QSAR-Assisted Virtual Screening of Lead-Like Molecules from Marine and Microbial Natural Sources for Antitumor and Antibiotic Drug Discovery
Abstract
:1. Introduction
2. Results and Discussion
2.1. Establishment of QSAR Classification Models
| SVM a | CT | Rf b | ||
|---|---|---|---|---|
| Training set/Test Set I/AntiMarin Set | ||||
| Descriptors | Class Size | Correct Predictions | ||
| Sensitivity c | ||||
| Specificity d | ||||
| G-mean e | ||||
| CDK f | Active 1612/798/315 Non-active 134/65/103 | Active | Active | Active |
| 1199/646/242 | 1599/782/312 | 1114/566/251 | ||
| Non-active | Non-active | Non-active | ||
| 79/34/30 | 32/5/1 | 93/53/33 | ||
| 0.74/0.81/0.77 | 0.99/0.98/0.99 | 0.69/0.71/0.80 | ||
| 0.59/0.52/0.29 | 0.24/0.08/0.01 | 0.69/0.82/0.32 | ||
| 0.66/0.65/0.47 | 0.49/0.27/0.10 | 0.69/0.76/0.51 | ||
| PM6 g | Active | Active | Active | |
| 1043/535/252 | 1603/795/311 | 1061/532/248 | ||
| Non-active | Non-active | Non-active | ||
| 60/40/23 | 14/3/1 | 63/38/23 | ||
| 0.65/0.67/0.80 | 0.99/1.00/0.99 | 0.66/0.67/0.79 | ||
| 0.45/0.62/0.22 | 0.10/0.05/0.01 | 0.47/0.58/0.22 | ||
| 0.54/0.64/0.42 | 0.32/0.21/0.10 | 0.56/0.62/0.42 | ||
| CDK+PM6 | Active | Active | Active | |
| 1244/649/236 | 1598/764/305 | 1124/577/256 | ||
| Non-active | Non-active | Non-active | ||
| 75/37/29 | 49/13/3 | 89/50/27 | ||
| 0.77/0.81/0.75 | 0.99/0.96/0.97 | 0.70/0.72/0.81 | ||
| 0.56/0.57/0.28 | 0.37/0.20/003 | 0.66/0.77/0.26 | ||
| 0.66/0.68/0.46 | 0.60/0.44//0.17 | 0.68/0.75/0.46 | ||
| SVM a | CT | Rf b | ||
|---|---|---|---|---|
| Training set/Test Set I/AntiMarin Set | ||||
| Descriptors | Class Size | Correct Predictions | ||
| Sensitivity c | ||||
| Specificity d | ||||
| G-mean e | ||||
| CDK f | Active 880/438/58 Non-active 866/425/360 | Active | Active | Active |
| 707/352/15 | 735/354/12 | 763/366/22 | ||
| Non-active | Non-active | Non-active | ||
| 556/291/220 | 631/296/245 | 675/348/247 | ||
| 0.80/0.80/0.26 | 0.84/0.81/0.17 | 0.87/0.84/0.38 | ||
| 0.64/0.68/0.61 | 0.73/0.70/0.68 | 0.78/0.82/0.69 | ||
| 0.72/0.74/0.40 | 0.78/0.75//0.34 | 0.82/0.83/0.51 | ||
| PM6 g | Active | Active | Active | |
| 533/256/23 | 663/306/30 | 543/273/22 | ||
