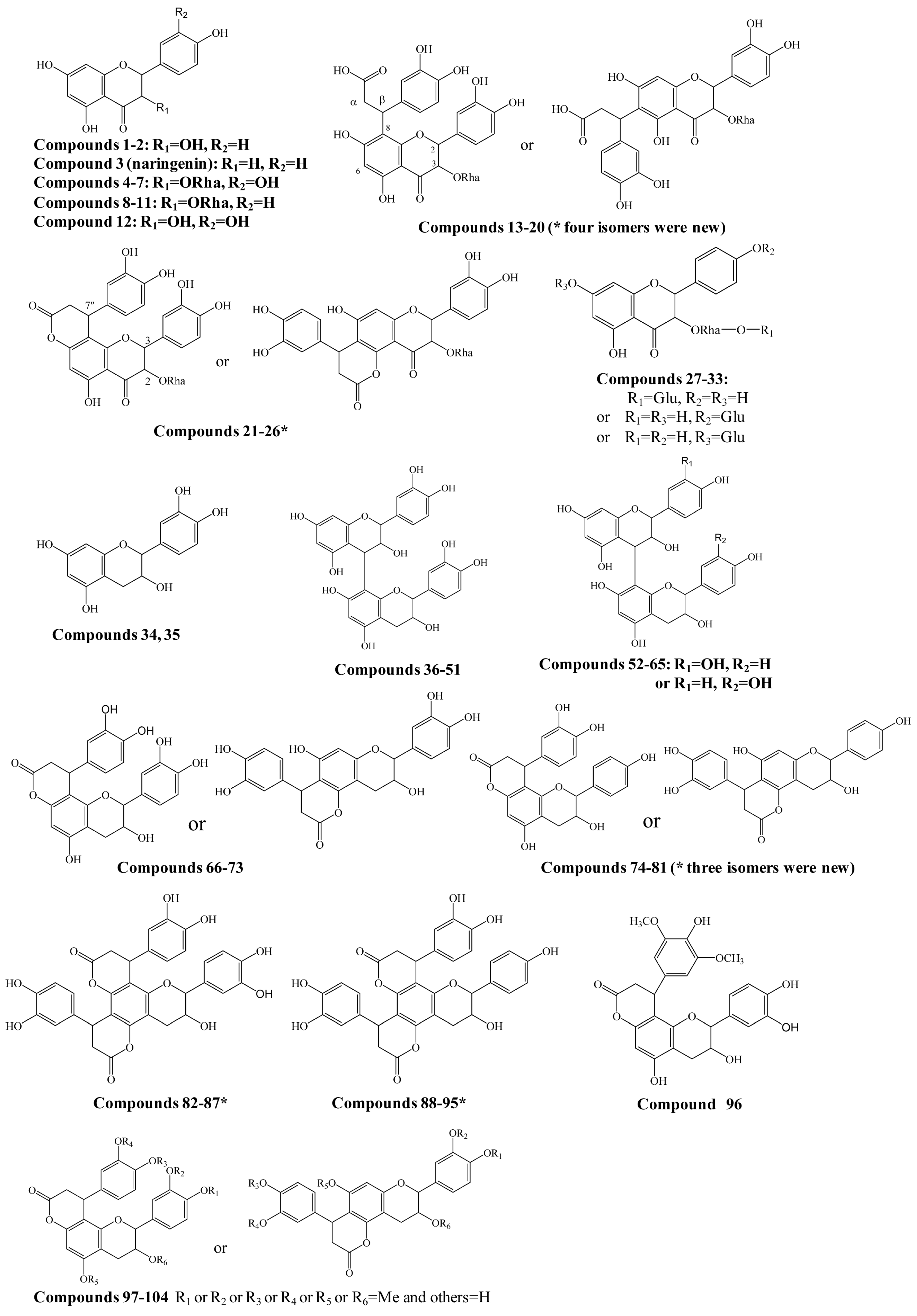
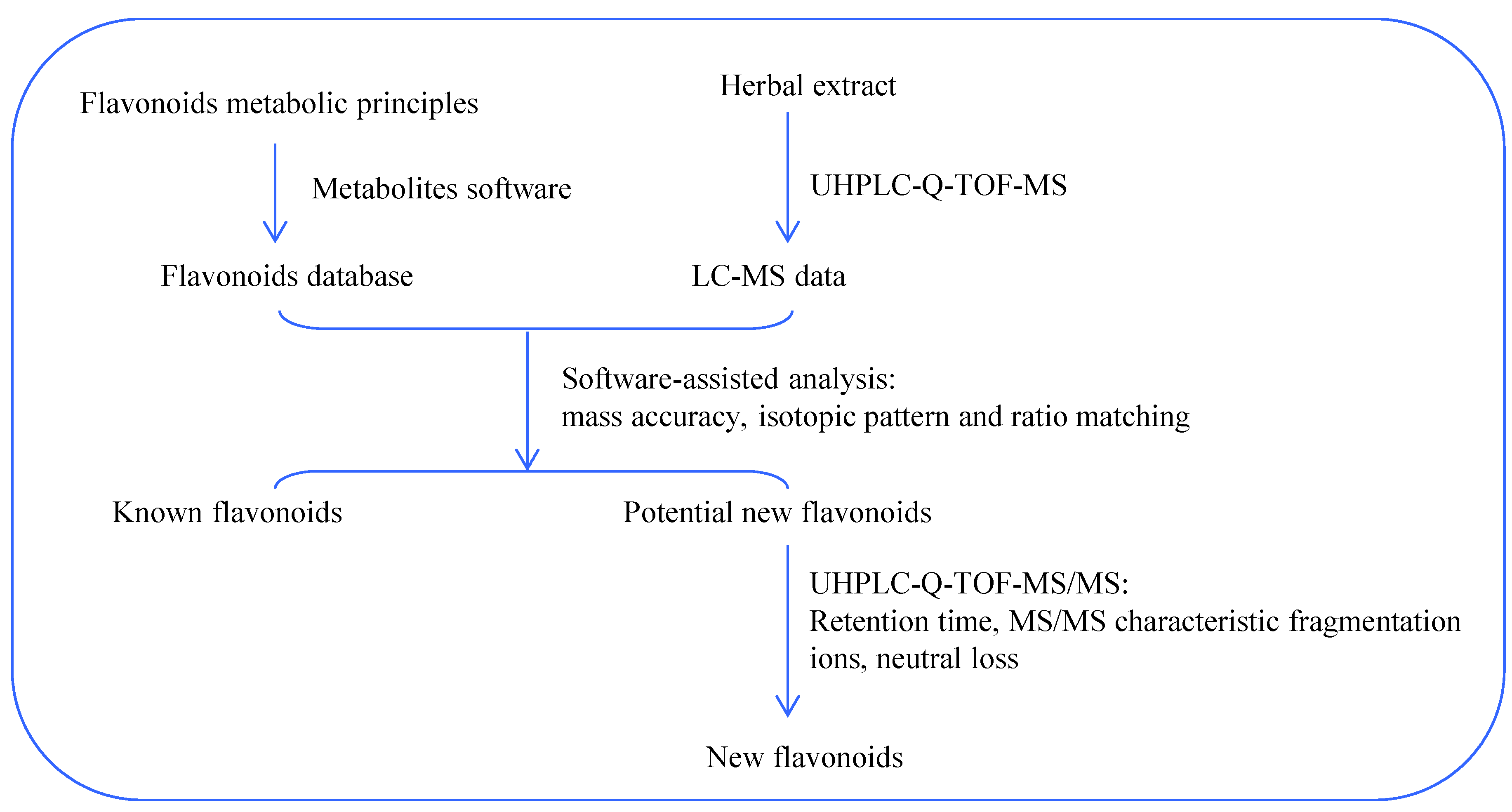
Figure 3.
Flow chart of metabolites software-assisted flavonoids hunting in Smilax glabra.
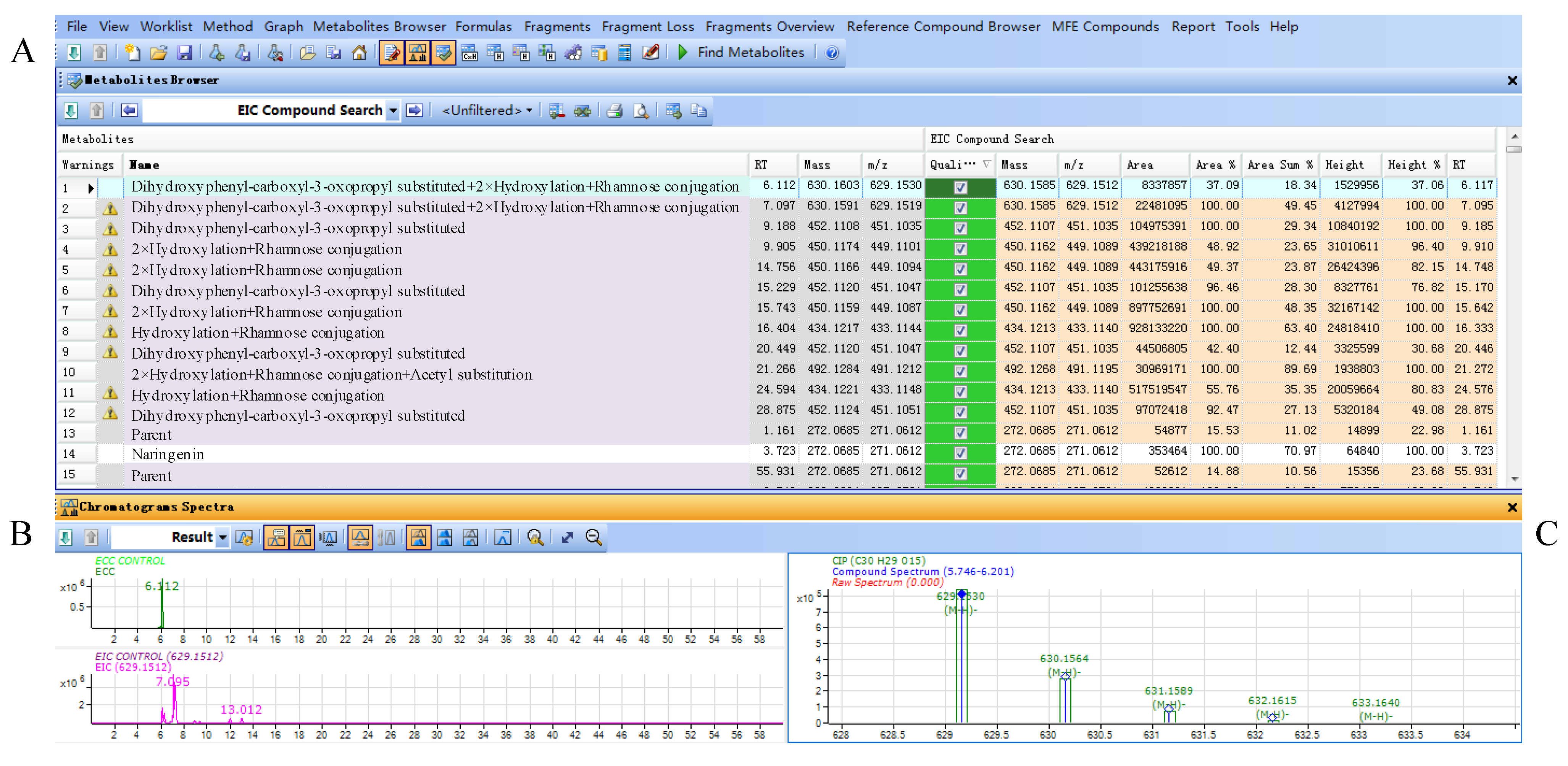
Figure 4.
(A) Result table for filtered compounds by flavonoid database in Smilax glabra; (B) ECC and EIC chromatograms of selected compound; (C) mass spectrum of selected compound.
A total of 454 flavanonols and 170 flavanols were searched, and among them the structures of 104 compounds were further confirmed by the comparison of retention times with suitable standards and/or the interpretation of MS/MS spectra (
Figure 1 and
Supplementary Table S1). At least 27 potential new compounds were identified as four isomers of dihydroxyphenylpropanoic acid-substituted neoastilbin, astilbin, neoisoastilbin, and isoastilbin (compounds
13–
20), dihydroxyphenylpropanoid-substituted astilbin and five isomers (compounds
21–
26), three dihydroxyphenylpropanoid-substituted tetrahydroxyflavanol isomers (
74,
77,
78,
80 or
81), 4,8,10-tris(dihydroxyphenyl)-11-hydroxy-3,4,7,8,11,12-hexahydro-2
H,6
H,10
H-dipyrano[2,3-
f:2',3'-
h]chromene-2,6-dione (
