N-Hydroxycinnamide Derivatives of Osthole Ameliorate Hyperglycemia through Activation of AMPK and p38 MAPK
Abstract
:1. Introduction
2. Results and Discussion
2.1. OHC-4p and OHC-2m Were Superior to Osthole in Activating AMPK and Increasing Glucose Uptake in Skeletal Muscle Cells
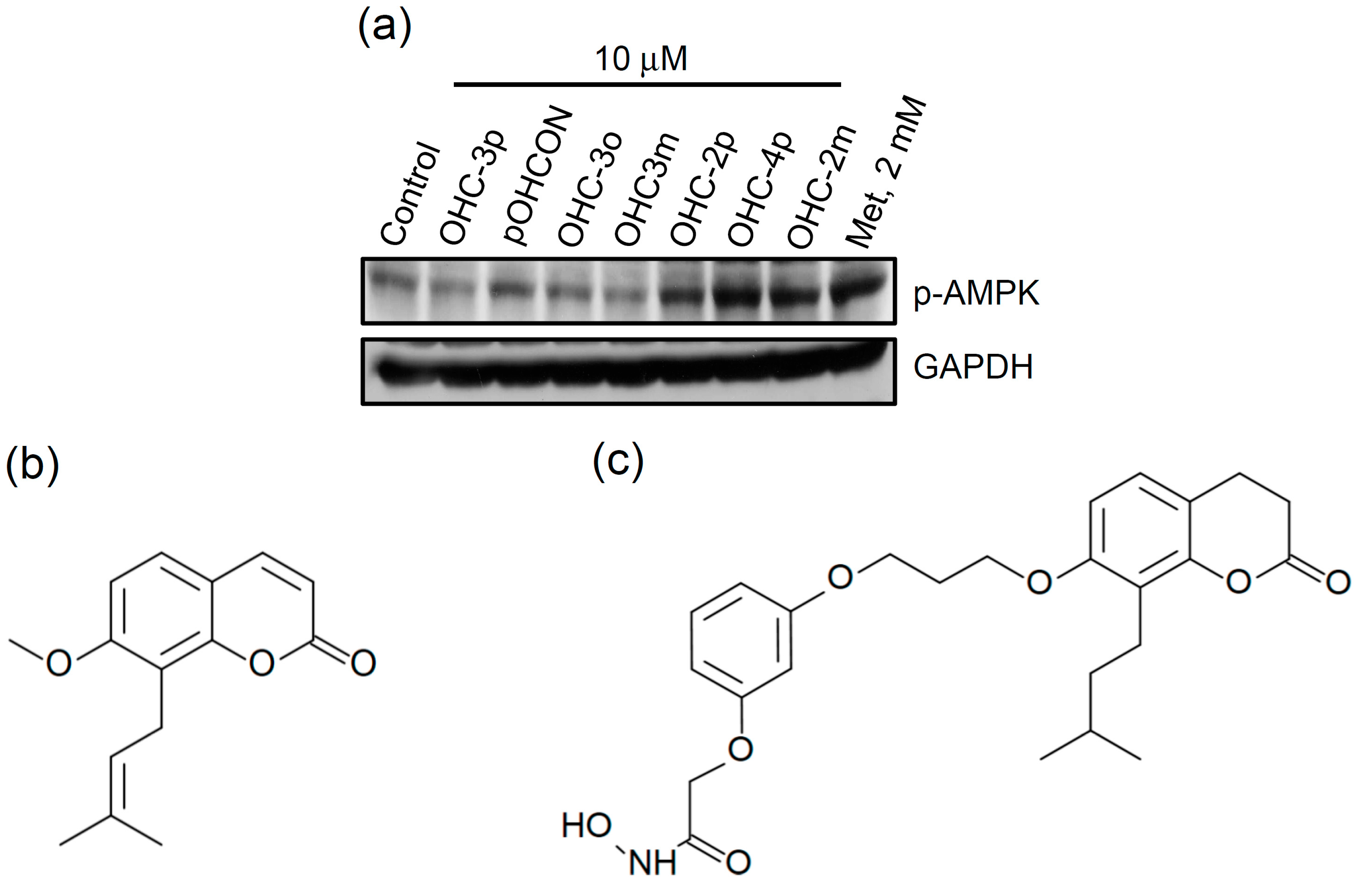

2.2. OHC-4p and OHC-2m Stimulated Glucose Uptake that Depended on Activation of AMPK and p38 MAPK in Skeletal Muscle Cells

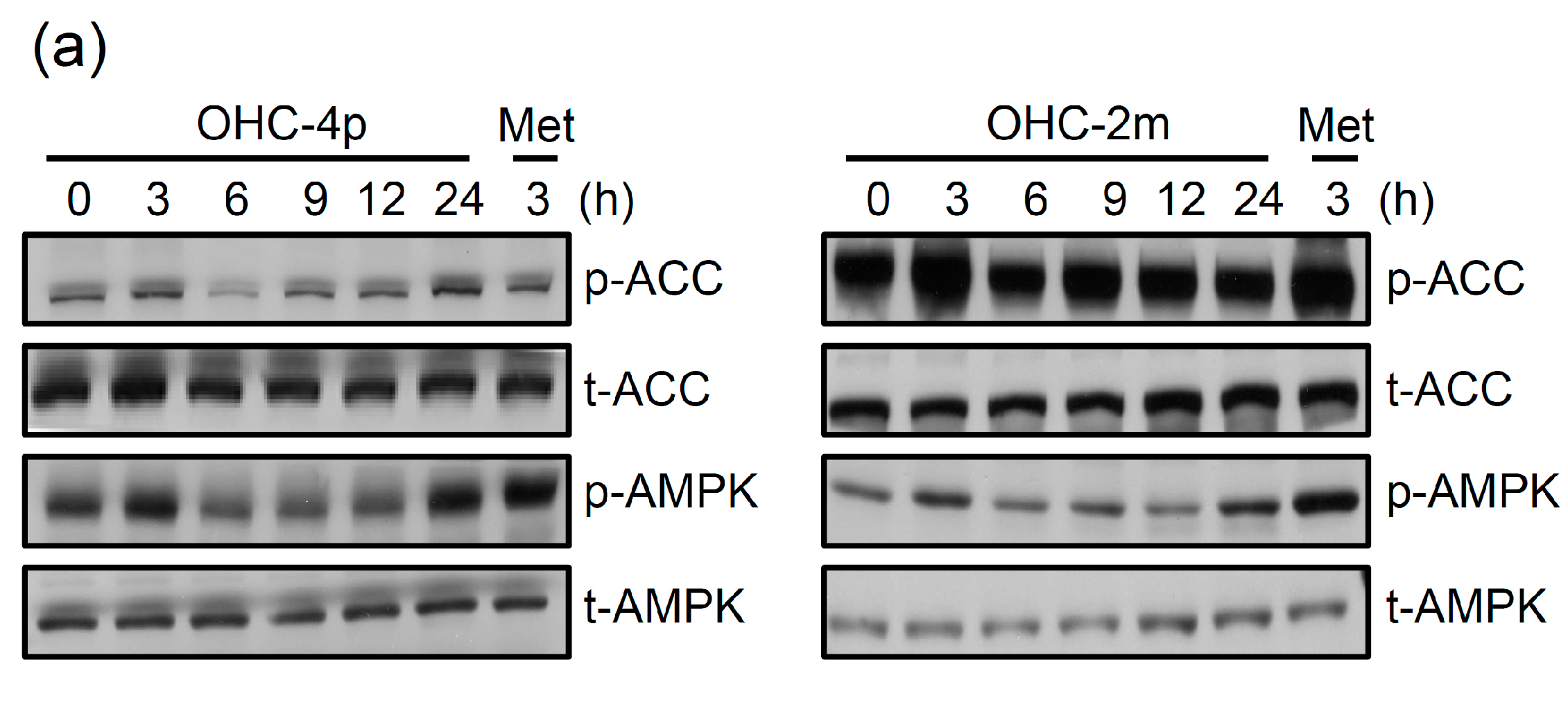
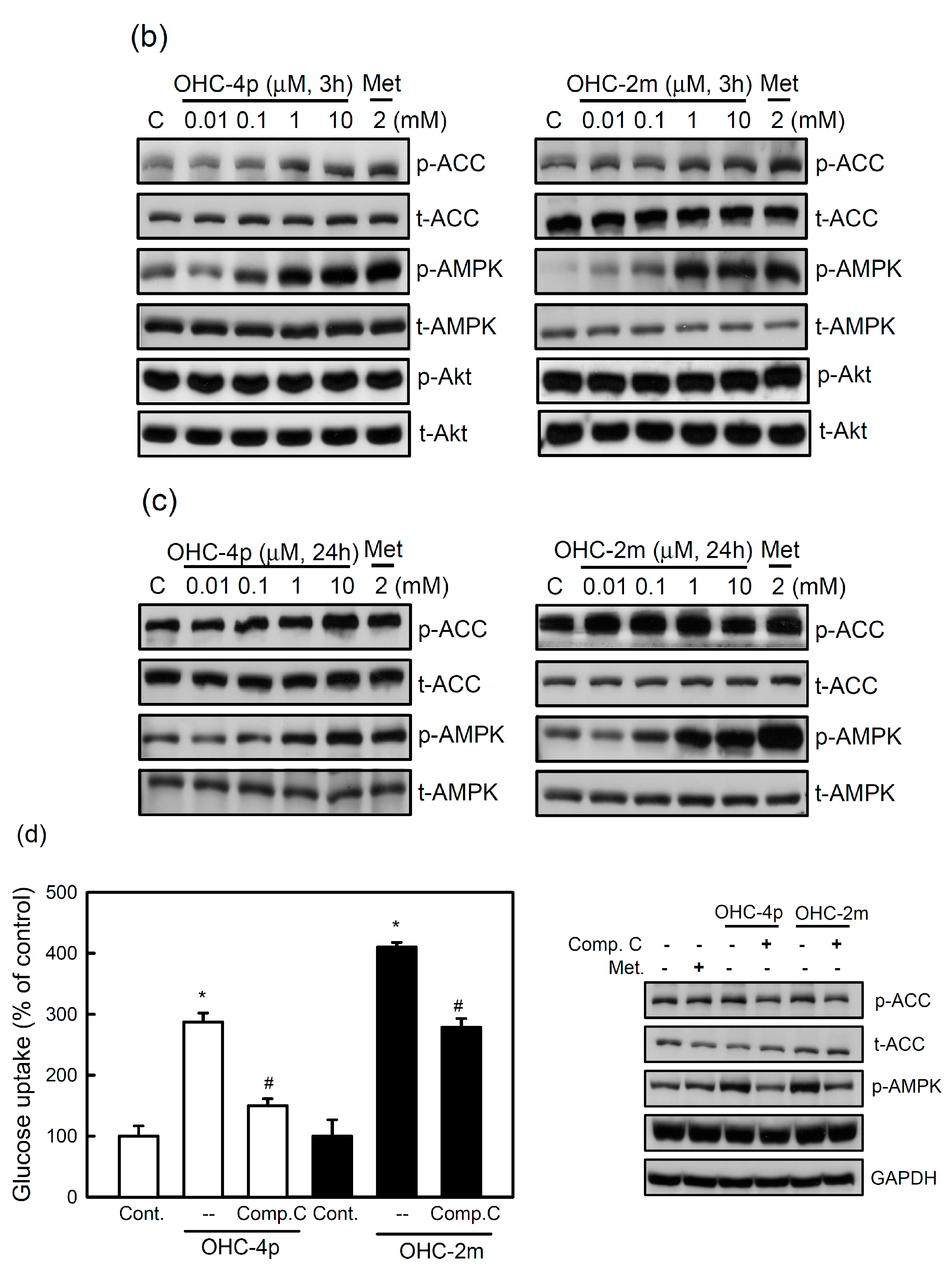
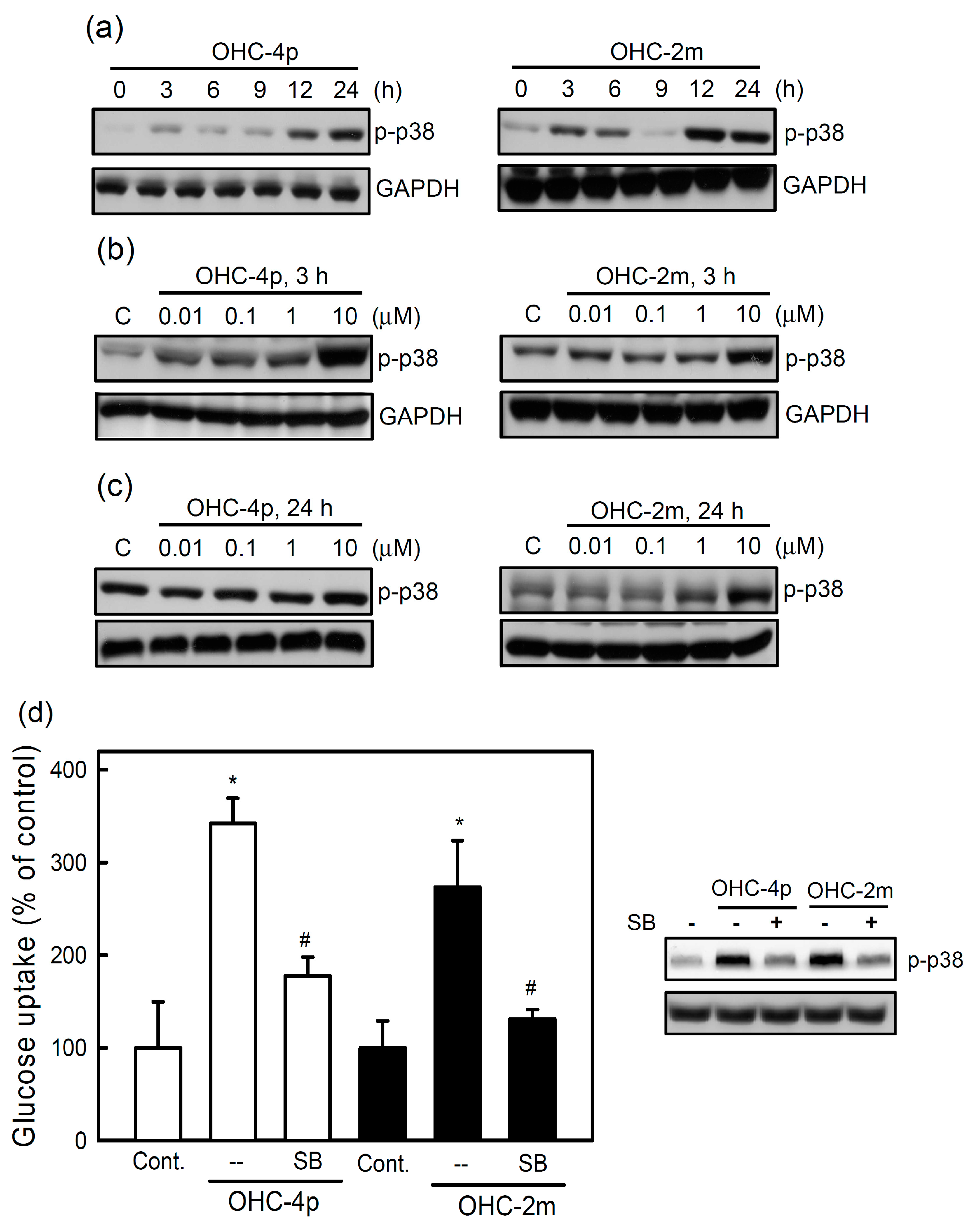
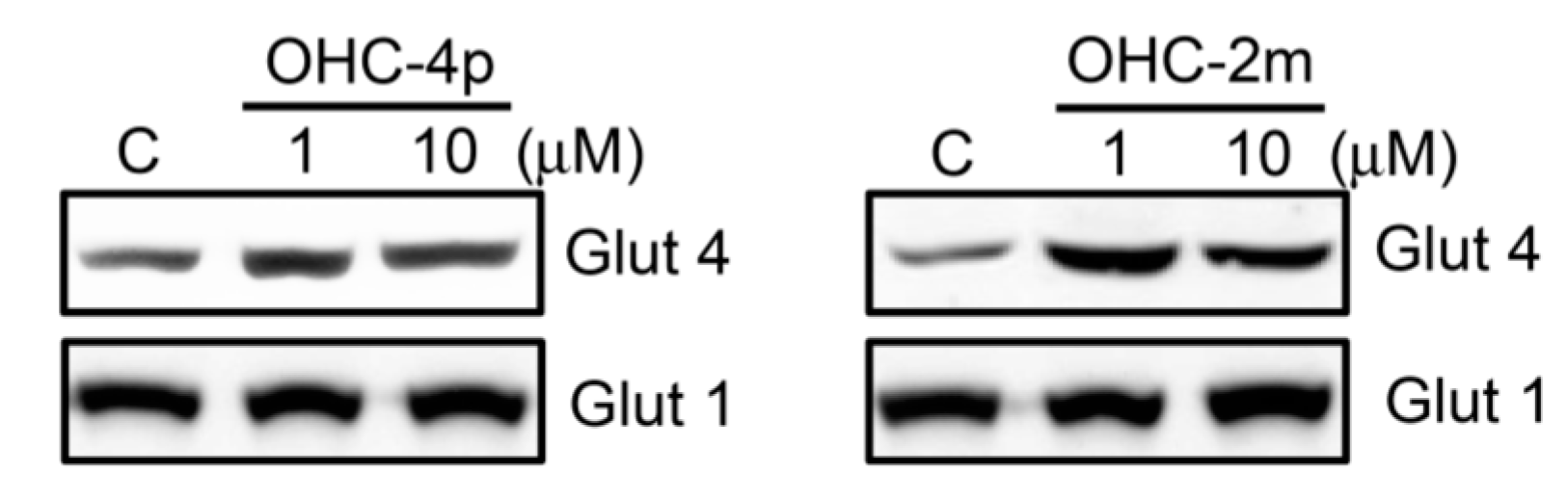
2.3. OHC-4p and OHC-2m Improved Hyperglycemia in Mice with STZ-Induced Diabetes
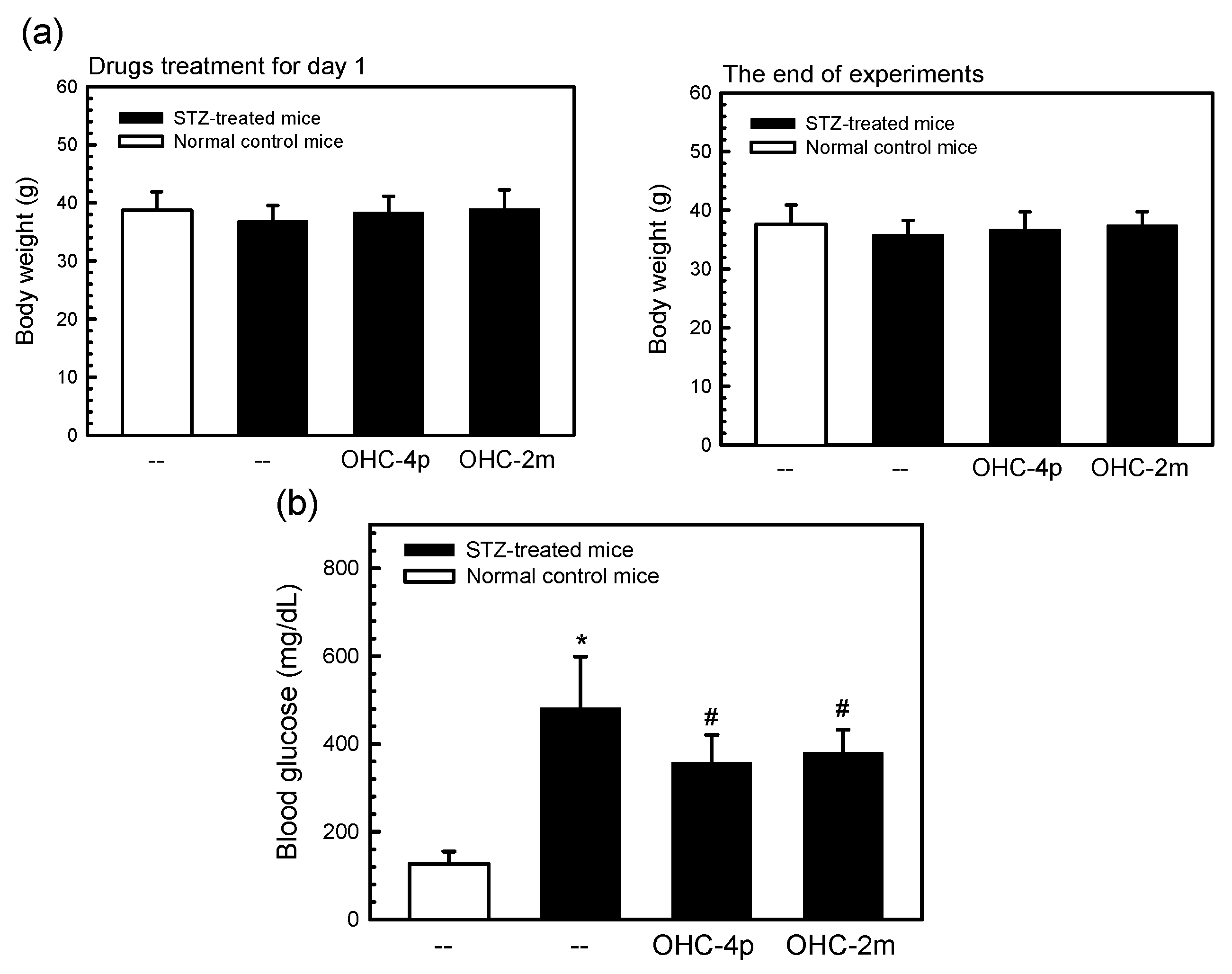
2.4. Discussion
3. Experimental Section
3.1. Materials
3.2. Cell Cultures and Myoblast Differentiation
3.3. Cell Viability Assay
3.4. Preparation of Membrane Proteins and Western Blot Analysis
3.5. Glucose Uptake Assay
3.6. Induction of STZ-Induced Diabetes in Mice and Determination of Plasma Glucose Levels
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Towler, M.C.; Hardie, D.G. AMP-activated protein kinase in metabolic control and insulin signaling. Circ. Res. 2007, 100, 328–341. [Google Scholar] [CrossRef] [PubMed]