| Non-active | Non-active | Non-active | ||
| 511/255/209 | 475/204/157 | 507/247/188 | ||
| 0.61/0.58/0.40 | 0.75/0.70/0.52 | 0.62/0.62/0.38 | ||
| 0.59/0.60/0.58 | 0.55/0.48/0.44 | 0.59/0.58/0.52 | ||
| 0.60/0.59/0.48 | 0.64/0.58/0.48 | 0.60/0.60/0.44 | ||
| CDK+PM6 | Active | Active | Active | |
| 689/344/12 | 735/354/12 | 763/374/18 | ||
| Non-active | Non-active | Non-active | ||
| 620/313/244 | 631/296/245 | 679/344/256 | ||
| 0.78/0.79/0.21 | 0.84/0.81/0.17 | 0.87/0.85/0.31 | ||
| 0.72/0.74/0.68 | 0.73/0.70/0.68 | 0.78/0.81/0.71 | ||
| 0.75/0.76/0.37 | 0.78/0.75//0.34 | 0.82/0.83/0.47 | ||
| SVM a | CT | Rf b | ||
|---|---|---|---|---|
| Training set/Test Set I/AntiMarin Set | ||||
| Descriptors | Class Size | Correct Predictions | ||
| Sensitivity c | ||||
| Specificity d | ||||
| G-mean e | ||||
| CDK f | Active 642/326/228 Non-active 1104/537/190 | Active | Active | Active |
| 483/236/159 | 514/246/150 | 535/268/163 | ||
| Non-active | Non-active | Non-active | ||
| 997/485/126 | 1049/484/123 | 1045/502/118 | ||
| 0.75/0.72/0.70 | 0.80/0.76/0.66 | 0.83/0.82/0.71 | ||
| 0.90/0.90/0.66 | 0.95/0.90/0.65 | 0.95/0.93/0.62 | ||
| 0.82/0.81/0.68 | 0.87/0.83//0.65 | 0.89/0.88/0.67 | ||
| PM6 g | Active | Active | Active | |
| 440/227/144 | 182/83/48 | 432/229/136 | ||
| Non-active | Non-active | Non-active | ||
| 642/302/64 | 995/484/153 | 668/307/66 | ||
| 0.69/0.70/0.63 | 0.28/0.26/0.21 | 0.67/0.70/0.60 | ||
| 0.58/0.56/0.34 | 0.90/0.90/0.81 | 0.60/0.57/0.35 | ||
| 0.63/0.63/0.46 | 0.50/0.48/0.41 | 0.64/0.63/0.46 | ||
| CDK+PM6 | Active | Active | Active | |
| 485/236/161 | 514/246/150 | 536/266/161 | ||
| Non-active | Non-active | Non-active | ||
| 994/485/124 | 1049/484/123 | 1047/504/120 | ||
| 0.76/0.72/0.71 | 0.80/0.76/0.66 | 0.83/0.82/0.71 | ||
| 0.90/0.90/0.65 | 0.95/0.90/0.65 | 0.95/0.94/0.63 | ||
| 0.82/0.81/0.68 | 0.87/0.83//0.65 | 0.89/0.88/0.67 | ||
| CDK a | PM6 b | CDK+PM6 | ||
|---|---|---|---|---|
| Model | Class Size | Correct Predictions | ||
| Sensitivity c | ||||
| Specificity d | ||||
| G-mean e | ||||
| Overall | Active 183 Non-active 0 | Active | Active | Active |
| 114 | 148 | 121 | ||
| Non-active | Non-active | Non-active | ||
