide | 7.14 | 351 | 477.0670 | 301.0277/151.0034 |
| Quercetin-3-O-glucoside | 7.20 | 352 | 463.0843 | 301.0277/151.0034 |
| Kaempferol derivative | 7.27 | 345 | 475.0753 | 447.0968/285.0187 |
| Quercetin-3-O-hexoside | 7.32 | 352 | 463.0843 | 301.0277/151.0034 |
| Luteolin-3-O-glucoronide | 7.49 | 340 | 461.0710 | 285.0187 |
| Quercetin-3-O-pentoside | 7.88 | 352 | 433.0777 | 301.0277/151.0034 |
| Quercetin-3-[6''-(3-hydroxy-3-methylglutaroyl)-galactoside | 7.94 | 345 | 607.1293 | 463.0843/301.0277/151.0034 |
| Quercetin-3-O-pentoside | 8.12 | 352 | 433.0777 | 301.0277/151.0034 |
| Kaempferol-3-O-rutinoside | 8.27 | 350 | 593.1559 | 447.0968/285.0187 |
| Kaempferol-3-O-glucuronide | 8.43 | 346 | 461.0710 | 285.0187 |
| Methyl ellagic acid pentose | 8.6 | 360 | 477.1082 | 314.0421/300.9996 |
| Quercetin-3-O-6-acetylglucoside | 8.76 | 350 | 505.0980 | 447.0397/301.0277/151.0034 |
| Apigenin-3-O-glucoronide | 8.90 | 338 | 445.0710 | 269.0450 |
| Caffeoyldihexoside | 9.20 | 324 | 503.1190 | 341.0773/179.0321 |
| Caffeic acid derivative | 9.66 | 324 | 459.094 | 179.0321/161.0241 |
| Kaempferol-3-O-6-acetylglucoside | 10.19 | 345 | 489.1042 | 284.0313 |
2.2. Phenolic Compounds from Wild Blackberry Leaves
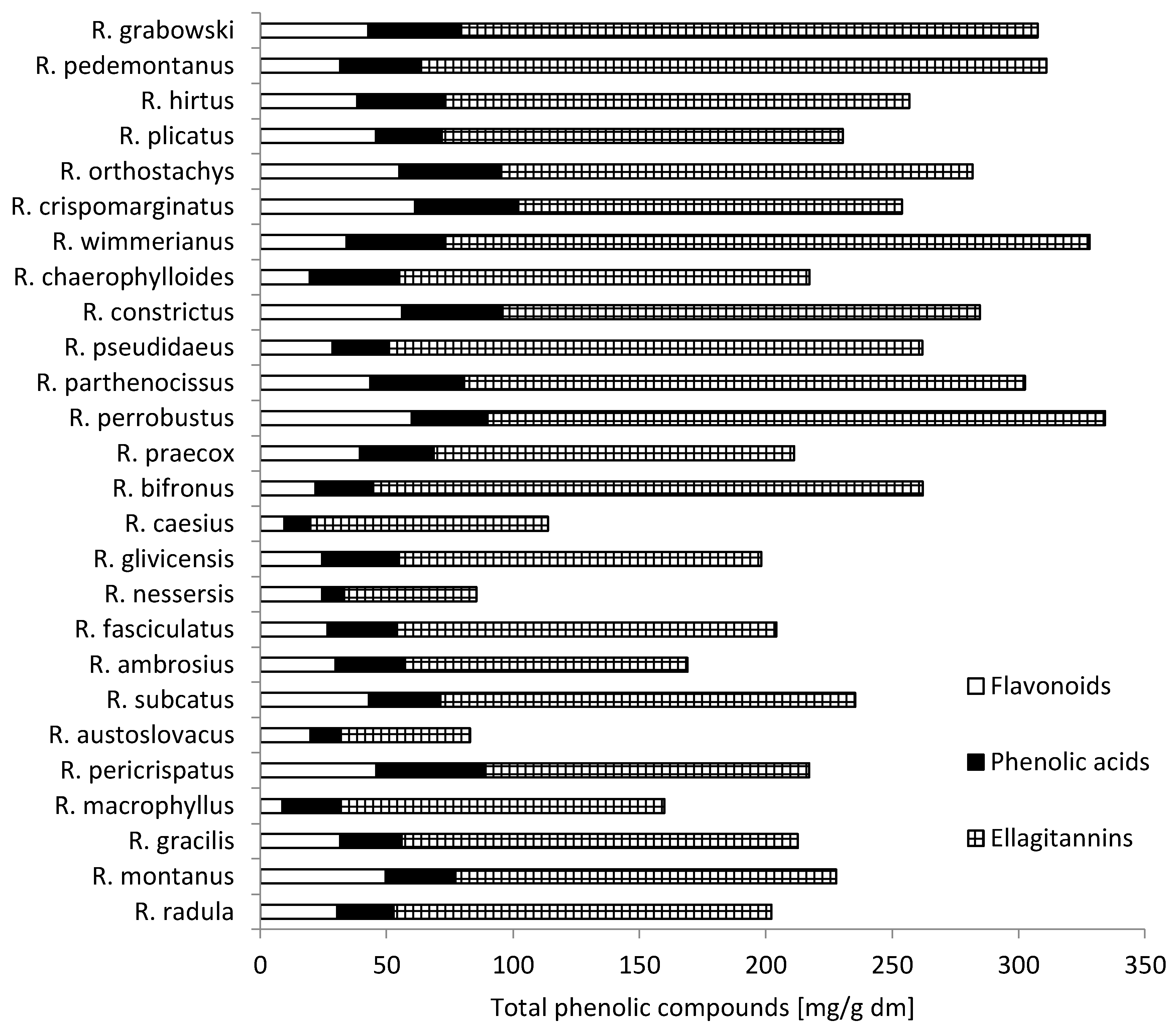
| Blackberry Species | Phenolic Acid | Ellagitanins | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| pCA der | NChA | ChA | C-hex | p-CqA | Chex | p-CA | EAp | EA | EArha | mEApen | Cdihex | CAd | SH6 | ELC | Ehex | |
| R. radula | 4.07 † | 7.64 | 0.39 | 0.82 | 2.72 | 1.77 | 1.28 | 0.83 | 2.33 | 0.06 | 0.12 | 0.18 | 0.27 | 16.66 | 71.08 | 61.56 |
| R. montanus | 4.65 | 8.68 | 0.44 | 3.27 | 3.24 | 3.55 | 1.71 | 0.00 | 1.56 | 0.09 | 0.11 | 0.06 | 0.23 | 16.95 | 66.43 | 67.20 |
| R. gracilis | 3.52 | 8.14 | 1.86 | 2.32 | 1.85 | 3.84 | 0.72 | 0.24 | 1.43 | 0.04 | 0.12 | 0.30 | 0.11 | 18.07 | 71.06 | 67.43 |
| R. macrophyllus | 5.11 | 11.07 | 0.55 | 0.23 | 1.91 | 1.56 | 0.68 | 0.34 | 1.12 | 0.04 | 0.05 | 0.18 | 0.07 | 14.48 | 46.92 | 66.84 |
| R. pericrispatus | 3.36 | 17.50 | 0.80 | 4.50 | 6.01 | 5.47 | 3.05 | 0.20 | 1.18 | 0.03 | 0.15 | 0.30 | 0.60 | 14.49 | 55.51 | 58.06 |
| R. austoslovacus | 2.57 | 3.56 | 0.19 | 0.72 | 1.37 | 1.02 | 0.40 | 0.40 | 1.24 | 0.02 | 0.06 | 0.01 | 0.09 | nd | 16.75 | 34.84 |