- Richter, E.A.; Hargreaves, M. Exercise, GLUT4, and skeletal muscle glucose uptake. Physiol. Rev. 2013, 93, 993–1017. [Google Scholar] [CrossRef] [PubMed]
- Govers, R. Cellular regulation of glucose uptake by glucose transporter GLUT4. Adv. Clin. Chem. 2014, 66, 173–240. [Google Scholar] [PubMed]
- Sakamoto, K.; Holman, G.D. Emerging role for AS160/TBC1D4 and TBC1D1 in the regulation of GLUT4 traffic. Am. J. Physiol. Endocrinol. Metab. 2008, 295, E29–E37. [Google Scholar] [CrossRef] [PubMed]
- Zou, M.H.; Kirkpatrick, S.S.; Davis, B.J.; Nelson, J.S.; Wiles, W.G., IV; Schlattner, U.; Neumann, D.; Brownlee, M.; Freeman, M.B.; Goldman, M.H. Activation of the AMP-activated protein kinase by the anti-diabetic drug metformin in vivo role of mitochondrial reactive nitrogen species. J. Biol. Chem. 2004, 279, 43940–43951. [Google Scholar]
- Konrad, D.; Rudich, A.; Bilan, P.J.; Patel, N.; Richardson, C.; Witters, L.A.; Klip, A. Troglitazone causes acute mitochondrial membrane depolarisation and an AMPK-mediated increase in glucose phosphorylation in muscle cells. Diabetologia 2005, 48, 954–966. [Google Scholar] [CrossRef] [PubMed]