| 69 | 35 | 62 | ||
| 0.62 | 0.81 | 0.66 | ||
| na f | na | na | ||
| 0.79 | 0.90 | 0.81 | ||
| Antitumor | Active 68 Non-active 115 | Active | Active | Active |
| 55 | 41 | 56 | ||
| Non-active | Non-active | Non-active | ||
| 26 | 41 | 26 | ||
| 0.81 | 0.60 | 0.82 | ||
| 0.23 | 0.36 | 0.23 | ||
| 0.43 | 0.46 | 0.43 | ||
| Antibiotic | Active 29 Non-active 154 | Active | Active | Active |
| 17 | 27 | 18 | ||
| Non-active | Non-active | Non-active | ||
| 150 | 68 | 149 | ||
| 0.59 | 0.93 | 0.62 | ||
| 0.97 | 0.44 | 0.97 | ||
| 0.76 | 0.64 | 0.77 | ||
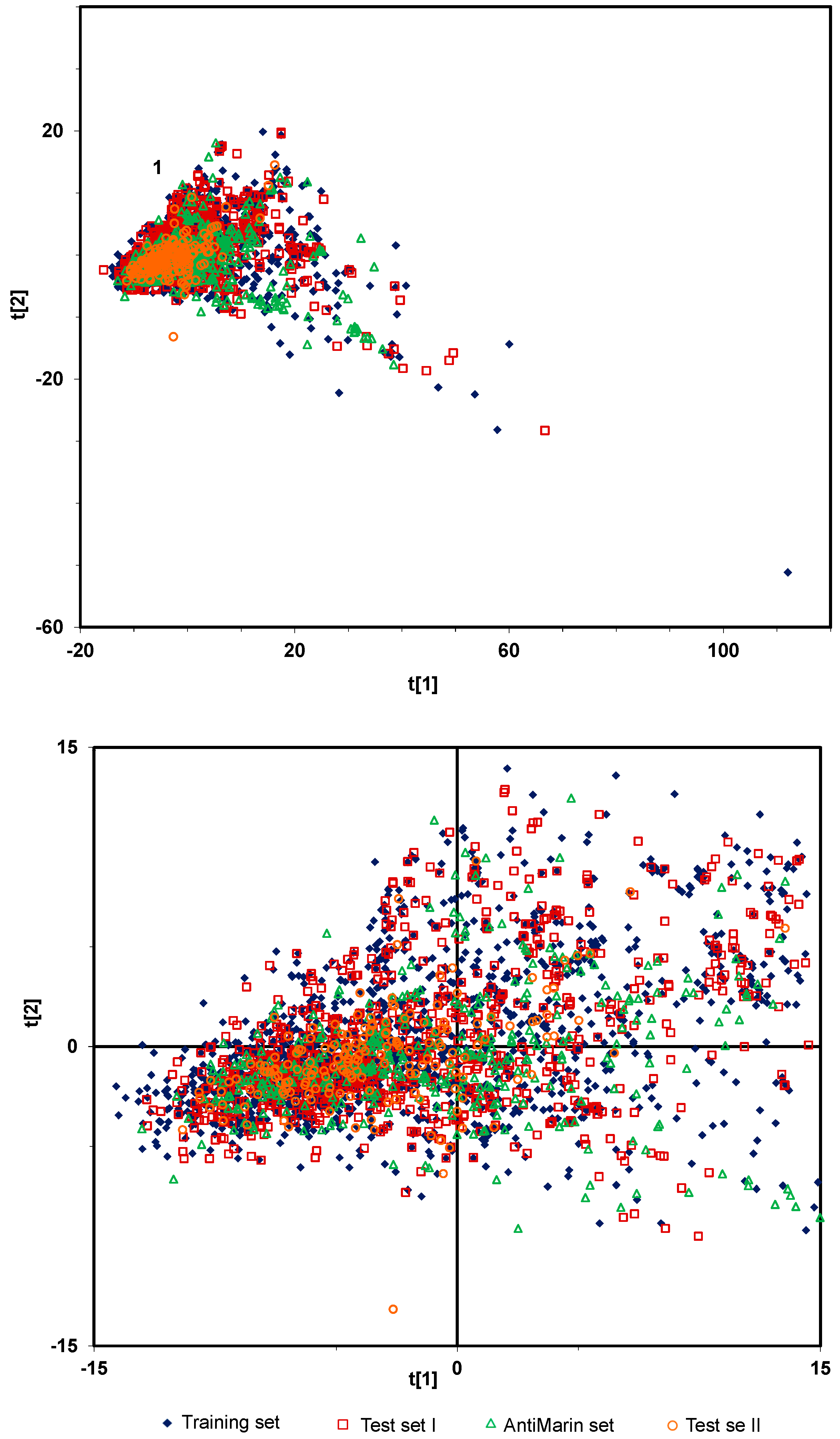
2.1.1. Overall Biological Activity Model
| Models | CDK Descriptors | PM6 Descriptors | CDK+PM6 Descriptors | ||
|---|---|---|---|---|---|
| Overall biological activity | SVM a | 20D: ALogp2; BCUTc-1l; BCUTp-1h; PPSA-2; DPSA-3; FPSA-3; TPSA; Wlambda2.unity; Weta1.unity; ATSc3; SCH-5; SP-6; VP-7; khs.ssCH2; khs.dsCH; khs.sssCH; khs.aaaC; khs.sNH2; MDEC-33; TopoPSA | 8D: εHOMO; εLUMO; Mulliken electronegativity (χ); Parr & Pople absolute hardness; Schuurmann MO shift alpha; hardness (η); chemical potential (μ); electrophilicity index (ω) | 21D: ALogp2; BCUTc-1l; BCUTp-1h; PPSA-2; DPSA-3; FPSA-3; TPSA; Wlambda2.unity; Weta1.unity; ATSc3; SCH-5; SP-6; VP-7; khs.ssCH2; khs.dsCH; khs.sssCH; khs.aaaC; khs.sNH2; MDEC-33; TopoPSA; εHOMO | |
| Rfs b | c | FMF; BCUTp-1l; BCUTw-1h; khs.sF; Weta1.unity; VCH-7; HybRatio; FPSA-1; BCUTc-1h; LOBMAX | η; Parr & Pople absolute hardness; ω; χ; εLUMO; Schuurmann MO shift alpha; εHOMO; μ | khs.sssCH; MDEO-12; XlogP; TopoPSA; MDEO-11; VC-6; FMF; ATSc5; VCH-7; VC-5 | |
| CT | 8D: SP-6; BCUTc-1h; Weta1.unity; Wnu1.unity; MDEC-11; SC-5; VP-7; MDEC-22 | 3D: εHOMO; Parr & Pople absolute hardness; εLUMO | 11D: SP-6; εHOMO; BCUTc-1h; Wnu1.unity; FNSA-3; THSA; Wlambda2.unity; MDEC-33; FPSA-3; ATSp5; ω | ||
| Antitumor activity | SVM | 42D: ALogp2; AMR; BCUTw-1h; BCUTp-1l; PNSA-3; FPSA-3; FNSA-2; WNSA-3; THSA; TPSA; naAromAtom; nAromBond; ATSc2; ATSc3; ATSc4; ATSc5; bpol; C1SP2; C2SP2; SCH-4; SCH-5; VCH-4; VCH-7; VC-6; SPC-5; FMF; HybRatio; khs.dsCH; khs.aaCH; khs.sssCH; khs.tsC; khs.sNH2; khs.dO; khs.ssO; khs.sF; MDEC-12; MDEC-13; MDEC-22; MDEO-11; MDEO-12; MDEO-22; TopoPSA | 8D: εHOMO; εLUMO; χ; Parr & Pople absolute hardness; Schuurmann MO shift alpha; η; μ; ω | 44D: ALogp2; AMR; BCUTw-1h; BCUTp-1l; PNSA-3; FPSA-3; FNSA-2; WNSA-3; THSA; TPSA; Wnu1.unity; naAromAtom; nAromBond; ATSc2; ATSc3; ATSc4; ATSc5; bpol; C1SP2; C2SP2; SCH-4; SCH-5; VCH-4; VCH-7; VC-6; SPC-5; FMF; HybRatio; khs.dsCH; khs.aaCH; khs.sssCH; khs.tsC; khs.sNH2; khs.dO; khs.ssO; khs.sF; MDEC-12; MDEC-13; MDEC-22; MDEO-11; MDEO-12; MDEO-22; TopoPSA; εHOMO | |
| Rfs | c | khs.sssCH; BCUTp-1l; TopoPSA; VC-5; FMF; MDEO-12; VC-6; RNCS; VCH-7; BCUTw-1l | Parr & Pople absolute hardness; η; εLUMO; εHOMO; χ; Schuurmann MO shift alpha; μ; ω | khs.sssCH; MDEO-12; XlogP; TopoPSA; MDEO-11; VC-6; FMF; ATSc5; VCH-7; VC-5 | |
| CT | 9D: MDEO-12; Khs.sssCH; MDEO-11; SCH-7; nAromBond; VC-6; BCUTc-1h; C2SP2; BCUTp-1l | 4D: εHOMO; Parr & Pople absolute hardness; εLUMO; χ; | 9D: MDEO-12; Khs.sssCH; MDEO-11; SCH-7; nAromBond; VC-6; BCUTc-1h; C2SP2; BCUTp-1l | ||
| Antibiotic activity | SVM | 41D: ALogP; BCUTw-1h; BCUTp-1l; DPSA-3; FPSA-3; RPCG; RNCS; TPSA; Wnu1.unity; nAromBond; ATSc1; ATSc3; ATSc5; ATSm1; ATSm5; nBase; C2SP2; C3SP3; SCH-4; SCH-5; VCH-4; VCH-7; SC-4; SC-5; VPC-5; nHBAcc; khs.sssCH; khs.tsC; khs.dssC; khs.sOH; khs.ssO; khs.sF; nAtomLC; MDEC-13; MDEC-22; MDEO-11; MDEO-12; MDEO-22; MOMI-XZ; TopoPSA; XlogP | 8D: εHOMO; εLUMO; χ; Parr & Pople absolute hardness; Schuurmann MO shift alpha; η; μ; ω | 42D: ALogP; BCUTw-1h; BCUTp-1l; DPSA-3; FPSA-3; RPCG; RNCS; TPSA; Wnu1.unity; nAromBond; ATSc1; ATSc3; ATSc5; ATSm1; ATSm5; nBase; C2SP2; C3SP3; SCH-4; SCH-5; VCH-4; VCH-7; SC-5; VPC-5; nHBAcc; khs.sssCH; khs.tsC; khs.dssC; khs.sOH; khs.ssO; khs.sF; nAtomLC; MDEC-13; MDEC-22; MDEC-24; MDEO-11; MDEO-12; MDEO-22; MOMI-XZ; TopoPSA; XlogP; εHOMO | |
| Rfs | c | MDEC-22; MDEO-12; C2SP2; TopoPSA; khs.dsCH; khs.sssCH; FMF; VC-5; C4SP3; MDEC-33 | Parr_&_Pople_absolute_hardness; η; εLUMO; εHOMO; χ; Schuurmann MO shift alpha; μ; ω | MDEC-22; MDEO-12; C2SP2; khs.sssCH; khs.dsCH; TopoPSA; khs.dssC; MDEC-33; FMF; C4SP3 | |