| R. subcatus | 5.71 | 6.12 | 0.88 | 4.40 | 1.68 | 4.47 | 1.79 | 0.25 | 2.02 | nd | 0.55 | 0.07 | 0.15 | 59.79 | 44.99 | 59.36 |
| R. ambrosius | 4.43 | 9.06 | 0.68 | 4.69 | 0.92 | 4.81 | 0.75 | 0.08 | 1.61 | nd | 0.30 | 0.11 | 0.03 | 21.11 | 39.37 | 51.24 |
| R. fasciculatus | 5.73 | 0.89 | 4.40 | 1.96 | 5.26 | 3.13 | 1.42 | 1.31 | 1.67 | 0.03 | 0.13 | 1.09 | 0.28 | 23.24 | 62.66 | 64.38 |
| R. nessersis | 2.29 | 0.65 | 1.02 | 0.26 | 0.40 | 0.64 | 0.52 | 0.62 | 2.10 | 0.01 | 0.07 | 0.02 | 0.03 | 12.22 | 5.69 | 34.47 |
| R. glivicensis | 4.33 | 7.30 | 5.26 | 1.48 | 3.58 | 4.14 | 2.60 | 0.33 | 1.16 | 0.02 | 0.10 | 0.19 | 0.09 | 48.46 | 36.29 | 58.50 |
| R. caesius | 5.06 | 0.74 | 0.44 | 0.31 | 0.93 | 0.26 | 0.11 | 0.85 | 1.25 | 0.06 | 0.04 | 0.09 | 0.04 | 5.79 | 36.26 | 51.99 |
| R. bifronus | 4.31 | 12.44 | 0.23 | 0.32 | 1.34 | 1.98 | 0.72 | 0.09 | 1.52 | nd | 0.03 | 0.06 | 0.01 | 39.48 | 63.73 | 114.07 |
| R. praecox | 4.64 | 1.45 | 0.26 | 7.39 | 2.34 | 8.90 | 0.78 | 0.33 | 1.76 | nd | 0.16 | 0.66 | 0.49 | 18.49 | 52.36 | 71.61 |
| R. perrobustus | 3.16 | 4.39 | 1.06 | 6.82 | 3.85 | 6.65 | 1.61 | 0.18 | 1.59 | nd | 0.32 | 0.08 | 0.10 | 53.02 | 123.41 | 67.96 |
| R. parthenocissus | 3.79 | 22.07 | 0.41 | 2.98 | 1.23 | 4.01 | 0.40 | 0.26 | 1.63 | nd | 0.12 | 0.13 | 0.04 | 11.41 | 95.06 | 115.44 |
| R. pseudidaeus | 3.12 | 1.33 | 2.38 | 0.75 | 3.59 | 5.40 | 0.36 | 3.30 | 1.61 | nd | 0.21 | 0.48 | 0.04 | 15.07 | 78.00 | 117.86 |
| R. constrictus | 5.91 | 7.69 | 1.92 | 6.57 | 3.02 | 8.69 | 1.12 | 0.27 | 2.94 | nd | 0.77 | 0.40 | 0.38 | 24.38 | 61.83 | 102.64 |
| R. chaerophylloides | 3.86 | 3.93 | 6.34 | 3.18 | 4.54 | 7.97 | 1.97 | 0.22 | 1.80 | nd | 0.12 | 1.44 | 0.13 | 13.96 | 44.72 | 103.46 |
| R. wimmerianus | 3.84 | 15.80 | 2.67 | 3.31 | 4.64 | 4.38 | 1.36 | 0.27 | 2.42 | nd | 0.07 | 0.30 | 0.11 | 64.44 | 76.12 | 114.46 |
| R. crispomarginatus | 2.73 | 6.82 | 0.38 | 7.54 | 3.10 | 12.11 | 1.71 | 0.54 | 2.85 | nd | 0.14 | 1.32 | 1.45 | 7.38 | 60.16 | 84.43 |
| R. orthostachys | 4.47 | 3.96 | 2.80 | 6.03 | 3.92 | 7.21 | 1.51 | 4.07 | 2.40 | nd | nd | 3.13 | 0.43 | 45.60 | 57.01 | 84.12 |
