Preliminary Biological Evaluation of 18F-FBEM-Cys-Annexin V a Novel Apoptosis Imaging Agent
Abstract
:1. Introduction
2. Results and Discussion
2.1. Radiolabeling
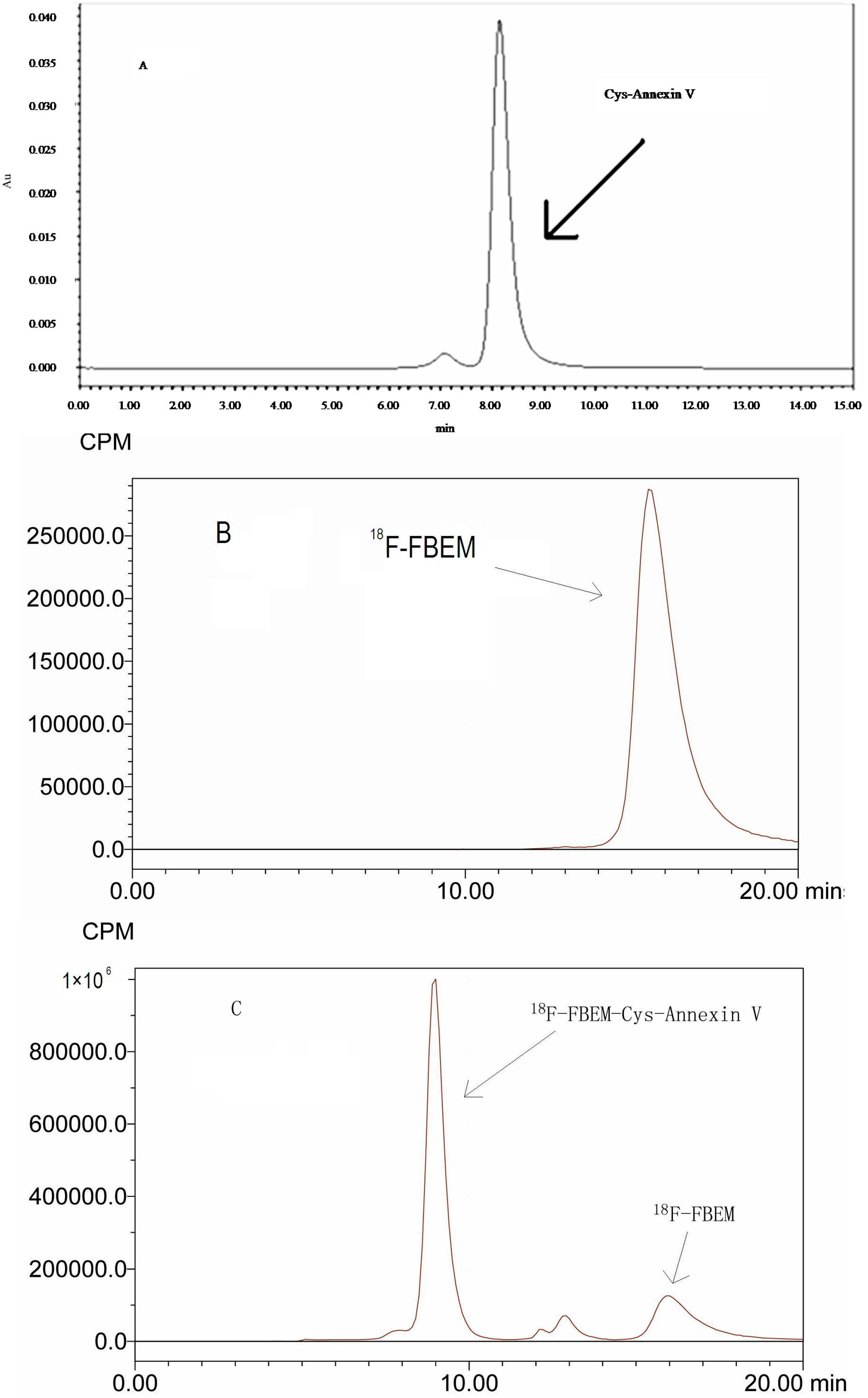
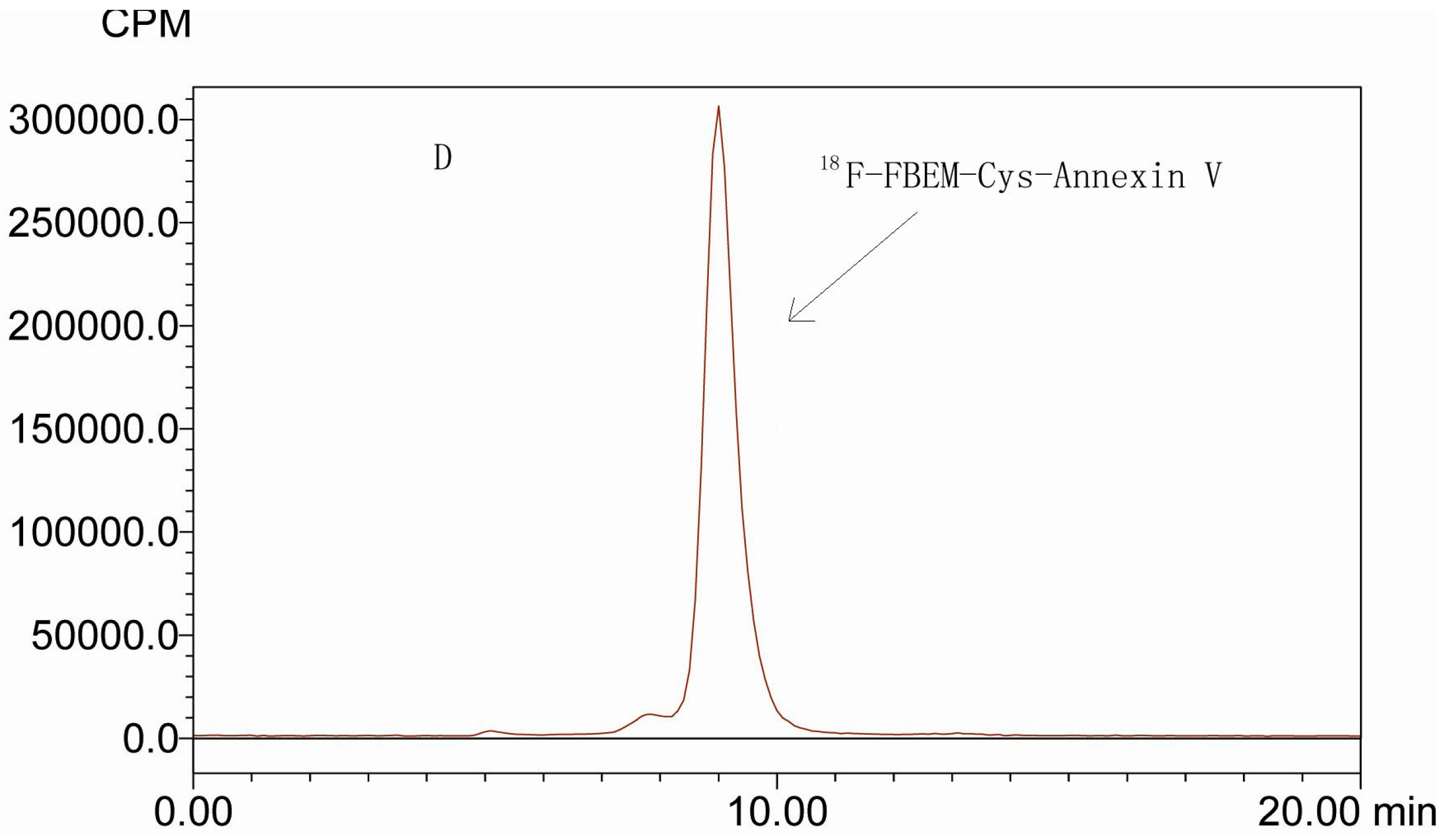
2.2. In Vitro Stability of 18F-FBEM-Cys-Annexin V
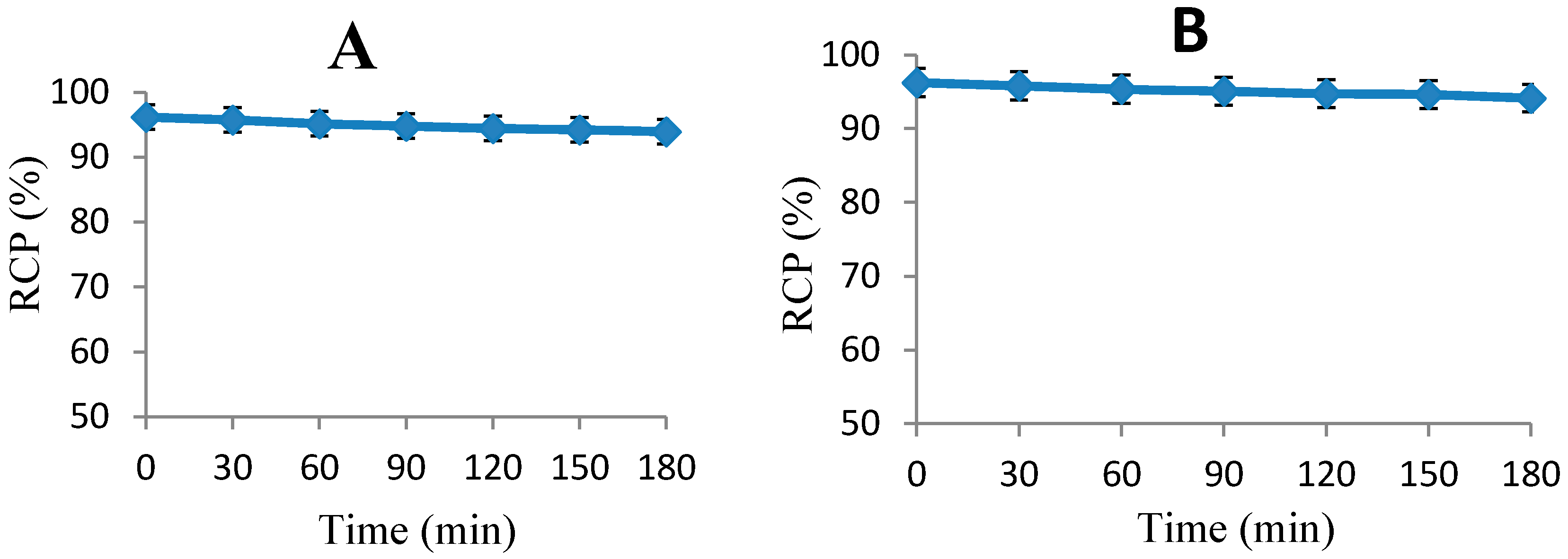
2.3. Blood Kinetics Studies
| Parameter (units) | 18F-FEBM-Cys-Annexin V |
|---|---|
| K12 (min−1) | 0.02 |
| K21 (min−1) | 0.038 |
| Ke (min−1) | 0.009 |
| CL (%ID/g/min) | 0.031 |
| T1/2α (min) | 11.261 |
| T1/2β (min) | 134.62 |
| AUC (%ID/g·min) | 634.123 |
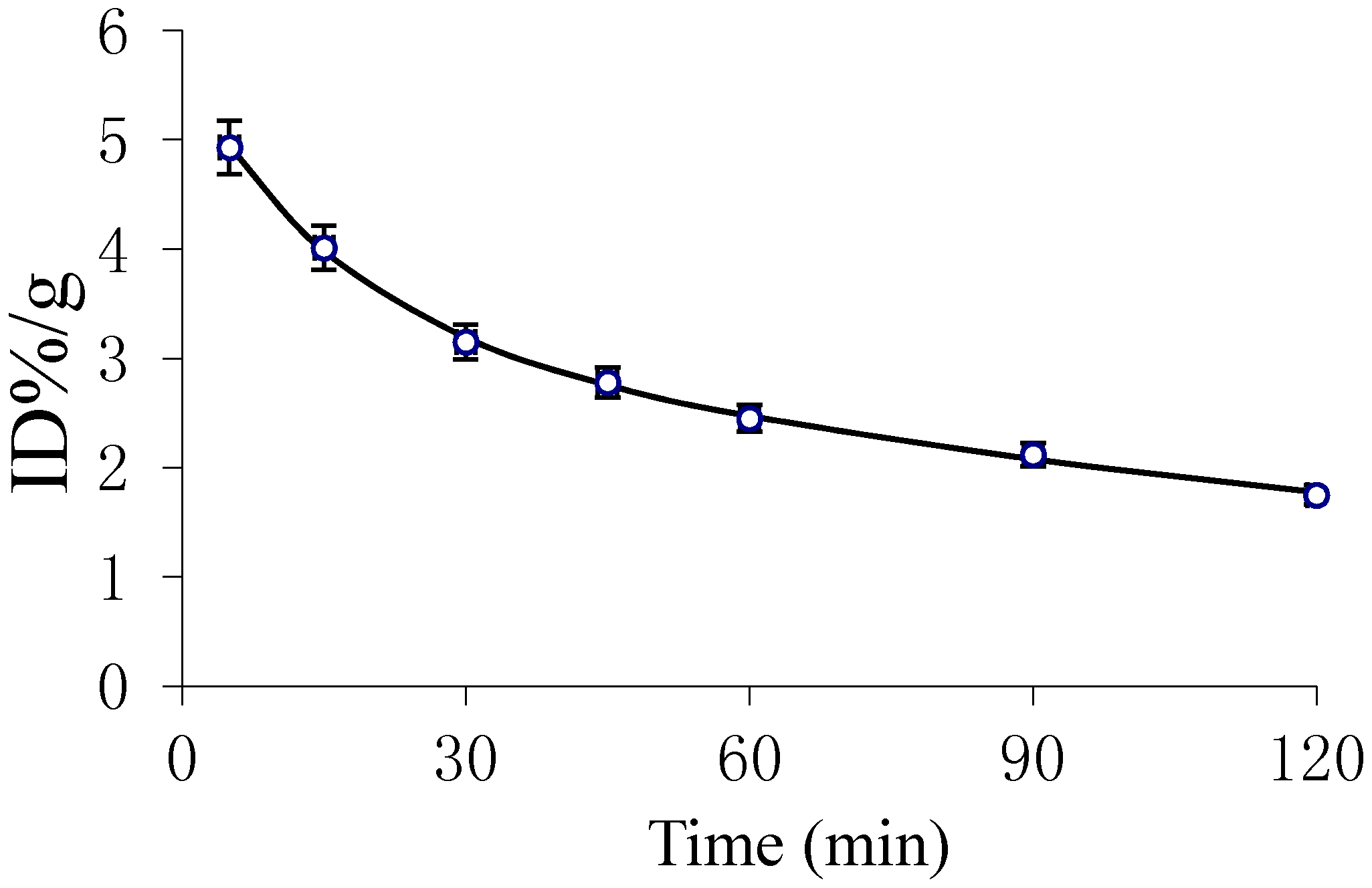
2.4. Dynamic MicroPET Images of Normal ICR Mice
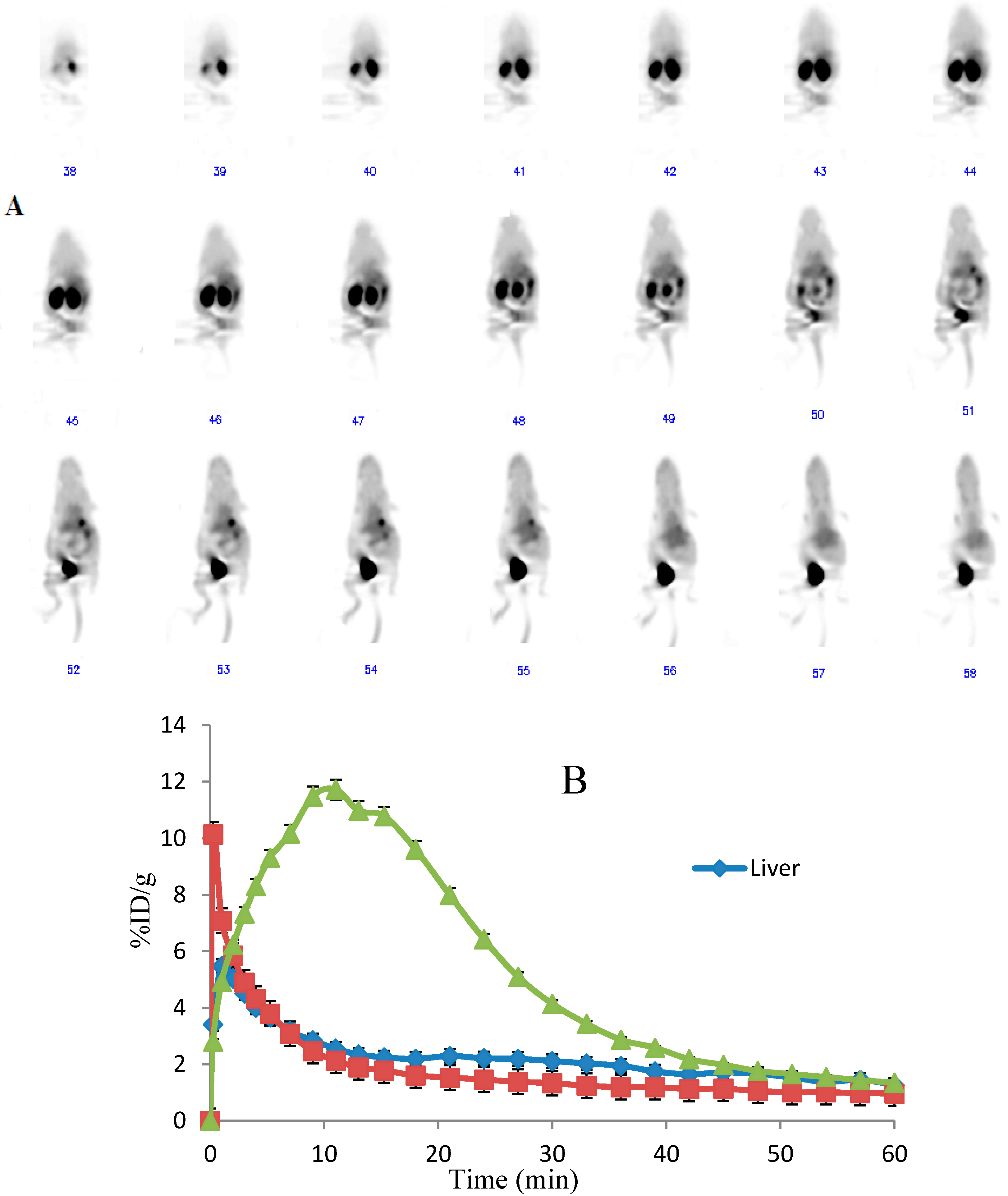
2.5. Imaging of Rat Model of Apoptosis


3. Experimental Section
3.1. General Information
3.2. Preparation of 18F-N-[2-(4-Fluorobenzamido)ethyl]maleimide
3.3. Preparation of 18F-FBEM-Cys-Annexin V
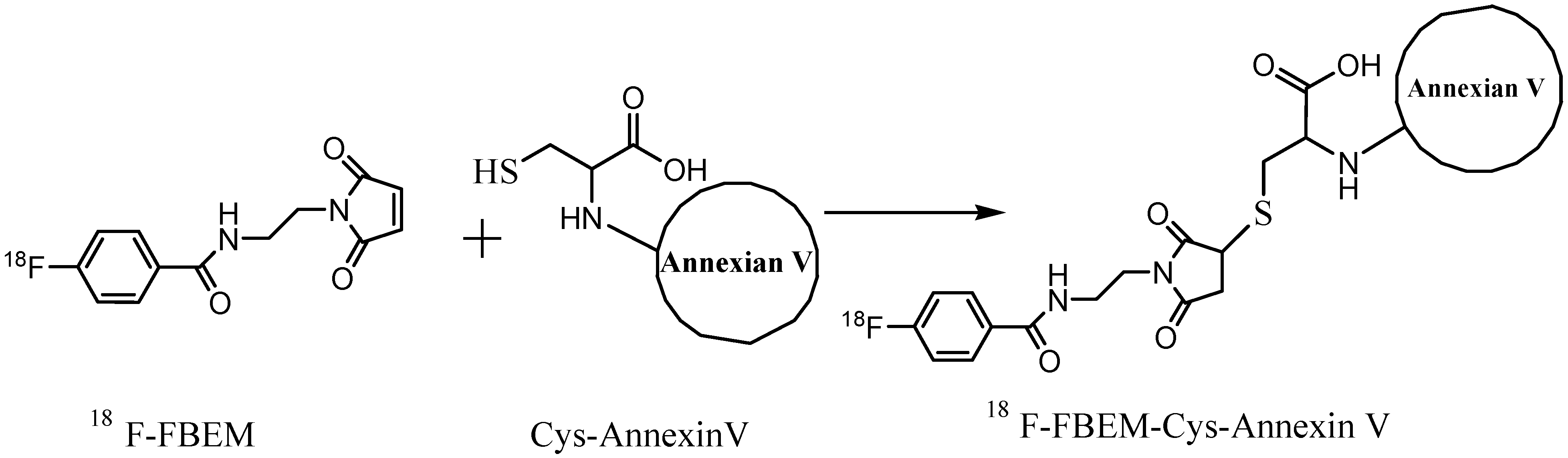
3.4. In Vitro Stability of 18F-FBEM-Cys-Annexin V
3.5. Blood Kinetics Studies of 18F-FBEM-Cys-Annexin V in Normal Mice
3.6. Dynamical MicroPET Images of Normal Mice
