Novel Orally Active Analgesic and Anti-Inflammatory Cyclohexyl-N-Acylhydrazone Derivatives
Abstract
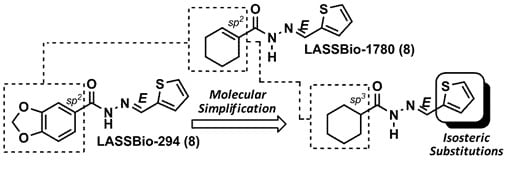
:1. Introduction
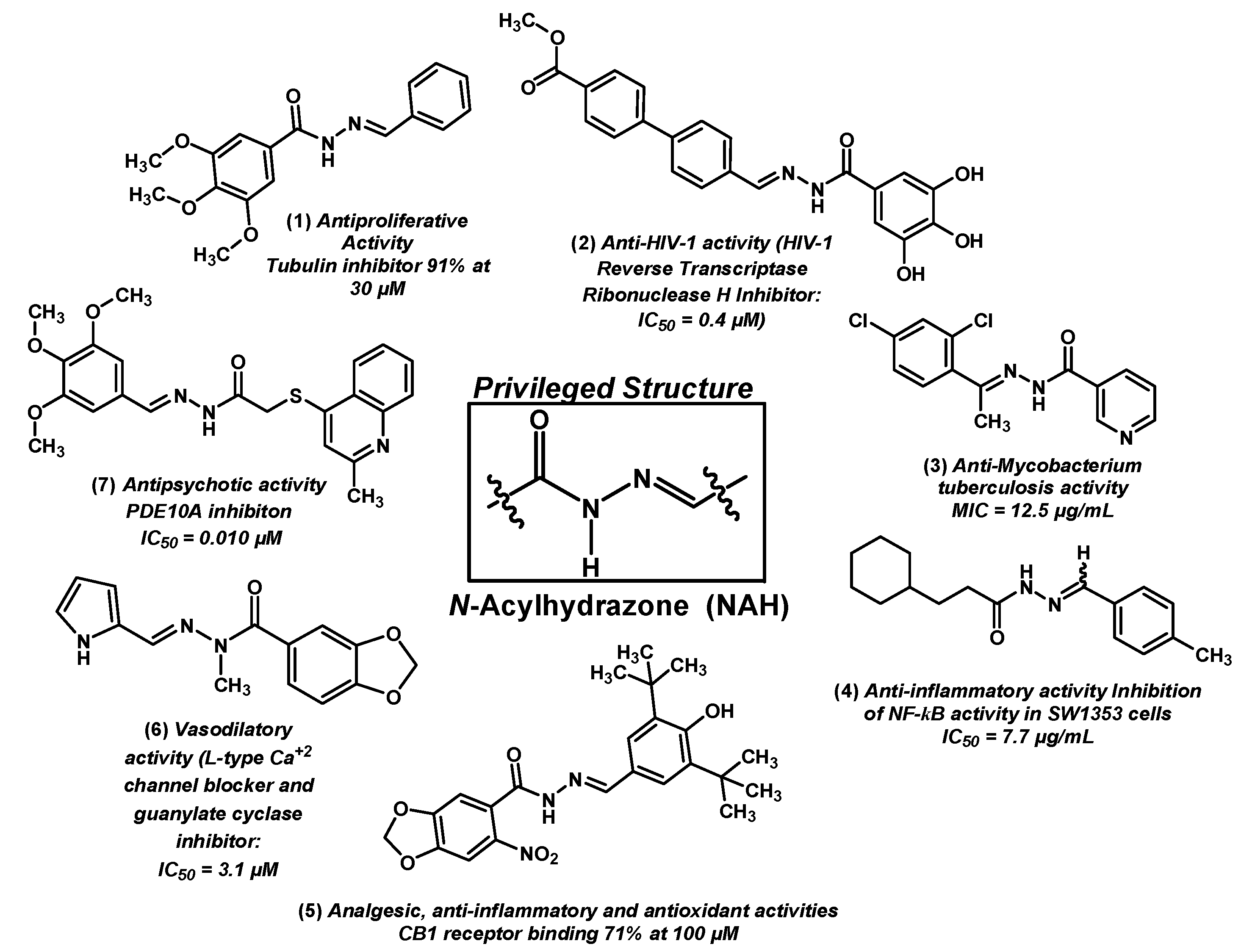

2. Results and Discussion
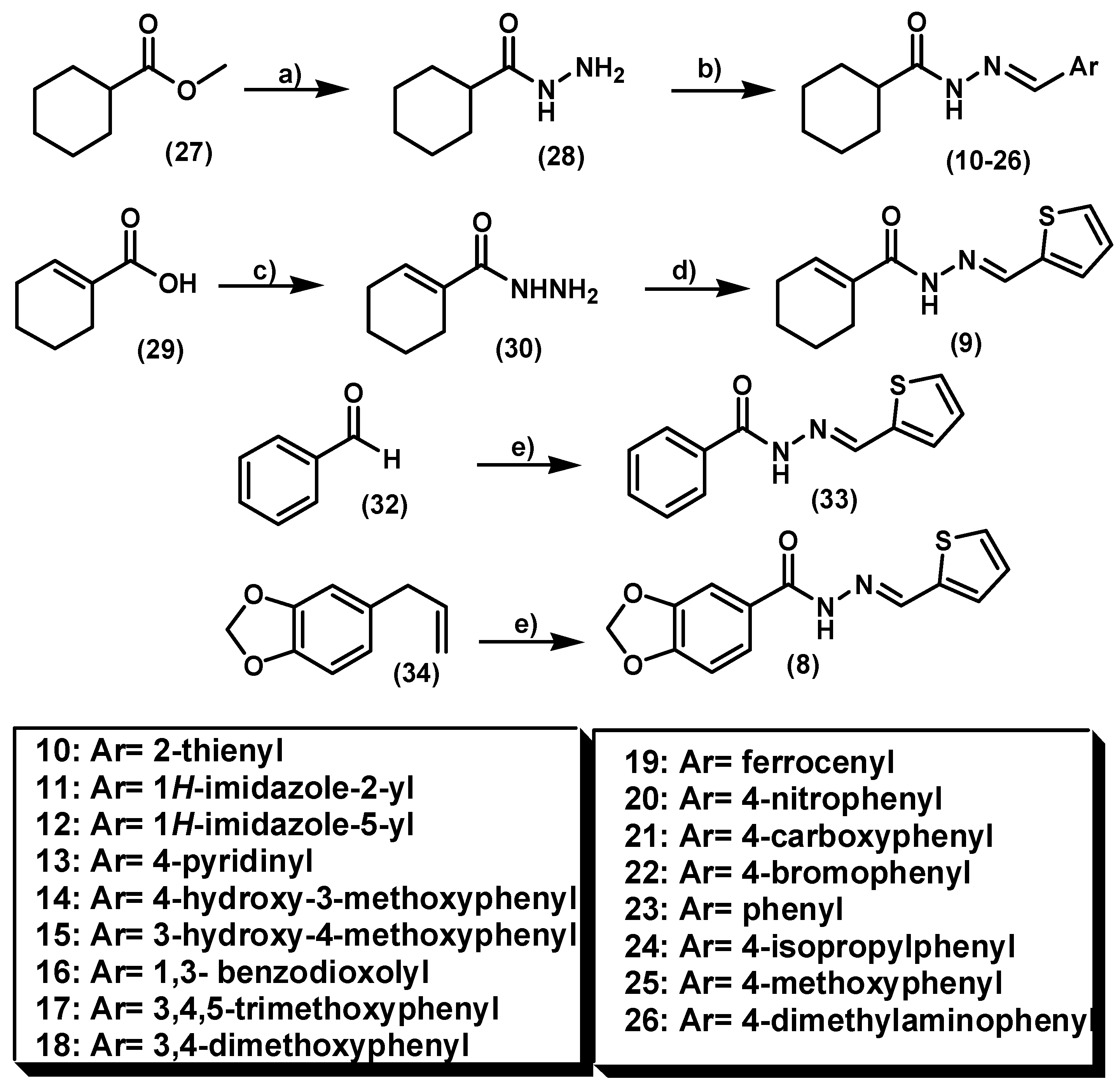
2.1. Chemistry
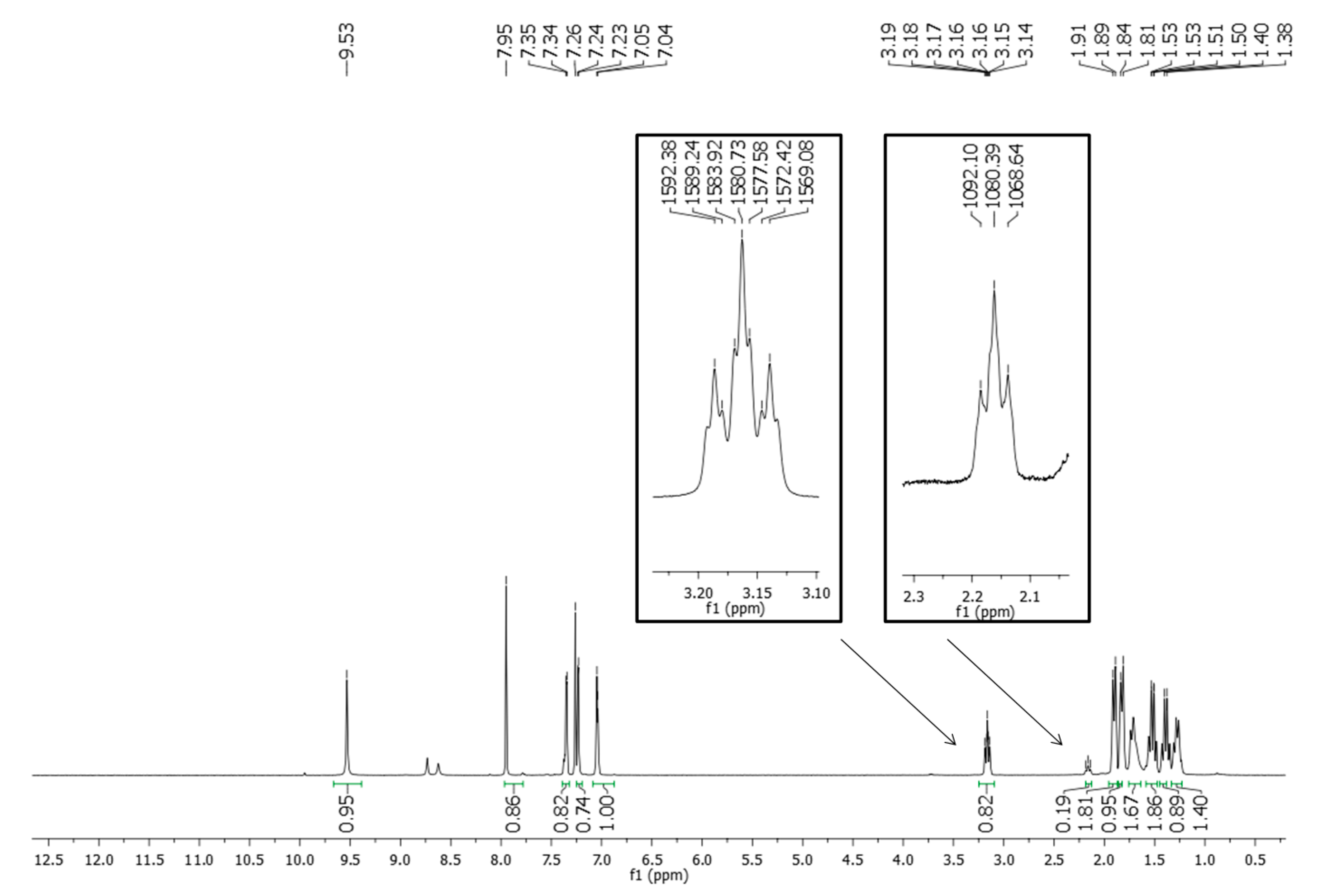
2.2. Pharmacological Activities
| Biological Tests | |||||||
|---|---|---|---|---|---|---|---|
 | Binding A2a | Carrageenan-induced peritonitis | Acetic acid-induced writhing test | Formalin-induced pain test | |||
| Compounds | (R1) | (Ar) | % of inhibition a | % of inhibition b | % of inhibition b | Phase I % of inhibition b | Phase II % of inhibition b |
| Dipyrone or Indomethacin | - | 65.1 ± 4.0 ** dipyrone | 82.5 ± 6.2 ** indomethacin | 23.5 ± 7.9 indomethacin | 57.4 ± 5.8 ** indomethacin | ||