| R. plicatus | 4.82 | 5.68 | 1.52 | 3.62 | 1.15 | 5.83 | 0.54 | 0.35 | 1.74 | nd | nd | 0.41 | 0.16 | 58.48 | 42.17 | 58.14 |
| R. hirtus | 3.35 | 5.48 | 1.82 | 0.93 | 4.93 | 11.87 | 3.13 | 0.63 | 1.53 | nd | nd | 0.37 | 0.65 | 73.92 | 34.67 | 75.00 |
| R. pedemontanus | 3.17 | 5.00 | 5.27 | 1.07 | 8.28 | 2.97 | 2.68 | 0.44 | 1.56 | 0.05 | nd | 0.53 | 0.42 | 63.51 | 71.67 | 112.73 |
| R. grabowski | 4.92 | 2.12 | 2.49 | 6.49 | 6.50 | 7.69 | 1.91 | 0.37 | 2.09 | 0.21 | nd | 0.72 | 0.54 | 49.77 | 64.84 | 114.00 |
| ANOVA P value | *** | * | ** | * | ** | * | *** | ** | *** | *** | *** | ** | ** | * | * | * |
| Blackberry Species | Flavonoids | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Q-m-hex | K-glu-rha-rha | Q-rut | Q-gal | Q-gluc | Q-glu | Kd | Q-hex | L-gluc | Q-pen | Q-m-gal | Q-pen | K-rut | K-gluc | Q-a-glu | A-gluc | K-a-glu | |
| R. radula | 0.79 | 0.21 | 0.14 | 0.19 | nd | 6.43 | nd | 0.70 | 1.63 | 0.51 | 0.22 | 0.17 | 0.48 | 11.56 | 0.59 | 6.60 | 0.21 |
| R. montanus | nd | nd | 0.09 | 0.91 | nd | 23.87 | nd | 0.85 | 6.03 | 3.54 | 0.39 | 0.11 | 0.21 | 11.07 | 1.04 | 1.16 | 0.36 |
| R. gracilis | 0.22 | nd | 1.16 | 1.36 | nd | 7.36 | nd | 1.54 | 2.35 | 0.99 | 1.24 | 0.44 | 1.04 | 7.03 | 0.89 | 5.34 | 0.61 |
| R. macrophyllus | 0.27 | 0.06 | 0.08 | 0.04 | 0.06 | 0.73 | nd | 0.04 | 0.40 | 0.09 | 0.03 | 0.07 | 0.33 | 1.25 | 0.03 | 5.17 | 0.03 |
| R. pericrispatus | 0.16 | nd | 0.44 | 0.84 | nd | 18.91 | nd | 2.50 | 5.56 | 2.68 | 0.83 | 0.03 | 0.57 | 11.07 | 0.90 | 1.46 | 0.04 |
| R. austoslovacus | 0.14 | 0.04 | 0.22 | 0.24 | nd | 2.67 | nd | 0.12 | 0.60 | 0.49 | 0.08 | 0.02 | 0.25 | 6.03 | 0.27 | 8.56 | 0.04 |
| R. subcatus | 0.19 | 0.03 | 0.25 | 0.73 | nd | 10.81 | nd | 2.39 | 7.31 | 2.29 | 0.92 | 0.03 | 0.99 | 7.33 | 3.55 | 3.13 | 3.19 |
| R. ambrosius | nd | nd | 0.32 | 0.49 | 0.41 | 7.98 | nd | 0.28 | 6.88 | 1.60 | 0.37 | 0.23 | 0.20 | 5.08 | 0.93 | 4.81 | 0.19 |
| R. fasciculatus | 0.30 | 0.08 | 0.09 | 0.12 | nd | 5.09 | 3.10 | 2.56 | 1.58 | 0.35 | 0.12 | 0.03 | 0.35 | 9.30 | 0.31 | 3.04 | 0.11 |
| R. nessersis | nd | 0.01 | 0.16 | 0.07 | nd | 0.71 | nd | 0.20 | 0.60 | 0.18 | 0.21 | 0.34 | 0.58 | 1.93 | 0.05 | 19.03 | 0.43 |
| R. glivicensis | 0.17 | 0.15 | 0.07 | 0.30 | nd | 5.56 | nd | 0.33 | 1.17 | 0.54 | 0.18 | 0.12 | 0.11 | 10.81 | 0.52 | 4.30 | 0.08 |