82–
87), and 4,8-bis(dihydroxyphenyl)-11-hydroxy-10-hydroxyphenyl-3,4,7,8,11,12-hexahydro-2
H,6
H,10
H-dipyrano- [2,3-
f:2',3'-
h]chromene-2,6-dione (compounds
88–
95) (
Figure 1). In addition, by comparison with the corresponding references [
8,
9,
10], engeletin glucoside and isomers (compounds
27–
33), 15 isomers of procyanidin B (
36–
51), 13 isomers of tetrahydroxyflavan(4→8)-catechin (
52–
65), six isomers of cinchonains Ia and Ib (
66–
73), three isomers of corbulains Ia and Ib (
75,
76, and
79), and six isomers of smilglabrone B (
97–
104) were detected from
Smilax glabra for the first time (
Figure 1 and
Supplementary Table S1).
2.2.1. Identification of Flavanonols
Naringenin was selected as parent compound in the flavanonols database. Aside from naringenin, 32 other flavanonols, including at least 10 new structures, were automatically searched by the combination of database and UHPLC-Q-TOF-MS, and further identified by their retention times and MS/MS spectra including characteristic MS/MS fragmentation ions and neutral losses.
Astilbin, engeletin, and isomers. Compound
5 at 10.6 min had a quasi-molecular [M-H]
− ion at
m/
z 449.1107 and was identified as a “2× hydroxylation and rhamnose conjugation” derivative of naringenin,
i.e., astilbin or an analog. The mass differences between the calculated (theoretical) and observed values were less than 4 ppm for the molecular ion and the other three isotopic ions (
Supplementary Table S1) and the abundance ratios of the observed isotopic peaks matched well with the calculated values. Moreover, the retention time was same with that of a standard of astilbin. Thus, compound
5 was definitely identified as astilbin. Similarly, three isomers of astilbin—neoastilbin (
4), neoisoastilbin (
6) and isoatilbin (
7)—as well as engeletin (
9), were also determined by comparison of retention times and mass spectra with the corresponding standards. The main fragmentation ions in the MS/MS spectra were produced from the neutral losses of a rhamnose substituted at 3-OH (loss of 146 and 164 Da). Besides, compounds
8,
10 and
11 were deduced as the isomers of engeletin, neoengeletin (
8), neoisoengeletin (
10) and isoengeletin (
11) from the retention times and MS/MS spectra, in which the same fragmentation ions as engeletin were observed [
10].