| (8) LASSBio-294 | 1,3-benzodioxolyl | 2-thienyl | (Ki = 8.2µM) | 55.3 ± 4.8 ** | 68.2 ± 13.6 ** | 37.4 ± 4.9 | 42.0 ± 4.7 ** |
| (33) LASSBio-382 | phenyl | 2-thienyl | 17% at 10 µM | 40.3 ± 2.3 ** | 57.2 ± 8.5 ** | 12.3 ± 6.1 | 23.2 ± 4.1 |
| (9) LASSBio-1780 | cyclohexenyl | 2-thienyl | 7% at 10 µM | 21.4 ± 5.4 ** | 77.3 ± 3.2 ** | - | - |
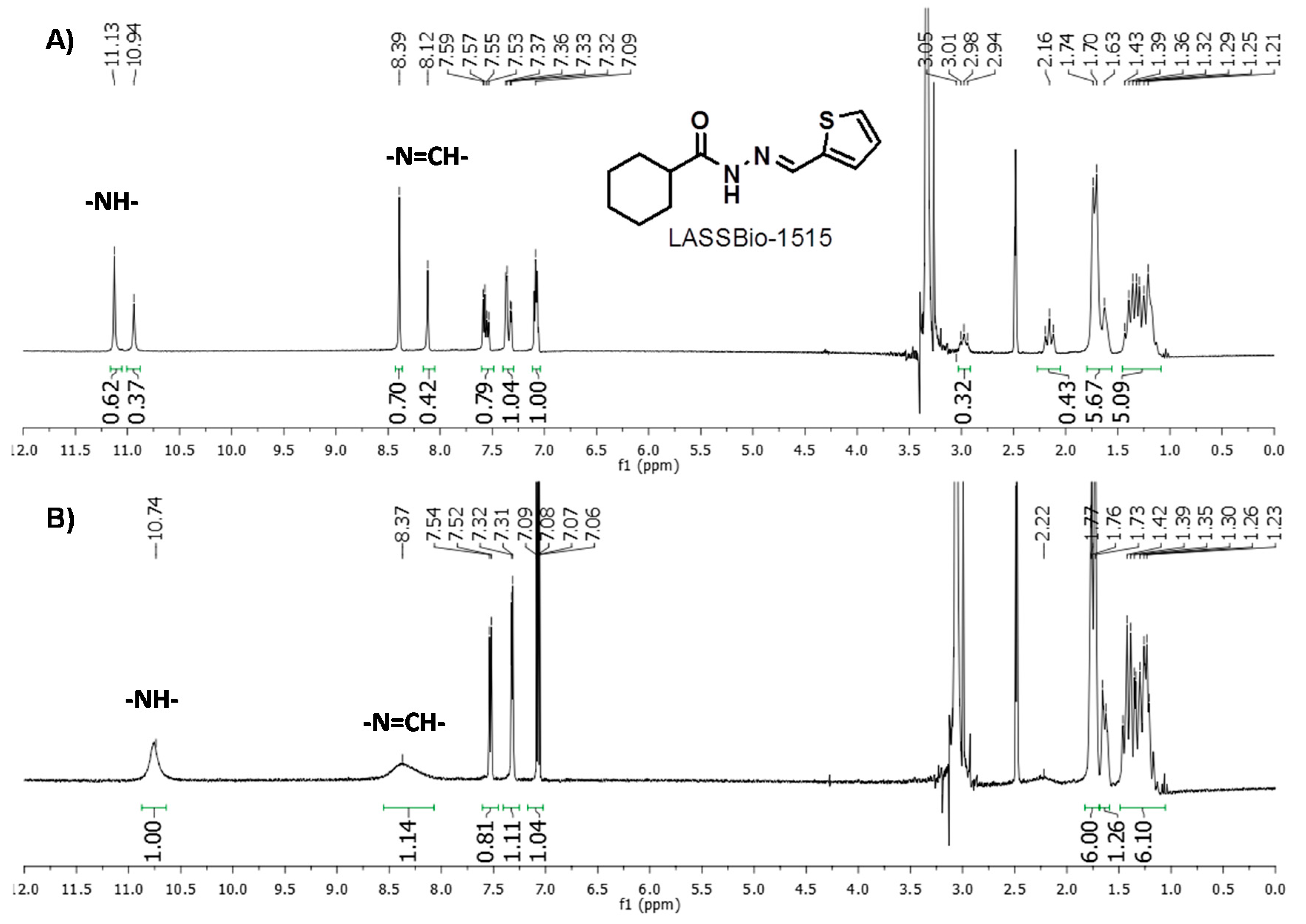
| (10) LASSBio-1515 | cyclohexyl | 2-thienyl | 13% at 30 µM | 79.2 ± 2.7 ** | 87.9 ± 3.4 ** | 32.9 ± 11.5 | 61.5 ± 2.9 ** |
| (11) LASSBio-1600 | cyclohexyl | 1H-imidazole-5-yl | 36% at 10 µM | 39.8 ± 4.9 ** | 20.9 ± 5.7 * | 5.2 ± 3.2 | 15.4 ± 10.6 |
| (12) LASSBio-1602 | cyclohexyl | 1H-imidazole-2-yl | 0% at 10 µM | 37.6 ± 1.9 ** | 69.2 ± 7.9 ** | 14.2 ± 5.8 | 5.7 ± 2.4 |