| R. caesius | 0.35 | 0.06 | 0.07 | 0.02 | 0.04 | 1.12 | 0.79 | 0.63 | 0.56 | 0.16 | 0.02 | 0.02 | 0.13 | 1.46 | 0.09 | 4.02 | nd |
| R. bifronus | 0.27 | 0.07 | 0.20 | 0.11 | 0.13 | 4.96 | nd | 0.12 | 1.73 | 0.48 | 0.08 | 0.05 | 0.49 | 8.12 | 0.40 | 4.56 | nd |
| R. praecox | 0.22 | nd | 0.38 | 0.64 | 22.26 | nd | nd | 1.03 | 0.47 | 1.91 | nd | 0.09 | 0.58 | 9.29 | 0.54 | 1.96 | 0.16 |
| R. perrobustus | 0.22 | nd | 0.25 | 0.55 | 17.63 | nd | nd | 9.89 | 5.78 | 2.61 | nd | nd | 2.67 | 11.46 | 4.27 | 1.25 | 3.45 |
| R. parthenocisus | 0.12 | nd | 0.31 | 0.58 | 12.94 | nd | nd | 1.13 | 2.84 | 2.02 | nd | nd | 0.55 | 17.88 | 1.51 | 3.51 | 0.16 |
| R. pseudidaeus | nd | nd | 0.05 | 0.18 | 8.69 | 1.96 | nd | 3.68 | 0.23 | 1.27 | nd | nd | 0.26 | 9.43 | 1.26 | 1.49 | nd |
| R. constrictus | nd | nd | 0.55 | 0.59 | 15.83 | 4.58 | nd | nd | 9.35 | 2.72 | nd | nd | 3.85 | 10.51 | 3.13 | 2.36 | 2.64 |
| R. chaerophylloides | 0.19 | nd | 0.30 | 0.28 | 7.57 | 1.67 | nd | nd | 0.35 | 0.20 | nd | nd | 0.52 | 6.39 | 0.14 | 1.74 | 0.20 |
| R. wimmerianus | 0.40 | nd | 0.06 | 0.13 | 3.52 | nd | nd | 2.07 | 0.90 | 0.34 | nd | nd | 2.13 | 10.68 | 1.55 | 7.60 | 4.66 |
| R. crispomarginatus | 0.28 | nd | 0.59 | 1.84 | 28.08 | nd | nd | 3.51 | 4.66 | 5.11 | nd | nd | 0.50 | 13.67 | 1.10 | 1.92 | nd |
| R. orthostachys | nd | nd | nd | 0.65 | 24.59 | 4.64 | nd | nd | 3.63 | 4.90 | nd | nd | 0.33 | 12.60 | 0.76 | 3.01 | nd |
| R. plicatus | 0.20 | nd | 2.13 | 2.99 | 11.20 | 3.84 | nd | nd | 9.84 | 2.66 | nd | nd | 1.57 | 4.90 | 3.55 | 1.39 | 1.63 |
| R. hirtus | 0.22 | nd | 0.24 | 0.05 | 8.09 | 4.30 | nd | nd | 0.26 | 0.12 | nd | nd | 0.46 | 16.51 | 0.12 | 8.13 | nd |
| R. pedemontanus | 0.42 | nd | 0.05 | 0.10 | 3.52 | 0.87 | nd | nd | 0.85 | 0.14 | nd | nd | 0.17 | 11.66 | 0.30 | 13.52 | nd |
| R. grabowski | 0.11 | nd | 0.46 | 0.72 | 19.10 | nd | nd | 1.15 | 3.49 | 2.65 | nd | nd | 0.35 | 11.40 | 0.82 | 2.55 | nd |
| ANOVA P value | ** | * | * | * | * | * | ** | ** | ** | * | * | * | * | *** | *** | *** | ** |
2.3. Antioxidant Activity

2.4. Cluster Analysis

3. Experimental Section
3.1. Reagents and Standards
3.2. Plant Material
| Blackberry Species | Origin | Geographical Location |