3.7. MicroPET Images of Rat Model of Apoptosis
3.8. TUNEL Staining
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Fink, S.L.; Cookson, B.T. Apoptosis, pyroptosis, and necrosis: Mechanistic description of dead and dying eukaryotic cells. Infect. Immun. 2005, 73, 1907–1916. [Google Scholar] [CrossRef] [PubMed]
- Hofstra, L.; Liem, I.H.; Dumont, E.A.; Boersma, H.H.; van Heerde, W.L.; Doevendans, P.A.; DeMuinck, E.; Wellens, H.J.J.; Kemerink, G.J.; Reutelingsperger, C.P.M.; et al. Visualisation of cell death in vivo in patients with acute myocardial infarction. Lancet 2000, 356, 209–212. [Google Scholar]
- Thiagarajan, P.; Tait, J.F. Binding of Annexin-V Placental Anticoagulant ProteinI to Platelets—Evidence for Phosphatidylserine Exposure in The Procoagulant Response of Activated Platelets. J. Biol. Chem. 1990, 265, 17420–17423. [Google Scholar] [PubMed]
- Tait, J.F.; Gibson, D.; Fujikawa, K. Phospholipid Binding-Properties of Human Placental Anticoagulant Protein-I, A Member of the Lipocortin Family. J. Biol. Chem. 1989, 264, 7944–7949. [Google Scholar] [PubMed]