Dihydroxyphenylpropanoic acid-substituted astilbin and isomers. Eight peaks at 6.1, 6.3, 7.1, 7.2, 8.9, 9.3, 12.0, and 13.0 min (compounds
13–
20) with the formula C
30H
30O
15 were identified as the products of “dihydroxylphenylpropanoic acid substituted+2×hydroxylation+rhamnose conjugation” of narigenin. The [M−H]
− molecular ion of compound
13 was observed at
m/
z 629.1511, along with three isotopic ions at
m/
z 630.1541, 631.1554 and 632.1568, which matched well with the calculated values (<5 ppm). Moreover, the abundances of observed ion peaks matched well with the calculated values and the relative errors were 0%–2.5%. The main fragmentation ions at
m/
z 303.0511, 285.0406 and 151.0032 indicated that the skeleton should be dihydroquercetin (
Figure 5) [
20]. The product ions at
m/
z 475.1258 and 449.1096 produced from the neutral losses of CO
2+C
6H
6O
2 and C
9H
8O
4, respectively, confirmed the existence of a dihydroxylphenylpropanoic acid substitution in compound
13, which connected with the skeleton by C-β. Furthermore, the mass differences of 146 and 164 Da from
m/
z 449.1096 to 303.0511 and 285.0406, respectively, demonstrated the presence of a rhamnose unit at 3-OH, which was further confirmed by the product ions at
m/
z 329.0669 and 311.0535 produced from
m/
z 475.1258. The product ion at
m/
z 177.0190 indicated that the dihydroxylphenylpropanoic acid group should be substituted at C-6 or C-8 (
Figure 5C). Therefore, compound
13 was identified as β-dihydroxyphenyl-α-carboxyl-3-oxopropyl-substituted 3,5,7,3ʹ,4ʹ-pentahydroxyl-flavanonol 3-
O-rhamnoside,
i.e., dihydroxyphenylpropanoic acid-substituted astilbin or an isomer. Compounds
15 and
16 had similar MS and MS/MS spectra as compound
13 (
Supplementary Table S1), and were identified as isomers of compound
13, where the differences might be the configurations at C-2, C-3 and C-β, or the substitution position of the dihydroxyphenylpropanoic acid moiety on ring A. In addition, five trace compounds,
14, and
17–
20 were also assigned as the same structure by the software-assisted mass data matching.
Figure 5.
(A) Extracted ion chromatogram (EIC) of compound 13–20; (B) MS/MS spectrum of compound 13; (C) proposed fragmentation pathway of compound 13.
Figure 5.
(A) Extracted ion chromatogram (EIC) of compound 13–20; (B) MS/MS spectrum of compound 13; (C) proposed fragmentation pathway of compound 13.
These eight compounds can be divided into four groups from retention times 6.1 min (
13) and 6.3 min (
14); 7.1 min (
15) and 7.2 min (
16); 8.9 min (
17) and 9.3 min (
18); 12.0 min (
19) and 13.0 min (
20), and the retention times within the groups were very close, while they were significantly different among the groups. The pattern was very similar to that of astilibin and three isomers, in which the elution order was neoastilbin (9.9 min), astilbin (10.6 min), neoisoastilbin (14.8 min) and isoastilbin (15.9 min). Thus,
13 and
14 should be β-dihydroxyphenyl-α-carboxyl-3-oxopropyl-substituted neoastilbin, and the difference lies on the configuration of C-β or the substitution position of the dihydroxyphenylpropanoic acid unit on ring A. Similarly, six other compounds were identified as β-dihydroxyphenyl-α-carboxyl-3-oxopropyl-substituted astilbins (
15 and
16), β-dihydroxyphenyl-α-carboxyl-3-oxopropyl-substituted neoisoastilbins (
17 and
18), and β-dihydroxyphenyl-α-carboxyl-3-oxopropyl-substituted neoisoastilbins (
19 and
20). Four of them were detected for the first time [
10].