- Merrill, G.F.; Kurth, E.J.; Hardie, D.G.; Winder, W.W. AICA riboside increases AMP-activated protein kinase, fatty acid oxidation, and glucose uptake in rat muscle. Am. J. Physiol. 1997, 273, E1107–E1112. [Google Scholar] [PubMed]
- Park, C.E.; Kim, M.J.; Lee, J.H.; Min, B.I.; Bae, H.; Choe, W.; Kim, S.S.; Ha, J. Resveratrol stimulates glucose transport in C2C12 myotubes by activating AMP-activated protein kinase. Exp. Mol. Med. 2007, 39, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Park, J.M.; Kim, E.K.; Lee, J.O.; Lee, S.K.; Jung, J.H.; You, G.Y.; Park, S.H.; Suh, P.G.; Kim, H.S. Curcumin stimulates glucose uptake through AMPK-p38 MAPK pathways in L6 myotube cells. J. Cell Physiol. 2010, 223, 771–778. [Google Scholar] [PubMed]
- Cai, E.P.; Lin, J.K. Epigallocatechin gallate (EGCG) and rutin suppress the glucotoxicity through activating IRS2 and AMPK signaling in rat pancreatic beta cells. J. Agric. Food Chem. 2009, 57, 9817–9827. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.H.; Xiong, Y.; Collins, Q.F.; Liu, H.Y. p38 mitogen-activated protein kinase plays a critical role in the control of energy metabolism and development of cardiovascular diseases. Zhong Nan Da Xue Xue Bao Yi Xue Ban 2007, 32, 1–14. (In Chinese) [Google Scholar] [PubMed]