| CT | 16D: TopoPSA; C2SP2; VC-5; MDEC-22; XlogP; BCUTp-1h; VP-0; SCH-7; DPSA-1; Khs.dssC; MDEC-12; Khs.sssCH; THSA; MDEO-12; C2SP3; HybRatio | 3D: εHOMO; Parr & Pople absolute hardness; εLUMO | 16D: TopoPSA; C2SP2; VC-5; MDEC-22; XlogP; BCUTp-1h; VP-0; SCH-7; DPSA-1; Khs.dssC; MDEC-12; Khs.sssCH; THSA; MDEO-12; C2SP3; HybRatio | ||
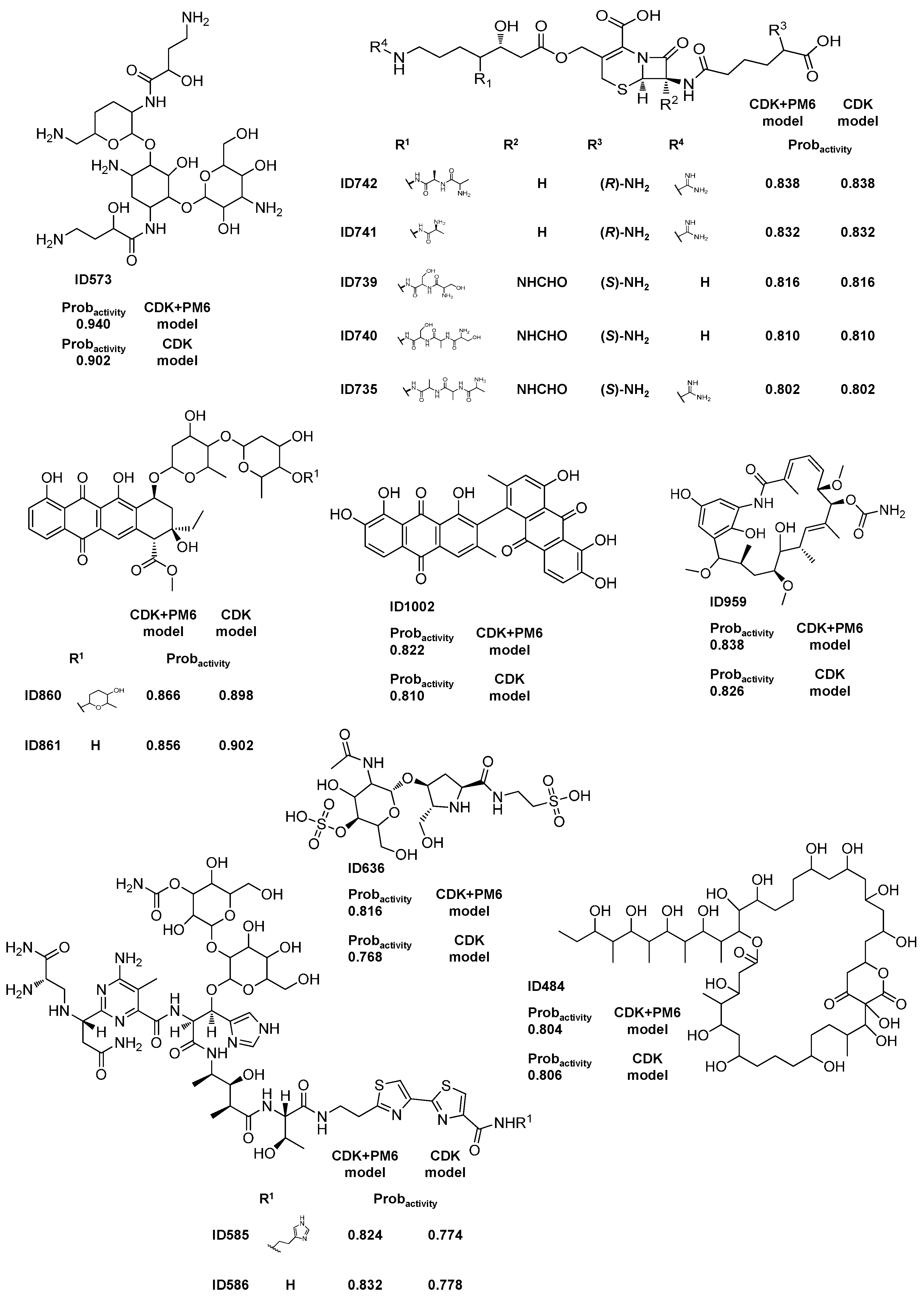
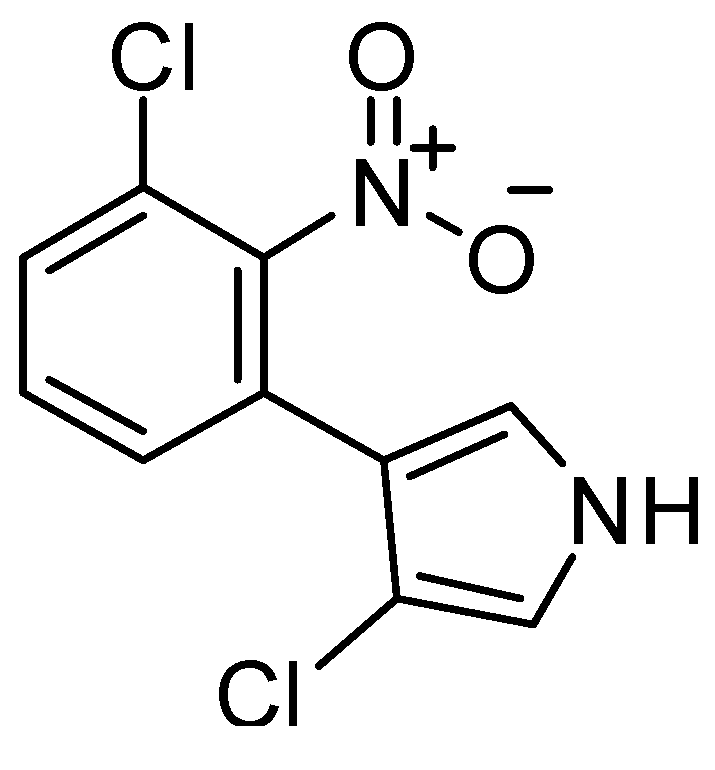
2.1.2. Antitumor Activity Model
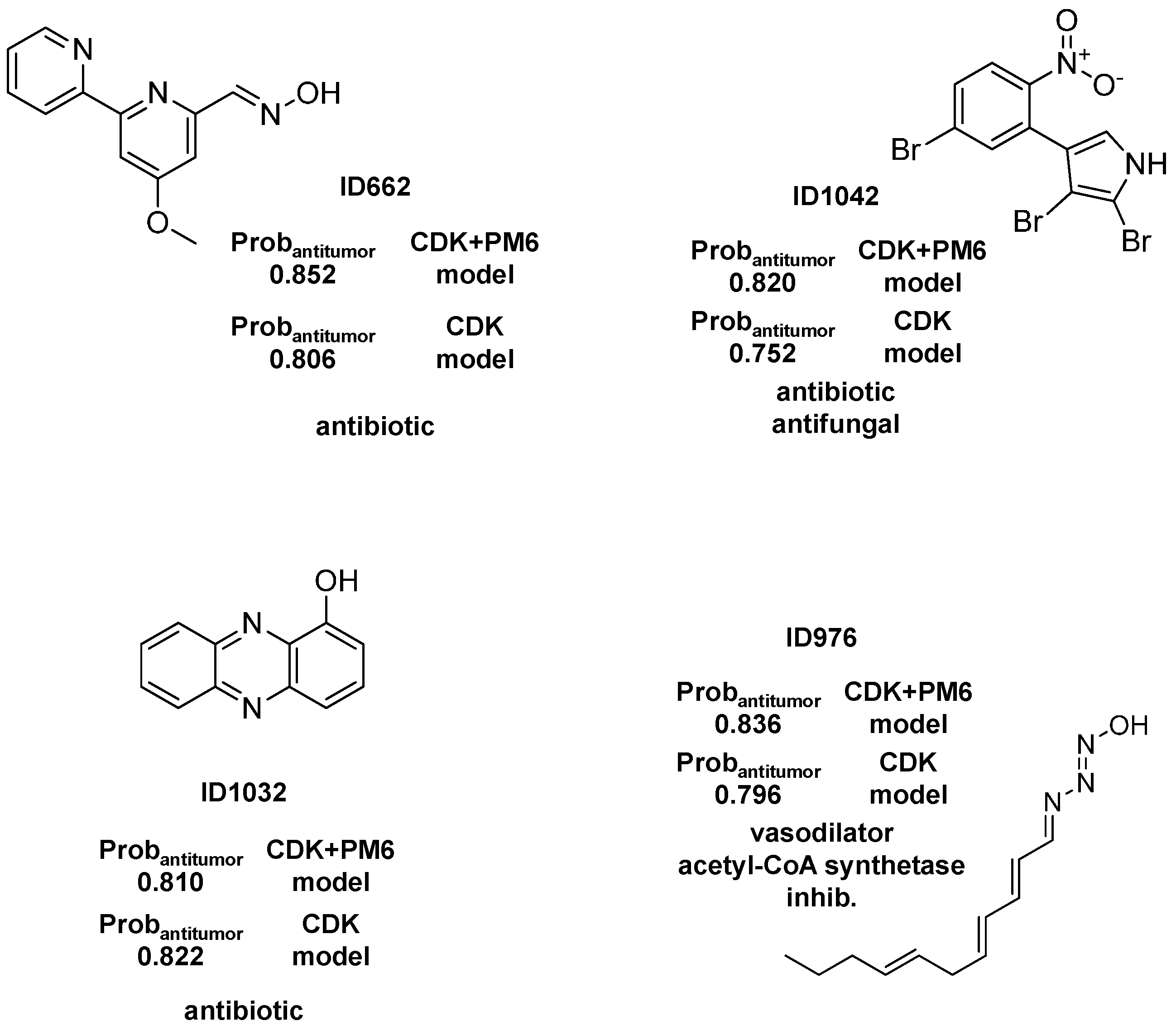
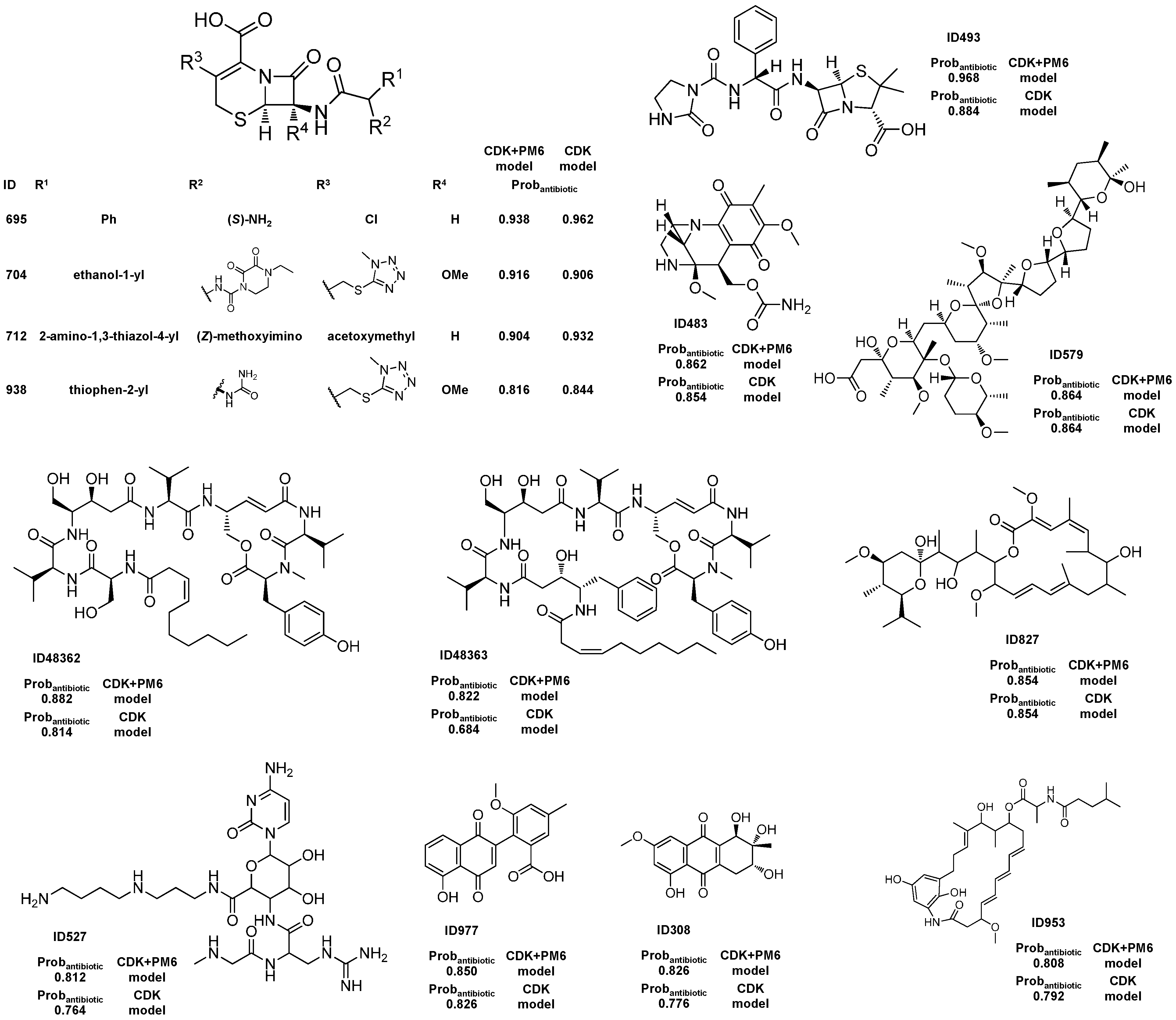
2.1.3. Antibiotic Activity Model
2.2. Analysis of Empirical and Quantum Descriptors Identified as Relevant for Modeling Overall Biological Activity, Antitumor and Antibiotic Activities
3. Experimental Section
3.1. Data Sets and Descriptors
3.2. Molecular Descriptors
3.3. Selection of Descriptors and Optimization of QSAR Classification Methods
3.4. ML Techniques
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cragg, G.M.; Newman, D.J. Natural products: A continuing source of novel drug leads. Biochim. Biophys. Acta 2013, 1830, 3670–3695. [Google Scholar] [CrossRef] [PubMed]
- Bérdy, J. Bioactive microbial metabolites. J. Antibiot. 2005, 58, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Fenical, W.; Jensen, P.R. Developing a new resource for drug discovery: Marine actinomycete bacteria. Nat. Chem. Biol. 2006, 2, 666–673. [Google Scholar] [CrossRef] [PubMed]
- Cragg, G.M.; Grothaus, P.G.; Newman, D.J. New horizons for old drugs and drug leads. J. Nat. Prod. 2014, 77, 703–723. [Google Scholar] [CrossRef] [PubMed]
- Mayer, S.; Glaser, K.B.; Cuevas, C.; Jacobs, R.S.; Kem, W.; Little, R.D.; McIntosh, J.; Newman, D.J.; Potts, B.C.; Shuster, D.E. The odyssey of marine pharmaceuticals: A current pipeline perspective. Trends Pharmacol. Sci. 2010, 31, 255–265. [Google Scholar] [PubMed]
- MarinLit. Available online: http://www.chem.canterbury.ac.nz/marinlit/marinlit.shtml (accessed on 29 January 2015).
- Gerwick, W.H.; Moore, B.S. Lessons from the past and charting the future of marine natural products drug discovery and chemical biology. Chem. Biol. 2012, 19, 85–98. [Google Scholar] [CrossRef] [PubMed]
- Koch, M.A.; Schuffenhauer, A.; Scheck, M.; Wetzel, S.; Casaulta, M.; Odermatt, A.; Ertl, P.; Waldmann, H. Charting biologically relevant chemical space: A structural classification of natural products (SCONP). Proc. Natl. Acad. Sci. USA 2005, 102, 17272–17277. [Google Scholar] [CrossRef] [PubMed]