- Gerke, V.; Moss, S.E. Annexins: From structure to function. Physiol. Rev. 2002, 82, 331–371. [Google Scholar] [PubMed]
- Koopman, G.; Reutelingsperger, C.P.M.; Kuijten, G.A.M.; Keehnen, R.M.J.; Pals, S.T.; Vanoers, M.H.J. Annexin-V For Flow Cytometric Detection of Phosphatidylserine Expression on B-cells Undergoing Apoptosis. Blood 1994, 84, 1415–1420. [Google Scholar] [PubMed]
- Kemerink, G.J.; Boersma, H.H.; Thimister, P.W.; Hofstra, L.; Liem, I.H.; Pakbiers, M.T.; Janssen, D.; Reutelingsperger, C.P.; Heidendal, G.A. Biodistribution and dosimetry of 99mTc-BTAP-annexin-V in humans. Eur. J. Nucl. Med. 2001, 28, 1373–1378. [Google Scholar] [CrossRef] [PubMed]
- Boersma, H.H.; Liem, I.H.; Kemerink, G.J.; Thimister, P.W.L.; Hofstra, L.; Stolk, L.M.L.; van Heerde, W.L.; Pakbiers, M.T.W.; Janssen, D.; Beysens, A.J.; et al. Comparison between human pharmacokinetics and imaging properties of two conjugation methods for Tc-99m-Annexin A5. Br. J. Radiol. 2003, 76, 553–560. [Google Scholar]