- Somwar, R.; Perreault, M.; Kapur, S.; Taha, C.; Sweeney, G.; Ramlal, T.; Kim, D.Y.; Keen, J.; Cote, C.H.; Klip, A.; et al. Activation of p38 mitogen-activated protein kinase alpha and beta by insulin and contraction in rat skeletal muscle: potential role in the stimulation of glucose transport. Diabetes 2000, 49, 1794–1800. [Google Scholar]
- Goel, A.; Nag, P.; Rahuja, N.; Srivastava, R.; Chaurasia, S.; Gautam, S.; Chandra, S.; Siddiqi, MI.; Srivastava, A.K. Discovery of biaryl-4-carbonitriles as antihyperglycemic agents that may act through AMPK-p38 MAPK pathway. Mol. Cell Endocrinol. 2014, 394, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Hwang, J.T.; Park, H.S.; Kwon, D.Y.; Kim, M.S. Capsaicin stimulates glucose uptake in C2C12 muscle cells via the reactive oxygen species (ROS)/AMPK/p38 MAPK pathway. Biochem. Biophys. Res. Commun. 2013, 439, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhang, Y.; Qu, D.; Jiang, T.; Li, S. Osthole induces G2/M arrest and apoptosis in lung cancer A549 cells by modulating PI3K/Akt pathway. J. Exp. Clin. Cancer Res. 2011, 30, 33. [Google Scholar] [CrossRef] [PubMed]