- Ertl, P.; Roggo, S.; Schuffenhauer, A. Natural product-likeness score and its application for prioritization of compound libraries. J. Chem. Inf. Model. 2008, 46, 68–74. [Google Scholar] [CrossRef]
- Wetzel, S.; Bon, R.S.; Kumar, K.; Waldmann, H. Biology-oriented synthesis. Angew. Chem. Int. Ed. 2011, 50, 10800–10826. [Google Scholar] [CrossRef]
- Liu, B.; Zhou, J. SARS-CoV protease inhibitors design using virtual screening method from natural products libraries. J. Comput. Chem. 2005, 26, 484–490. [Google Scholar] [CrossRef] [PubMed]
- Wright, A.D.; de Nys, R.; Angerhofer, C.K.; Pezzuto, J.M.; Gurrath, M. Biological activities and 3D QSAR studies of a series of Delisea pulchra (cf. fimbriata) derived natural products. J. Nat. Prod. 2006, 69, 1180–1187. [Google Scholar]
- Hussain, A.; Melville, J.L.; Hirst, J.D. Molecular docking and QSAR of aplyronine A and analogues: Potent inhibitors of actin. J. Comput. Aided Mol. Des. 2010, 24, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Hassan, H.M.; Elnagar, A.Y.; Khanfar, M.A.; Sallam, A.A.; Mohammed, R.; Shaala, L.A.; Youssef, D.T.A.; Hifnawy, M.S.; El Sayed, K.A. Design of semisynthetic analogues and 3D-QSAR study of eunicellin-based diterpenoids as prostate cancer migration and invasion inhibitors. Eur. J. Med. Chem. 2011, 46, 1122–1130. [Google Scholar] [CrossRef] [PubMed]
- Pereira, F.; Latino, D.A.R.S.; Gaudêncio, S.P. A chemoinformatics approach to the discovery of lead-like molecules from marine and microbial sources en route to antitumor and antibiotic drugs. Mar. Drugs 2014, 12, 757–778. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.-L.; Chan, D.S.-H.; Leung, C.-H. Drug repositioning by structure-based virtual screening. Chem. Soc. Rev. 2013, 42, 2130–2141. [Google Scholar] [CrossRef] [PubMed]
- AntiMarin Database; Blunt, J.W.; Munro, M.H.G.; Laatsch, H. (Eds.) University of Canterbury: Christchurch, New Zealand; University of Göttingen: Göttingen, Germany, 2007.
- AntiBase Home Page. Available online: http://wwwuser.gwdg.de/~hlaatsc/antibase.htm (accessed on 29 January 2015).