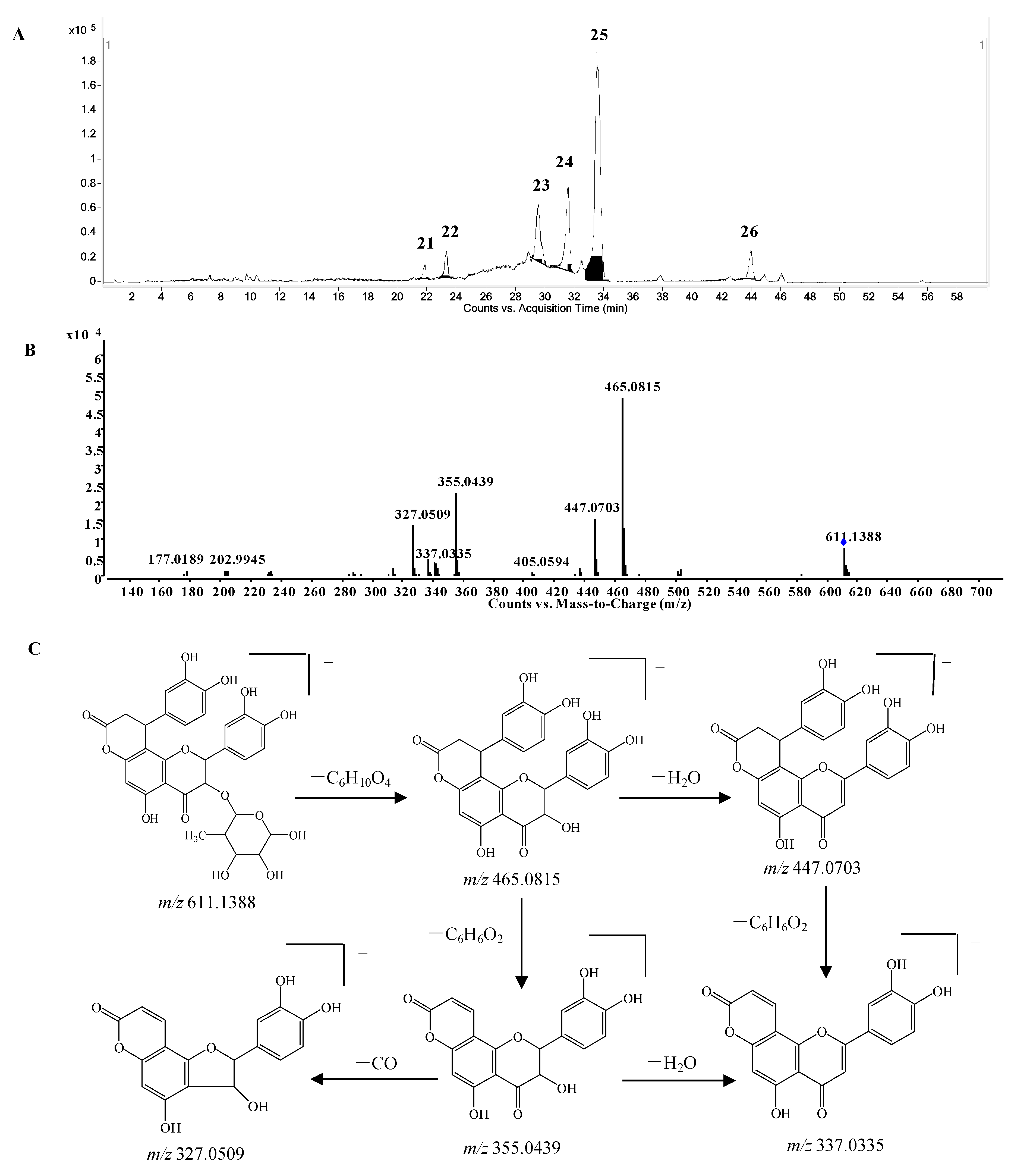
Phenylpropanoid-substituted astilbin and isomers. Six peaks at 21.9, 23.3, 29.5, 31.5, 33.7 and 43.8 min (compounds
21–
26) with the molecular formula of C
30H
28O
14 were identified as the products of “dihydroxyphenylpropanoid-substituted+2×hydroxylation+rhamnose conjugation” of narigenin. The [M−H]
− molecular ion of compound
24 was observed at
m/
z 611.1388, and three isotopic ions at
m/
z 612.1419, 613.1434 and 614.1438, which matched well with the calculated values, were also seen. Moreover, the abundances of the observed ion peaks matched well with the calculated values and the relative errors were 0%–4.5%. The main fragmentation ions at
m/
z 465.0815 [M-H-rhamnose]
− and 447.0703 [M-H-rhamnose-H
2O]
− (
Figure 6B) confirmed the presence of rhamnose, while the ions at
m/
z 355.0439 [M-H-rhamnose-C
6H
6O
2]
− and 337.0335 [M-H-rhamnose-H
2O-C
6H
6O
2]
− indicated the substitution of the dihydroxyphenyl group. The molecular formula C
30H
28O
14 has two hydrogens less and one oxygen more than that of glabraoside A (C
30H
30O
13), a dihydroxyphenylpropanoid-substituted catechin rhamnoside, and the fragmentation ions produced from the losses of rhamnose and dihydroxyphenyl group were also 14 Da more than the corresponding ions in glabraoside A [
19], so it should be a dihydroxyphenylpropanoid-substituted flavanonol rhamnoside,
i.e., dihydroxyphenyl-propanoid-substituted astilbin or an isomer. The product ions at
m/z 327.0509 produced from the loss of CO from the ion of
m/z 355.0439 further confirmed the existence of a carbonyl group at position 4 in compound
24. Compounds
23,
25 and
26 were identified as isomers of compound
24 by their similar MS and MS/MS spectra (
Supplementary Table S1), and the differences among them should be the configurations at C-2, C-3 and C-7", or the substitution position of the dihydroxyphenylpropanoid unit on ring A. This is the first report of phenylpropanoid-substituted astilbin and isomers.