- Liao, P.C.; Chien, S.C.; Ho, C.L.; Wang, E.I.; Lee, S.C.; Kuo, Y.H.; Jeyashoke, N.; Chen, J.; Dong, W.C.; Chao, L.K.; et al. Osthole regulates inflammatory mediator expression through modulating NF-κB, mitogen-activated protein kinases, protein kinase C, and reactive oxygen species. J. Agric. Food Chem. 2010, 58, 10445–10451. [Google Scholar]
- Li, X.X.; Hara, I.; Matsumiya, T. Effects of osthole on postmenopausal osteoporosis using ovariectomized rats, comparison to the effects of estradiol. Biol. Pharm. Bull 2002, 25, 738–742. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.J.; Suk, F.M.; Wang, C.K.; Hung, L.F.; Liu, D.Z.; Chen, N.Q.; Chen, Y.C.; Chang, C.C.; Liang, Y.C. Osthole, a potential antidiabetic agent, alleviates hyperglycemia in db/db mice. Chem. Biol. Interact. 2009, 181, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xie, M.; Xue, J.; Gu, Z. Osthole improves fat milk-induced fatty liver in rats: Modulation of hepatic PPAR-alpha/gamma-mediated lipogenic gene expression. Planta Med. 2007, 73, 718–724. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.H.; Lin, R.J.; Lin, S.Y.; Chen, Y.C.; Lin, H.M.; Liang, Y.C. Osthole Enhances Glucose Uptake through Activation of AMP-Activated Protein Kinase in Skeletal Muscle Cells. J. Agric. Food Chem. 2011, 59, 12874–12881. [Google Scholar] [CrossRef] [PubMed]
- Wagh, R.D.; Mahajan, H.S.; Kaskhedikar, S.G. Synthesis and antioxidant activity of substituted phenylalkene hydroxamic acids. Asian J. Chem. 2007, 19, 4188–4192. [Google Scholar]
- Huang, W.J.; Chen, C.C.; Chao, S.W.; Lee, S.S.; Hsu, F.L.; Lu, Y.L.; Hung, M.F.; Chang, C.I. Synthesis of N-hydroxycinnamides capped with a naturally occurring moiety as inhibitors of histone deacetylase. ChemMedChem 2010, 5, 598–607. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.J.; Chen, C.C.; Chao, S.W.; Yu, C.C.; Yang, C.Y.; Guh, J.H.; Lin, Y.C.; Kuo, C.I.; Yang, P.; Chang, C.I. Synthesis and evaluation of aliphatic-chain hydroxamates capped with osthole derivatives as histone deacetylase inhibitors. Eur. J. Med. Chem. 2011, 46, 4042–4049. [Google Scholar] [CrossRef] [PubMed]