- Vanderheyden, J.L.; Liu, G.; He, J.; Patel, B.; Tait, J.F.; Hnatowich, D.J. Evaluation of 99mTc-MAG3-annexin V: Influence of the chelate on in vitro and in vivo properties in mice. Nucl. Med. Biol. 2006, 33, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.J.; Azhdarinia, A.; Wu, P.; Yu, D.F.; Tansey, W.; Kalimi, S.K.; Kim, E.E.; Podoloff, D.A. In vivo and in vitro measurement of apoptosis in breast cancer cells using 99mTc-EC-annexin V. Cancer Biother. Radiopharm. 2001, 16, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Kemerink, G.J.; Liu, X.; Kieffer, D.; Ceyssens, S.; Mortelmans, L.; Verbruggen, A.M.; Steinmetz, N.D.; Vanderheyden, J.L.; Green, A.M.; Verbeke, K. Safety, biodistribution, and dosimetry of 99mTc-HYNIC-annexin V, a novel human recombinant annexin V for human application. J. Nucl. Med. 2003, 44, 947–952. [Google Scholar] [PubMed]
- De Saint-Hubert, M.; Wang, H.; Devos, E.; Vunckx, K.; Zhou, L.; Reutelingsperger, C.; Verbruggen, A.; Mortelmans, L.; Ni, Y.; Mottaghy, F.M. Preclinical Imaging of Therapy Response Using Metabolic and Apoptosis Molecular Imaging. Mol. Imaging Biol. 2011, 13, 995–1002. [Google Scholar] [CrossRef] [PubMed]