|---|---|---|
| R. radula | Albigowa Honie | N 50°0’19.28” E 22°10’22.06” |
| R. montanus | Berendowice | N 49°40’14.85” E 22°43’39.58” |
| R. gracilis | Las Niechciałka | N 50°5’45.38” E 22°35’45.06” |
| R. macrophyllus | Las Niechciałka | N 50°5’45.38” E 22°35’45.06” |
| R. pericrispatus | Kopystno | N 49°41’8.38” E 22°38’32.49” |
| R. austroslovacus | Długie k/Przemyśla | N 49°45’49.61” E 22°42’4.59” |
| R. sulcatus | Łazy k/Birczy | N 49°42’49.56” E 22°32’3.14” |
| R. ambrosius | Zmysłówka | N 50°9’58.91” E 22°22’43.39” |
| R. fasciculatus | Łazy k/Birczy | N 49°42’49.56” E 22°32’3.14” |
| R. nessensis | Las Niechciałka | N 50°5’45.38” E 22°35’45.06” |
| R. glivicensis | Zmysłówka | N 50°9’58.91” E 22°22’43.39” |
| R. caesius | Długie k/Przemyśla | N 49°45’49.61” E 22°42’4.59” |
| R. bifrons | Berendowice | N 49°40’26.44” E 22°43’6.76” |
| R. praecox | Ławy k/Birczy | N 49°42’49.56” E 22°32’3.14” |
| R. perrobustus | Łazy k/Birczy | N 49°42’49.56” E 22°32’3.14” |
| R. parthenocissus | Berendowice | N 49°40’26.44” E 22°43’6.76” |
| R. pseudidaeus | Białobrzeszki | N 50°7’18.26” E 22°31’29.98” |
| R. constrictus | Berendowice | N 49°40’14.85” E 22°43’39.58” |
| R. chaerophylloides | Gruszowa | N 49°40’27.7” E 22°41’36.99” |
| R. wimmerianus | Zmysłówka | N 50°9’58.91” E 22°22’43.39” |
| R. crispomarginatus | Łazy k/Birczy | N 49°42’49.56” E 22°32’3.14” |
| R. orthostachys | Berendowice | N 49°40’14.85” E 22°43’39.58” |
| R. plicatus | Łazy k/Birczy | N 49°42’49.56” E 22°32’3.14” |
| R. hirtus | Kolbuszowa | N 50°15’12,63” E 21°47’46,61” |
| R. pedemontanus | Zmysłówka | N 50°9’58.91” E 22°22’43.39” |
| R. grabowskii | Zmysłówka | N 50°9’58.91” E 22°22’43.39” |
3.3. Extraction Procedure by Pressurized Liquid Extraction (PLE)
3.4. Identification of Polyphenols by the Liquid Chromatography-Mass Spectrometry (LC-MS) Method
3.5. Analysis of Antioxidant Activity
3.6. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Strik, B.C. Berry crops: Worldwide area and production systems. In Berry Fruit Value Added Products for Health Promotion, 1st ed.; Zhao, Y., Ed.; CRC: Boca Raton, FL, USA, 2007; Volume 1, pp. 3–49. [Google Scholar]