| (13) LASSBio-1514 | cyclohexyl | 4-pyridinyl | 0% at 10 µM | 81.9 ± 2.2 ** | 65.7 ± 9.5 ** | 36.9 ± 8.5 | 43.0 ± 5.7 ** |
| (14) LASSBio-1688 | cyclohexyl | 3-hydroxy-4-methoxyphenyl | 15% at 30 µM | 74.3 ± 4.4 ** | 90.7 ± 2.5 ** | 36.9 ± 8.5 | 38.3 ± 5.8 ** |
| (15) LASSBio-1689 | cyclohexyl | 4-hydroxy-3-methoxyphenyl | - | 68.2 ± 4.9 ** | 55.6 ± 5.3 ** | 31.5 ± 8.3 | 63.1 ± 7.7 ** |
| (16) LASSBio-1691 | cyclohexyl | 1,3- benzodioxolyl | 33% at 30 µM | 64.7 ± 3.0 ** | 56.0 ± 11.9 ** | 31.3 ± 6.7 | 25.6 ± 5.9 |
| (17) LASSBio-1513 | cyclohexyl | 3,4,5-trimethoxyphenyl | 10% at 30 µM | 76.3 ± 3.8 ** | 78.3 ± 8.4 ** | 26.9 ± 5.8 | 38.5 ± 6.8 ** |
| (18) LASSBio-1509 | cyclohexyl | 3,4-dimethoxyphenyl | 9% at 10 µM | 63.7± 3.2 ** | 67.3 ± 8.5 ** | 13.1 ± 7.8 | 75.97 ± 4.4 ** |