- Karelson, M.; Lobanov, V.S.; Katritzky, A.R. Quantum-chemical descriptors in QSAR/QSPR studies. Chem. Rev. 1996, 96, 1027–1044. [Google Scholar] [CrossRef] [PubMed]
- Benigni, R.; Giuliani, A.; Franke, R.; Gruska, A. Structure-activity relationships of mutagenic and carcinogenic aromatic amines. Chem. Rev. 2000, 100, 3697–3714. [Google Scholar] [CrossRef] [PubMed]
- Benigni, R. Structure-activity relationship studies of chemical mutagens and carcinogens: Mechanistic investigations and prediction approaches. Chem. Rev. 2005, 105, 1767–1800. [Google Scholar] [CrossRef] [PubMed]
- Benigni, R.; Bossa, C. Mechanisms of chemical carcinogenicity and mutagenicity: A review with implications for predictive toxicology. Chem. Rev. 2011, 111, 2507–2536. [Google Scholar] [CrossRef] [PubMed]
- Mendoza-Wilson, A.M.; Glossman-Mitnik, D. CHIH-DFT study of the electronic properties and chemical reactiviy of quercetin. J. Mol. Struct. Theochem. 2005, 716, 67–72. [Google Scholar] [CrossRef]
- Mendoza-Wilson, A.M.; Glossman-Mitnik, D. Theoretical study of the molecular properties and chemical reactivity of (+)-catechin and (−)-epicatechin related to their antioxidants ability. J. Mol. Struct. Theochem. 2005, 761, 97–106. [Google Scholar] [CrossRef]
- Laufer, R.S.; Dmitrienko, G.I. Diazo group electrophilicity in kinamycins and lomaiviticin A: Potential insights into the molecular mechanism of antibacterial and antitumor activity. J. Am. Chem. Soc. 2002, 124, 1854–1855. [Google Scholar] [CrossRef] [PubMed]
- Pasha, F.A.; Srivastava, H.K.; Beg, Y.; Singh, P.P. DFT based electrophilicity index and QSAR study of phenols as anti leukemia agent. Am. J. Immunol. 2006, 2, 23–28. [Google Scholar] [CrossRef]
- Wang, L.H.; Yang, X.Y.; Zhang, X.; Mihalic, K.; Fan, Y-X.; Xiao, W.; Howard, O.M.Z.; Appella, E.; Maynard, A.T.; Farrar, W.L. Suppression of breast cancer by chemical modulation of vulnerable zinc fingers in estrogen receptor. Nat. Med. 2004, 10, 40–47. [Google Scholar] [CrossRef]
- Echeverria, C.; Santibanez, J.F.; Donoso-Tauda, O.; Escobar, C.A.; Ramirez-Tagle, R. Structural antitumoral activity relationships of synthetic chalcones. Int. J. Mol. Sci. 2009, 10, 221–223. [Google Scholar] [CrossRef] [PubMed]
- Pasha, F.A.; Muddassar, M.; Lee, C.; Cho, S. Structural antitumoral activity relationships of synthetic chalcones. Environ. Toxicol. Pharmacol. 2008, 26, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Soriano-Correa, C.; Raya, A.; Esquivel, R.O. Mechanism based QSAR studies of N-phenylbenzamides as antimicrobial agents. Int. J. Quant. Chem. 2008, 108, 1369–1379. [Google Scholar] [CrossRef]
- Pereira, F.; Latino, D.A.R.S.; Aires-de-Sousa, J. Estimation of mayr electrophilicity with a quantitative structure-property relationship approach using empirical and DFT descriptors. J. Org. Chem. 2011, 76, 9312–9319. [Google Scholar] [CrossRef] [PubMed]
- Lauria, A.; Ippolito, M.; Almerico, A.M. Combined use of PCA and QSAR/QSPR to predict the drugs mechanism of action. An application to the NCI ACAM database. QSAR Comb. Sci. 2009, 28, 387–395. [Google Scholar] [CrossRef]
- Larsen, S.B.; Omkvist, D.H.; Brodin, B.; Nielsen, C.U.; Steffansen, B.; Olsen, L.; Jorgensen, F.S. Discovery of ligands for the human intestinal di-tripeptide transporter (hPEPT1) using a QSAR-assisted virtual screening strategy. ChemMedChem 2009, 4, 1439–1445. [Google Scholar] [CrossRef] [PubMed]
- Fukui, K. Role of frontier orbitals in chemical reactions. Science 1982, 218, 747–754. [Google Scholar] [CrossRef] [PubMed]
- Bekhradnia, A.; Ala, S.; Bayat, B. Theoretical study on molecular orbital energy levels of h-2 blockers and correlation to their potency. Res. Pharm. Sci. 2012, 7, S615. [Google Scholar]
- Mingeot-Leclercq, M.-P.; Glupczynski, Y.; Tulkens, P.M. Aminoglycosides: Activity and resistance. Antimicrob. Agents Chemother. 1999, 43, 727–737. [Google Scholar] [PubMed]
- Umezawa, H.; Maeda, K.; Takeuchi, T.; Oakami, Y. New antibiotics bleomycin A and B. J. Antibiot. Ser. A 1996, 19, 200–209. [Google Scholar]
- Hecht, S.M. Bleomycin: New perspectives on the mechanism of action. J. Nat. Prod. 2000, 63, 158–168. [Google Scholar] [CrossRef] [PubMed]
- Kruft, B.I.; Greer, A. Photosensitization reactions in vitro and in vivo. Photochem. Photobiol. 2011, 87, 1204–1213. [Google Scholar] [CrossRef] [PubMed]
- PubChem Bioassay Web Page. Cerulomycin—Compound Summary. Available online: http://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?cid=5381230 (accessed on 29 January 2015).
- Mashima, T.; Sato, S.; Okabe, S.; Miyata, S.; Matsuura, M.; Sugimoto, Y.; Tsuruo, T.; Seimiya, H. Acyl-CoA synthetase as a cancer survival factor: Its inhibition enhances the efficacy of etoposide. Cancer Sci. 2009, 100, 1556–1562. [Google Scholar] [CrossRef] [PubMed]
- PubChem Bioassay Web Page. Pyrrolnitrin—Compound Summary. Available online: http://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?cid=13916 (accessed on 29 January 2015).