- Bonenber, G.K. Rośliny użyteczne człowiekowi; Instytut Wydawniczy Związków Zawodowych Warszawa: Warszawa, Poland, 1988; pp. 5–20. (In Polish) [Google Scholar]
- Patel, A.V.; Rojas-Vera, J.; Dacke, C.G. Therapeutic constituents and actions of Rubus species. Curr. Med. Chem. 2004, 11, 1501–1512. [Google Scholar] [CrossRef] [PubMed]
- Martini, S.; D’Addario, C.; Colacevich, A.; Focardi, S.; Borghini, F.; Santucci, A.; Figura, N.; Rossi, C. Antimicrobial activity against Helicobacter pylori strains and antioxidant properties of blackberry leaves (Rubus ulmifolius) and isolated compounds. Int. J. Antimicrob. Agents 2009, 34, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Larrosa, M.; García-Conesa, M.T.; Espín, J.C.; Tomás-Barberán, F.A. Ellagitannins, ellagic acid and vascular health. Mol. Asp. Med. 2010, 31, 513–539. [Google Scholar] [CrossRef]
- Wang, S.Y.; Lin, H.-S. Antioxidant activity in fruits and leaves of blackberry, raspberry and strawberry varies with cultivar and developmental stage. J. Agric. Food Chem. 2000, 48, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Siriwoharn, T.; Wrolstad, R.E. Characterisation of phenolics in Marion and Evergreen blackberries. J. Food Sci. 2004, 69, 233–240. [Google Scholar] [CrossRef]
- Sellappan, S.; Akoh, C.C.; Krewer, G. Phenolic compounds and antioxidant capacity of Georgia-grown blueberries and blackberries. J. Agric. Food Chem. 2002, 50, 2432–2438. [Google Scholar] [CrossRef] [PubMed]
- Cho, M.J.; Howard, L.R.; Prior, R.L.; Clark, J.R. Flavonoid glycosides and antioxidant capacity of various blackberry and red grape genotypes determined by high-performance liquid chromatogrphy/mass spectrometry. J. Sci. Food Agric. 2004, 84, 1771–1782. [Google Scholar] [CrossRef]
- Kao, M.W.S.; Woods, F.M.; Dozier, W.A., Jr.; Ebel, R.C.; Nesbitt, M.; Jee, J.; Fields, D. Phenolic content and antioxidant capacities of Alabama-grown thornless blackberries. Int. J. Fruit Sci. 2008, 7, 33–46. [Google Scholar] [CrossRef]
- Cho, M.J.; Howard, L.R.; Prior, R.L.; Clark, J.R. Flavonol glycosides and antioxidant capacity of various blackberry and blueberry genotypes determined by high-performance liquid chromatography/mass spectrometry. J. Sci. Food Agric. 2005, 85, 2149–2158. [Google Scholar] [CrossRef]