Engeletin glucoside and isomers. Compounds
27–
33 with the molecular formulae C
27H
32O
15 were identified as “hydroxylation+rhamnose+glucose conjugation” derivatives of narigenin. The exact masses of the molecular ions matched well with the theoretical values with an error of less than 5 ppm, and at least two isotopic peaks were observed, and their exact masses and abundances were consistent with the calculated values too (
Supplementary Table S1). These compounds have one glucose more than engeletin and isomers, neoengeletin, neoisoengeletin, and neoisoengeletin. According to the retention times, they were divided into four groups, 11.7 min (
27) and 11.9 min (
28); 12.3 min (
29) and 12.7 min (
30); 14.6 min (
31) and 15.0 min (
32); and 16.9 min (
33).
Figure 6.
(A) Extracted ion chromatogram (EIC) of compounds 21–26; (B) MS/MS spectrum of compound 24; (C) proposed fragmentation pathway of compound 24.
Figure 6.
(A) Extracted ion chromatogram (EIC) of compounds 21–26; (B) MS/MS spectrum of compound 24; (C) proposed fragmentation pathway of compound 24.
The pattern of these groups was very similar to that of neoengeletin (16.6 min), engeletin (19.2 min), neoisoengeletin (20.9) and isoengeletin (24.6 min), moreover, the retention times were shorter than those of the corresponding engeletin and isomers, so it was deduced that they were neoengeletin glucoside (27 and 28), engeletin glucoside (29 and 30), neoisoengeletin glucoside (31 and 32) and isoengeletin glucoside (33). The glucose might be a substituent at 7-OH, 4ʹ-OH or the rhamnose. No compound with the molecular formula of C27H32O15 was previously reported from S. glabra.
2.2.2. Identification of Flavanols
The flavanol database was established using catechin as parent compound. Two peaks (
34 and
35) with the molecular ions at
m/z 289.0718 (C
15H
13O
6) were detected at 3.8 and 4.8 min. They had identical fragmentation ions at
m/z 245.08, 205.05, and 179.03, which were same as those of catechin [
8]. Epicatechin eluted later than catechin under similar liquid chromatography conditions [
8], so compounds
34 and
35 were identified as catechin and epicatechin, respectively.
Flavanol polymers. Compounds
36–
51 with the molecular formula of C
30H
26O
12 (
m/z 577.13) were identified as “catechin conjugation” products of catechin with the assistance of the software by comparison of MS data (
Table S1). The MS/MS spectra further confirmed the structures, in which the main fragmentation ions were observed at
m/z 289.07, 407.07, 425.08, and 451.10 similar to those of procyanidin B [
8,
10]. Thus, compounds
36–
51 were identified as dimers of catechin,
i.e., procyanidin B and isomers. Similarly, compounds
52–
65 with the molecular formula of C
30H
26O
11 (
m/z 561.1402) were deduced to be “tetrahydroxyflavanol conjugation” derivatives of catechin and isomers. They had one oxygen less than compounds
36–
51 (
Supplementary Table S1). Unlike procyanidin B, the characteristic fragmentation ion of a catechin dimer at
m/z 451.10 was not observed [
8,
10], and instead an ion at
m/
z 435.10 was observed in the MS/MS spectra of compounds
52–
54, thus the upper unit of these three polymers should be tetrahydroxyflavanol, while the lower unit was catechin. This was further confirmed by the fragmentation ion at
m/
z 407.07 produced from the loss of ring-B-
2CH=
3CH-OH of the upper unit (C
8H
10O
3) [
21]. Therefore, compounds
52–
54 were identified as tetrahydroxyflavanol(4→8)-catechin and isomers. Compounds
55–
65 should be considered polymers of catechin and tetrahydroxyflavanol, although the linkage couldn’t be determined due to the lack of MS/MS information. Until now, just one isomer of C
30H
26O
12 (procyanidin B) and one of C
30H
26O
11 were found in
S. glabra [
8,
10], accordingly, the other 28 isomers were identified in this herb for the first time in this work.
Dihydroxyphenylpropanoid-substituted catechin and isomers. Compounds
66–
73 with the molecular formula of C
24H
20O
9 (
m/z 451.1035) at 9.2, 15.2, 15.4, 15.8, 20.5, 20.9, 28.9, and 30.4 min were assigned as “dihydroxyphenylpropanoid conjugation” products of catechin. They had similar MS/MS spectra, and the main product ions were observed at
m/z 341.06 and 217.01, which were similar to those of cinchonain Ib [
8,
10]. The ion at
m/z 341.06 was produced from the neutral loss of C
6H
6O
2 (110 Da), which confirmed the existence of a dihydroxyphenyl group. The ion at
m/z 217.01 was generated from the neutral losses of C
6H
6O
2 and C
7H
8O
2 (234 Da), which was produced from the elimination of dihydroxytoluene [
19]. Therefore, compounds
66–
73 were confirmed to be dihydroxylphenylpropanoid-substituted catechins and isomers,
i.e., cinchonains Ia, Ib, Ic, Id and isomers (
Supplementary Table S1). Until now, only two isomers, cinchonains Ia and Ib, were reported from
S. glabra [
8,
10].