| (19) LASSBio-1517 | cyclohexyl | ferrocenyl | 5% at 30 µM | 46.9 ± 6.7 ** | 97.6 ± 0.9 ** | 47.3 ± 4.7 ** | 27.1 ± 12.0 |
| (20) LASSBio-1603 | cyclohexyl | 4-nitrophenyl | 0% at 10 µM | 42.8 ± 1.9 ** | 47.6 ± 7.0 ** | 31.3 ± 8.6 | 25.9 ± 6.9 |
| (21) LASSBio-1516 | cyclohexyl | 4-carboxylic-phenyl | 0%, at 10 µM | 58.6 ± 5.3 ** | 87.4 ± 6.0 ** | 17.5 ± 5.9 | 54.7 ± 6.9 ** |
| (22) LASSBio-1511 | cyclohexyl | 4-bromophenyl | 3% at 10 µM | 70.7 ± 3.1 ** | 62.2 ± 2.1 ** | 53.4 ± 11.1 ** | 77.0 ± 3.9 ** |
| (23) LASSBio-1601 | cyclohexyl | phenyl | 8% at 10 µM | 28.7 ± 4.4 ** | 57.3 ± 9.5 ** | 20.3± 8.4 | NA |
| (24) LASSBio-1508 | cyclohexyl | 4-isopropylphenyl | 12% at 10 µM | 78.6 ± 3.3 ** | 64.9 ± 3.3 ** | 13.4 ± 8.2 | 38.5 ± 6.8 * |