- Zadernowski, R.; Naczk, M.; Nesterowicz, J. Phenolic acid profiles in some small berries. J. Agric. Food Chem. 2005, 53, 2118–2124. [Google Scholar] [CrossRef] [PubMed]
- Howard, L.R.; Hager, T.J. Berry fruit phytochemicals. In Berry fruit Value-Added Products for Health Promotion, 1st ed.; Zhao, Y., Ed.; CRC Press: Boca Raton, FL, USA, 2007; Volume 1, pp. 73–104. [Google Scholar]
- Hager, T.J.; Howard, L.R.; Liyanage, R.; Lay, J.O.; Prior, R.L. Ellagitannin composition of blackberry as determined by HPLC-ESI-MS and MALDI-TOF-MS. J. Agric. Food Chem. 2008, 56, 661–669. [Google Scholar] [CrossRef] [PubMed]
- Gasperotti, M.; Masuero, D.; Vrhovsek, U.; Guella, G.; Mattivi, F. Profiling and accurate quantification of Rubus ellagitannins and ellagic acid conjugates using direct UPLC-Q-TOF HDMS and HPLC-DAD analysis. J. Agric. Food Chem. 2010, 58, 4602–4616. [Google Scholar] [CrossRef] [PubMed]
- Seeram, N.P.; Lee, R.; Scheuller, H.S.; Heber, D. Identification of phenolic compounds in strawberries by liquid chromatography electrospray ionization mass spectroscopy. Food Chem. 2006, 97, 1–11. [Google Scholar] [CrossRef]
- Mullen, W.; McGinn, J.; Lean, M.E.J.; Mac Lean, M.R.; Gardner, P.; Duthie, G.G.; Yokota, T.; Crozier, A. Ellagitannins, flavonoids and other phenolics in red raspberries and their contribution to antioxidant capacity and vasorelaxation properties. J. Agric. Food Chem. 2002, 50, 5191–5196. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Tachibana, H.; Nonaka, G.; Nishioka, I.; Hsu, F.L.; Kohda, H. Tannins and related compounds. CXXII. New dimeric, trimeric and tetrameric ellagitannins, lambertianins A–D, from Rubus lambertianus SERINGE. Chem. Pharm. Bull. 1993, 47, 1214–1220. [Google Scholar] [CrossRef]
- Gudej, J.; Tomczyk, M. Determination of flavonoids, tannins and ellagic acid in leaves from Rubus L. Species Arch. Pharm. Res. 2004, 27, 1114–1119. [Google Scholar] [CrossRef]
- Hollman, P.C.H.; Hertog, M.G.L.; Katan, M.B. Analysis and health effects of flavonoids. Food Chem. 1996, 57, 43–46. [Google Scholar] [CrossRef]
- Maas, J.L.; Galletta, G.J.; Stoner, G.D. Ellagic acid, an anticarcinogen in fruits, especially in strawberries: A review. Hort. Sci. 1991, 26, 10–14. [Google Scholar]