Dihydroxyphenylpropanoid-substituted tetrahydroxyflavnol and isomers. Compounds
74–
81 with the molecular ions of
m/
z 435.10 at 13.7, 17.3, 21.9, 22.5, 23.2, 24.1, 39.6, and 40.2 min were assigned as “phenylpropanoid+hydroxylation” derivatives of catechin. The molecular formula C
24H
20O
8 has one oxygen less than compounds
66–
73 (
Supplementary Table S1). Like cinchonain Ib, the main fragmentation ion of compounds
74,
77,
78,
80 and
81 at
m/z 325.0730 was produced from the neutral loss of C
6H
6O
2. It was reported that the ion produced from the neutral loss of benzene or substituted benzene was the characteristic product ion of phenylpropanoid-substituted catechins, e.g., the neutral loss of C
6H
6O
2 (110 Da) was the characteristic fragmentation ion of a 3,4-dihydroxyphenylpropanoid-substituted catechin. Thus, the dihydroxyphenyl group should be a substituent at C-7ʺ, not C-2,
i.e., a dihydroxyphenylpropanoid-substituted tetrahydroxyflavanol. Until now, there were only two such isomers, corbulain Ia and Ib, that were reported from
S. corbularia [
22]. Three compounds among
74,
77,
78,
80 and
81 were potential new compounds, and the differences among them should be the configurations of C-2, C-3, and C-7ʺ, and the substitution position of the dihydroxyphenylpropanoid moiety on ring A.
Bis-dihydroxyphenylpropanoid-substituted catechin and isomers. Compounds
82–
87 had the same molecular formula of C
33H
26O
12, and were identified as “2×dihydroxyphenylpropanoid-substituted” products of catechin. The major fragmentation ions in the MS/MS spectra were observed at
m/z 503.10, 393.06, and 341.06 (
Supplementary Table S1). The product ions at
m/z 503.10 and 393.06 were attributed to the neutral losses of one and two molecules of C
6H
6O
2 (110 Da), respectively, which indicated the existence of two dihydroxylphenyl groups. Another major product ion at
m/z 341.06 produced from the neutral loss of C
6H
6O
2+C
9H
6O
3 was also observed as prominent fragmentation peak in MS/MS spectrum of cinchonain Ib. Hence, compounds
82–
87 should be bis-dihydroxylphenylpropanoid-substituted catechins, which were further confirmed by the fragmentation ions at
m/z 451.1037 (C
24H
19O
9) produced from the neutral loss of C
9H
6O
3. The structure of one compound was determined by Liquid Chromatography-Solid Phase Extraction-Nuclear Magnetic Resonance (LC-SPE-NMR) approach in our laboratory.
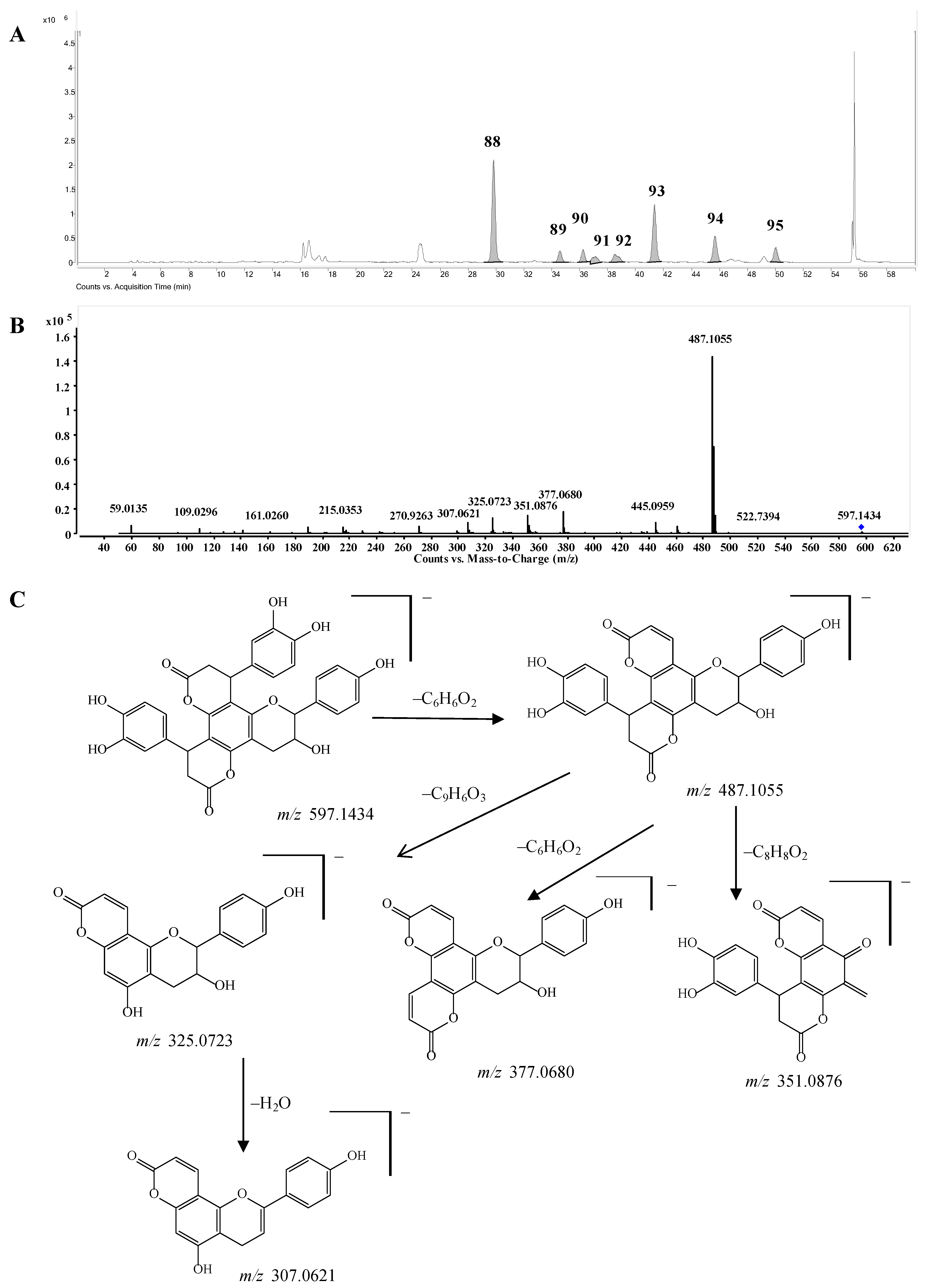
Bis-dihydroxyphenylpropanoid-substituted tetrahydroxyflavanol and isomers. Compounds
88–
95 had the same molecular formula C
33H
26O