- Tait, J.F.; Brown, D.S.; Gibson, D.F.; Blankenberg, F.G.; Strauss, H.W. Development and characterization of annexin V mutants with endogenous chelation sites for (99m)Tc. Bioconjugate Chem. 2000, 11, 918–925. [Google Scholar] [CrossRef]
- Ye, F.; Fang, W.; Wang, F.; Hua, Z.-C.; Wang, Z.; Yang, X. Evaluation of adenosine preconditioning with Tc-99m-His(10)-annexin V in a porcine model of myocardium ischemia and reperfusion injury: Preliminary study. Nucl. Med. Biol. 2011, 38, 567–574. [Google Scholar] [CrossRef] [PubMed]
- Tait, J.F.; Smith, C.; Levashova, Z.; Patel, B.; Blankenberg, F.G.; Vanderheyden, J.L. Improved detection of cell death in vivo with annexin V radiolabeled by site-specific methods. J. Nucl. Med. 2006, 47, 1546–1553. [Google Scholar] [PubMed]
- Lu, C.; Jiang, Q.; Hu, M.; Tan, C.; Ji, Y.; Yu, H.; Hua, Z. Preliminary Biological Evaluation of Novel 99mTc-Cys-Annexin A5 as a Apoptosis Imaging Agent. Molecules 2013, 18, 6908–6918. [Google Scholar] [CrossRef] [PubMed]
- Zijlstra, S.; Gunawan, J.; Burchert, W. Synthesis and evaluation of a 18F-labelled recombinant annexin-V derivative, for identification and quantification of apoptotic cells with