- Oh, D.-C.; Strangman, W.K.; Kauffman, C.A.; Jensen, P.R.; Fenical, W. Thalassospiramides A and B, immunosuppressive peptides from the marine bacterium Thalassospira sp. Org. Lett. 2007, 9, 1525–1528. [Google Scholar] [CrossRef] [PubMed]
- Stanton, D.T.; Jurs, P.C. Development and use of charged partial surface area structural descriptors in computer assisted quantitative structure property relationship studies. Anal. Chem. 1990, 62, 2323–2329. [Google Scholar] [CrossRef]
- Ertl, P.; Rohde, B.; Selzer, P. Fast calculation of molecular polar surface area as a sum of fragment-based contributions and its application to the prediction of drug transport properties. J. Med. Chem. 2000, 43, 3714–3717. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Fedorowicz, A.; Singh, H.; Soderholm, S.C. Application of the random forest method in studies of local lymph node assay based skin sensitization data. J. Chem. Inf. Model. 2005, 45, 952–964. [Google Scholar] [CrossRef] [PubMed]
- Pearlman, R.S.; Smith, K.M. Metric validation and the receptor relevant subspace concept. J. Chem. Inf. Comput. Sci. 1999, 39, 28–35. [Google Scholar] [CrossRef]
- Todeschini, R.; Consonni, V. Molecular Descriptors for Chemoinformatics, 2nd ed.; Wiley-VCH: Weinheim, Germany, 2009; Volumes 1 and 2. [Google Scholar]
- Dutta, D.; Guha, R.; Wild, D.; Chen, T. Ensemble feature selection: Consistent descriptor subsets for multiple QSAR models. J. Chem. Inf. Model. 2007, 47, 989–997. [Google Scholar] [CrossRef] [PubMed]
- PubChem Home Page. Available online: http://pubchem.ncbi.nlm.nih.gov (accessed on 29 January 2015).
- Wang, Y.; Xiao, J.; Suzek, T.O.; Zhang, J.; Wang, J.; Zhou, Z.; Han, L.; Karapetyan, K.; Dracheva, S.; Shoemaker, B.A. PubChem’s bioAssay database. Nucleic Acids Res. 2012, 40, D400–D412. [Google Scholar] [CrossRef] [PubMed]
- CDK Descriptor Calculator. Version 1.3.2. Available online: http://cdk.sourceforge.net/ (accessed on 29 January 2015).
- Steinbeck, C.; Hoppe, C.; Kuhn, S.; Floris, M.; Guha, R.; Willighagen, E.L. Recent developments of the chemistry development kit (CDK)—An open-source java library for chemo- and bioinformatics. Curr. Pharm. Des. 2006, 12, 2111–2120. [Google Scholar] [CrossRef] [PubMed]
- Stewart, J.J.P. MOPAC2009. Stewart Computational Chemistry: Colorado Springs, CO, USA, 2008. Available online: http://openmopac.net (accessed on 29 January 2015).
- Stewart, J.J.P. Optimization of parameters for semiempirical methods V: Modification of NDDO approximations and application to 70 elements. J. Mol. Model. 2007, 13, 1173–1213. [Google Scholar] [CrossRef] [PubMed]
- Parr, R.G.; Szentpaly, L.V.; Liu, S. Electrophilicity index. J. Am. Chem. Soc. 1999, 121, 1922–1924. [Google Scholar] [CrossRef]
- Akaike, H. A new look at the statistical model identification. IEEE Trans. Autom. Control 1974, 19, 716–723. [Google Scholar] [CrossRef]
- Hall, M.; Frank, E.; Holmes, G.; Pfahringer, B.; Reutemann, P.; Witten, I.H. The WEKA data mining software: An update. SIGKDD Explor. 2009, 11, 10–18. [Google Scholar] [CrossRef]
- Weka. Available online: http://www.cs.waikato.ac.nz/ml/weka/ (accessed on 29 January 2015).
- Hall, M.A.; Smith, L.A. Correlation-based feature selection for machine learning. In Proceedings of the Twelfth International FLAIRS Conference, Orlando, FL, USA, 1–5 May 1999; AAAI Press: Menlo Park, CA, USA, 1999; pp. 235–239. [Google Scholar]
- Aha, D.W.; Kibler, D.; Albert, M.K. Instance-based learning algorithms. Mach. Learn. 1991, 6, 37–66. [Google Scholar]
- Breiman, L.; Friedman, J.H.; Olshen, R.A.; Stone, C.J. Classification and Regression Trees; Chapman & Hall/CRC: Boca Raton, FL, USA, 2000. [Google Scholar]
- Development Core Team, R. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2004. Available online: http://www.r-project.org/ (accessed on 29 January 2015).
- Breiman, L. Random forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Cortes, C.; Vapnik, V. LIBSVM: A library for support vector machines. Mach. Learn. 1995, 20, 273–297. [Google Scholar]
- Chang, C.-C.; Lin, C.-J. LIBSVM: A library for support vector machines. ACM Trans. Intell. Syst. Technol. 2011, 2, 1–27. [Google Scholar] [CrossRef]
- LIBSVM. Available online: http://www.csie.ntu.edu.tw/~cjlin/libsvm/ (accessed on 29 January 2015).
- Manzalawy, Y.; El Honavar, V. WLSVM: Integrating LibSVM into Weka Environment 2005. Available online: http://www.cs.iastate.edu/~yasser/wlsvm/ (accessed on 29 January 2015).
- Sample Availability: Not available.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira, F.; Latino, D.A.R.S.; Gaudêncio, S.P. QSAR-Assisted Virtual Screening of Lead-Like Molecules from Marine and Microbial Natural Sources for Antitumor and Antibiotic Drug Discovery. Molecules 2015, 20, 4848-4873. https://doi.org/10.3390/molecules20034848
Pereira F, Latino DARS, Gaudêncio SP. QSAR-Assisted Virtual Screening of Lead-Like Molecules from Marine and Microbial Natural Sources for Antitumor and Antibiotic Drug Discovery. Molecules. 2015; 20(3):4848-4873. https://doi.org/10.3390/molecules20034848
Chicago/Turabian StylePereira, Florbela, Diogo A. R. S. Latino, and Susana P. Gaudêncio. 2015. "QSAR-Assisted Virtual Screening of Lead-Like Molecules from Marine and Microbial Natural Sources for Antitumor and Antibiotic Drug Discovery" Molecules 20, no. 3: 4848-4873. https://doi.org/10.3390/molecules20034848