11, and were predicted as “2×phenylpropanoid-substituted+3×hydroxylation” products of catechin. Like compounds
82–
87, the main fragmentation ions of compound
88 were produced from the neutral losses of one and two C
6H
6O
2 and C
6H
6O
2+C
9H
6O
3 at
m/z 487.1055, 377.0680, and 325.0723, respectively, which indicated the substitution of two dihydroxyphenylpropanoid units (
Figure 7C). The fragmentation ion at
m/
z 351.0876 produced from the loss of ring-B-
2CH=
3CH-OH from the ion of
m/
z 487.1055 further showed that only one hydroxyl group was substituted on ring B of compound
88, so compound
88 was determined as a bis-dihydroxyphenylpropanoid-substituted tetrahydroxyflavanol,
i.e., 4,8-bis(dihydroxy-phenyl)-11-hydroxy-10-hydroxyphenyl-3,4,7,8,11,12-hexahydro-2
H,6
H,10
H-dipyrano[2,3-
f:2',3'-
h]-chromene-2,6-dione. The other compounds
89–
95 might be the isomers of compound
88 from the MS data (
Supplementary Table S1), however, the substitution positions of the hydroxyl group couldn’t be determined due to the lack of MS/MS spectra. This is the first report concerning this type of structures.
Methylated cinchonains. Eight peaks at 24.3, 25.3, 25.5, 25.9, 27.2, 31.4, 32 and 38.5 min (compounds
97–
104) had the same molecular formula of C
25H
22O
9 and were indicated as “methylation+dihydroxyphenylpropanoid-substituted” derivatives of catechin (
Supplementary Table S1). The MS/MS spectrum of compound
99 was almost same with that of cinchonain Ib, while the molecular formula had one methylene more than cinchonain Ib. Due to the characteristic fragmentation ions related to the loss of benzene from a dihydroxyphenylpropanoid unit, the methyl group should thus be on 3ʺ-OH or 4ʺ-OH,
i.e., smiglabrone B or an isomer (
Figure 1) [
9]. The difference among the other isomers (compounds
97–
98 and
100–
104) should be the substitution position of the methyl group or the configuration of the cinchonain skeletons.
Figure 7.
(A) Extracted ion chromatogram (EIC) of compounds 88–95; (B) MS/MS spectrum of compound 88; (C) Proposed fragmentation pathway of compound 88.
Figure 7.
(A) Extracted ion chromatogram (EIC) of compounds 88–95; (B) MS/MS spectrum of compound 88; (C) Proposed fragmentation pathway of compound 88.
Until now, only one such compound, smiglabrone B, was reported in
S. glabra [
9] and a stereoisomer of smiglabrone B was isolated from
Guibourtia coleosperma [
23]. Besides, a compound
96 with the molecular formula of C
26H
24O
10 was assigned as a “2×methylation+hydroxylation+ dihydroxyphenylpropanoid-substituted” derivative of catechin,
i.e., smiglabrone A or an isomer (
Figure 1) [
9].