PET. Appl. Radiat. Isot. 2003, 58, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Kiesewetter, D.O.; Zhu, L.; Guo, N.; Gao, H.; Liu, G.; Hida, N.; Lang, L.; Niu, G.; Chen, X. Longitudinal PET Imaging of Doxorubicin-Induced Cell Death with (18)F-Annexin V. Mol. Imaging Biol. 2012, 14, 762–770. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.C.; Wang, F.; Fang, W.; Hua, Z.C.; Wang, Z.Z. 18F-annexin V apoptosis imaging for detection of myocardium ischemia and reperfusion injury in a rat model. J. Radioanal. Nucl. Chem. 2013, 298, 1733–1738. [Google Scholar] [CrossRef]
- De Bruin, B.; Kuhnast, B.; Hinnen, F.; Yaouancq, L.; Amessou, M.; Johannes, L.; Samson, A.; Boisgard, R.; Tavitian, B.; Dolle, F. 1-[3-(2-[18F]-fluoropyridin-3-yloxy)propyl]pyrrole-2,5-dione: Design, synthesis, and radiosynthesis of a new [18F]-fluoropyridine-based maleimide reagent for the labeling of peptides and proteins. Bioconjugate Chem. 2005, 16, 406–420. [Google Scholar] [CrossRef]
- Gao, H.; Niu, G.; Yang, M.; Quan, Q.; Ma, Y.; Murage, E.N.; Ahn, J.M.; Kiesewetter, D.O.; Chen, X. PET of Insulinoma Using (18)F-FBEM-EM3106B, a New GLP-1 Analogue. Mol. Pharm. 2011, 8, 1775–1782. [Google Scholar] [CrossRef] [PubMed]