- Summers, J.B.; Kim, K.H.; Mazdiyasni, H.; Holms, J.H.; Ratajczyk, J.D.; Stewart, A.O.; Dyer, R.D.; Carter, G.W. Hydroxamic acid inhibitors of 5-lipoxygenase: Quantitative structure-activity relationships. J. Med. Chem. 1990, 33, 992–998. [Google Scholar] [CrossRef] [PubMed]
- McGee, S.L.; Sparling, D.; Olson, A.L.; Hargreaves, M. Exercise increases MEF2- and GEF DNA-binding activity in human skeletal muscle. FASEB J. 2006, 20, 348–349. [Google Scholar] [PubMed]
- McGee, S.L.; Hargreaves, M. Exercise and skeletal muscle glucose transporter 4 expression: molecular mechanisms. Clin. Exp. Pharmacol. Physiol. 2006, 33, 395–399. [Google Scholar] [CrossRef] [PubMed]
- Xi, X.; Han, J.; Zhang, J.Z. Stimulation of glucose transport by AMP-activated protein kinase via activation of p38 mitogen-activated protein kinase. J. Biol. Chem. 2001, 276, 41029–41034. [Google Scholar] [CrossRef] [PubMed]
- Somwar, R.; Koterski, S.; Sweeney, G.; Sciotti, R.; Djuric, S.; Berg, C.; Trevillyan, J.; Scherer, P.E.; Rondinone, C.M.; Klip, A. A dominant-negative p38 MAPK mutant and novel selective inhibitors of p38 MAPK reduce insulin-stimulated glucose uptake in 3T3–L1 adipocytes without affecting GLUT4 translocation. J. Biol. Chem. 2002, 277, 50386–50395. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.Y.; Huang, W.J.; Lin, R.J.; Lin, S.Y.; Liang, Y.C. N-Hydroxycinnamide derivatives of osthole presenting genotoxicity and cytotoxicity against human colon adenocarcinoma cells in vitro and in vivo. Chem. Res. Toxicol. 2013, 26, 1683–1691. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Not available.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, W.-H.; Wu, H.-H.; Huang, W.-J.; Li, Y.-N.; Lin, R.-J.; Lin, S.-Y.; Liang, Y.-C. N-Hydroxycinnamide Derivatives of Osthole Ameliorate Hyperglycemia through Activation of AMPK and p38 MAPK. Molecules 2015, 20, 4516-4529. https://doi.org/10.3390/molecules20034516
Lee W-H, Wu H-H, Huang W-J, Li Y-N, Lin R-J, Lin S-Y, Liang Y-C. N-Hydroxycinnamide Derivatives of Osthole Ameliorate Hyperglycemia through Activation of AMPK and p38 MAPK. Molecules. 2015; 20(3):4516-4529. https://doi.org/10.3390/molecules20034516
Chicago/Turabian StyleLee, Wei-Hwa, Hsueh-Hsia Wu, Wei-Jan Huang, Yi-Ning Li, Ren-Jye Lin, Shyr-Yi Lin, and Yu-Chih Liang. 2015. "N-Hydroxycinnamide Derivatives of Osthole Ameliorate Hyperglycemia through Activation of AMPK and p38 MAPK" Molecules 20, no. 3: 4516-4529. https://doi.org/10.3390/molecules20034516