| (25) LASSBio-1510 | cyclohexyl | 4-methoxyphenyl | 0% at 10 µM | 67.4 ± 4.9 ** | 86.3 ± 4.0 ** | 30.1 ± 6.0 | 70.1 ± 5.3 ** |
| (26) LASSBio-1512 | cyclohexyl | 4-dimethylaminophenyl | 0% at 10 µM | 63.9 ± 4.0 ** | 68.3 ± 13.4 ** | 38.9 ± 13.1 * | 54.4 ± 4.9 ** |

3. Experimental Section
3.1. Chemistry
3.1.1. General Methods
3.1.2. General Procedure for the Preparation of the 3,4-Methylenedioxybenzoylacylhydrazone LASSBio-294 (8) and the Methylenedioxybenzoylacylhydrazone LASSBio-382 (33)
3.1.3. Procedure for the Synthesis of Cyclohex-1-enecarbohydrazide Intermediate 30
3.1.4. Procedure for the Synthesis of (E)-N'-(Thiophen-2-ylmethylene)cyclohex-1-enecarbohydrazide (LASSBio-1780, 9)
3.1.5. Procedure for the Preparation of Cyclohexanecarbohydrazide (28)
3.1.6. General Procedure for Preparation of Cyclohexylacylhydrazones 10–26
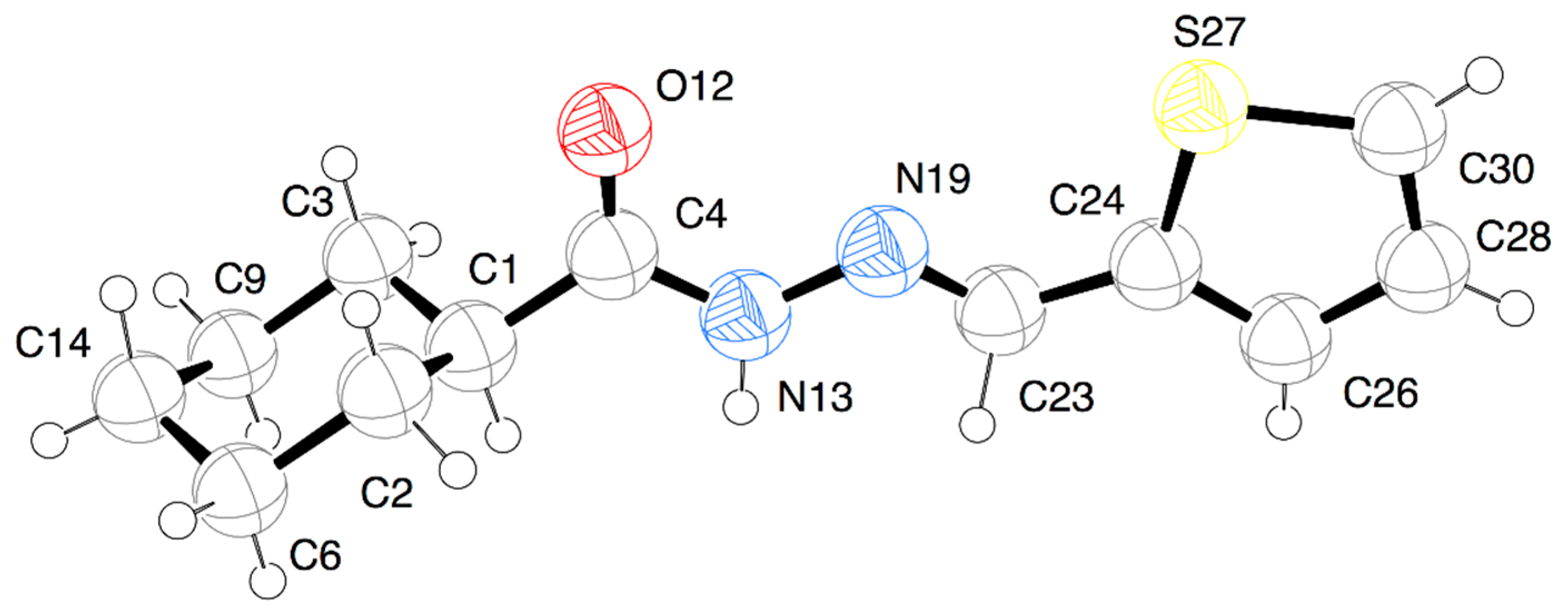
3.2. X-ray Crystallography
3.3. Binding Assay
3.4. Antinociceptive and Anti-Inflammatory Pharmacological Evaluation
3.4.1. Animals
3.4.2. Reagents
3.4.3. Acetic Acid-Induced Writhing Test
3.4.4. Formalin-Induced Nociception
3.4.5. Hot-Plate Test
3.4.6. Carrageenan-Induced Peritonitis
3.4.7. Statistical Analysis
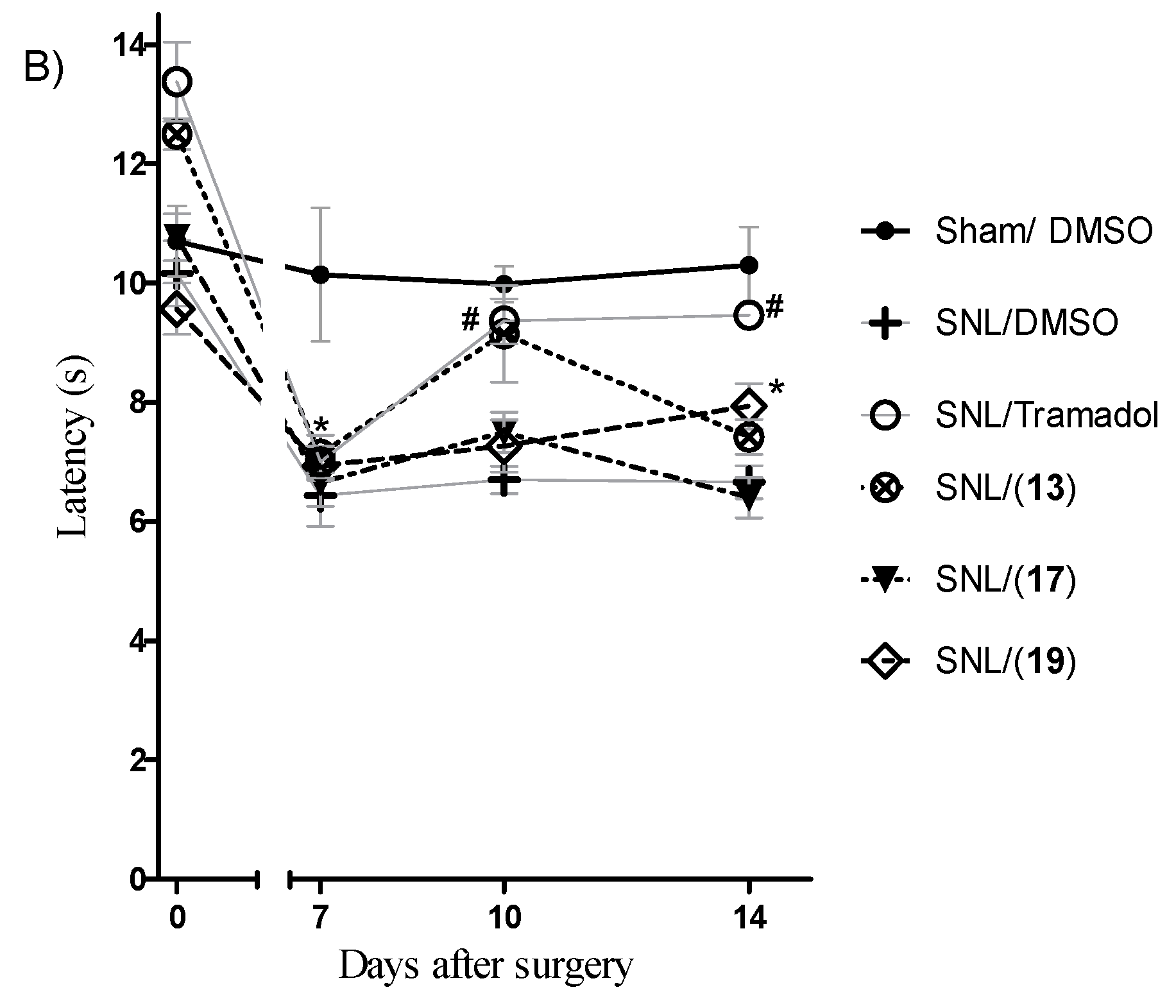
3.5. Thermal Sensitization and Mechanical Allodynia Induced by Spinal Nerve Ligation
3.5.1. Animals
3.5.2. Surgery
3.5.3. Behavioral Tests
3.5.4. Experimental Design
3.5.5. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Evans, B.E.; Rittle, K.E.; Bock, M.G.; DiPardo, R.M.; Freidinger, R.M.; Whitter, W.L.; Lundell, G.F.; Veber, D.F.; Anderson, P.S.; Chang, R.S.; et