- Kiesewetter, D.O.; Jacobson, O.; Lang, L.; Chen, X. Automated radiochemical synthesis of [18F]FBEM: A thiol reactive synthon for radiofluorination of peptides and proteins. Appl. Radiat. Isot. 2011, 69, 410–414. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Gao, H.; Guo, N.; Niu, G.; Ma, Y.; Kiesewetter, D.O.; Chen, X. Site-Specific Labeling of scVEGF with Fluorine-18 for Positron Emission Tomography Imaging. Theranostics 2012, 2, 607–617. [Google Scholar] [CrossRef] [PubMed]
- Keen, H.G.; Dekker, B.A.; Disley, L.; Hastings, D.; Lyons, S.; Reader, A.J.; Ottewell, P.; Watson, A.; Zweit, J. Imaging apoptosis in vivo using 124I-annexin V and PET. Nucl. Med. Biol. 2005, 32, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Bauwens, M.; De Saint-Hubert, M.; Devos, E.; Deckers, N.; Reutelingsperger, C.; Mortelmans, L.; Himmelreich, U.; Mottaghy, F.M.; Verbruggen, A. Site-specific Ga-68-labeled Annexin A5 as a PET imaging agent for apoptosis. Nucl. Med. Biol. 2011, 38, 381–392. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Niu, G.; Lang, L.; Guo, N.; Ma, Y.; Kiesewetter, D.O.; Backer, J.M.; Shen, B.; Chen, X. PET imaging of EGF receptors using 18F-FBEM-EGF in a head and neck squamous cell carcinoma model. Eur. J. Nucl. Med. Mol. Imaging 2012, 39, 300–308. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Sample of the compound Cys-Annexin V is available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, C.; Jiang, Q.; Hu, M.; Tan, C.; Yu, H.; Hua, Z. Preliminary Biological Evaluation of 18F-FBEM-Cys-Annexin V a Novel Apoptosis Imaging Agent. Molecules 2015, 20, 4902-4914. https://doi.org/10.3390/molecules20034902
Lu C, Jiang Q, Hu M, Tan C, Yu H, Hua Z. Preliminary Biological Evaluation of 18F-FBEM-Cys-Annexin V a Novel Apoptosis Imaging Agent. Molecules. 2015; 20(3):4902-4914. https://doi.org/10.3390/molecules20034902
Chicago/Turabian StyleLu, Chunxiong, Quanfu Jiang, Minjin Hu, Cheng Tan, Huixin Yu, and Zichun Hua. 2015. "Preliminary Biological Evaluation of 18F-FBEM-Cys-Annexin V a Novel Apoptosis Imaging Agent" Molecules 20, no. 3: 4902-4914. https://doi.org/10.3390/molecules20034902