- Nicoli, M.C.; Anese, M.; Parpinel, M. Influence of processing on the antioxidant properties of fruit and vegetables. Trends Food Sci. Technol. 1999, 10, 94–100. [Google Scholar] [CrossRef]
- Dai, J.; Mumper, R.J. Plant phenolics: Extraction, analysis and their antioxidant and anticancer properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef] [PubMed]
- Simpson, M.; Parsons, M.; Greenwood, J.; Wade, K. Raspberry leaf in pregnancy: Its safety and efficacy in labor. J. Midwifery Women Health 2001, 46, 51–59. [Google Scholar] [CrossRef]
- Oliveira, A.P.; Pereira, J.O.; Andrade, P.B.; Valentao, P.; Seabra, R.M.; Silva, B.M. Phenolic profile of Cydonia oblonga Miller leaves. J. Agric. Food Chem. 2007, 55, 7926–7930. [Google Scholar] [CrossRef] [PubMed]
- Tavares, L.; Figueira, I.; McDougall, G.J.; Vieira, H.L.A.; Stewart, D.; Alves, P.M.; Ferreira, R.B.; Santos, C.N. Neuroprotective effects of digested polyphenols from wild blackberry species. Eur. J. Nutr. 2013, 52, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the leaves are available from the corresponding authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oszmiański, J.; Wojdyło, A.; Nowicka, P.; Teleszko, M.; Cebulak, T.; Wolanin, M. Determination of Phenolic Compounds and Antioxidant Activity in Leaves from Wild Rubus L. Species. Molecules 2015, 20, 4951-4966. https://doi.org/10.3390/molecules20034951
Oszmiański J, Wojdyło A, Nowicka P, Teleszko M, Cebulak T, Wolanin M. Determination of Phenolic Compounds and Antioxidant Activity in Leaves from Wild Rubus L. Species. Molecules. 2015; 20(3):4951-4966. https://doi.org/10.3390/molecules20034951
Chicago/Turabian StyleOszmiański, Jan, Aneta Wojdyło, Paulina Nowicka, Mirosława Teleszko, Tomasz Cebulak, and Mateusz Wolanin. 2015. "Determination of Phenolic Compounds and Antioxidant Activity in Leaves from Wild Rubus L. Species" Molecules 20, no. 3: 4951-4966. https://doi.org/10.3390/molecules20034951
APA StyleOszmiański, J., Wojdyło, A., Nowicka, P., Teleszko, M., Cebulak, T., & Wolanin, M. (2015). Determination of Phenolic Compounds and Antioxidant Activity in Leaves from Wild Rubus L. Species. Molecules, 20(3), 4951-4966. https://doi.org/10.3390/molecules20034951