al. Methods for drug discovery: Development of potent, selective, orally effective cholecystokinin antagonists. J. Med. Chem. 1988, 31, 2235–2246. [Google Scholar] [CrossRef] [PubMed]
- Asif, M.; Husain, A. Analgesic, anti-Inflammatory, and antiplatelet profile of hydrazones containing synthetic molecules. J. Appl. Chem. 2013, 2013. [Google Scholar] [CrossRef]
- Rollas, S.; Küçükgüzel, S. Biological activities of hydrazone derivatives. Molecules 2007, 12, 1910–1939. [Google Scholar] [CrossRef] [PubMed]
- Kaplancikli, Z.A.; Altintop, M.D.; Ozdemir, A.; Turan-Zitouni, G.; Khan, S.I.; Tabanca, N. Synthesis and biological evaluation of some hydrazone derivatives as anti-inflammatory agents. Lett. Drug Des. Discov. 2012, 9, 310–315. [Google Scholar] [CrossRef]
- Duarte, C.D.; Barreiro, E.J.; Fraga, C.A. Privileged structures: A useful concept for the rational design of new lead drug candidates. Mini Rev. Med. Chem. 2007, 7, 1108–1119. [Google Scholar] [CrossRef] [PubMed]
- Do Amaral, D.N.; Cavalcanti, B.C.; Bezerra, D.P.; Ferreira, P.M.P.; Castro, R.P.; Sabino, J.R.; Machado, C.M.L.; Chammas, R.; Pessoa, C.; Sant’Anna, C.M.R.; et al. Docking, synthesis and antiproliferative activity of N-acylhydrazone derivatives designed as combretastatin A4 analogues. PLoS One 2014, 9, e85380. [Google Scholar] [CrossRef] [PubMed]
- Fraga, C.A.; Barreiro, E.J. Medicinal chemistry of N-acylhydrazones: New lead-compounds of analgesic, antiinflammatory and antithrombotic drugs. Curr. Med. Chem. 2006, 13, 167–198. [Google Scholar] [CrossRef] [PubMed]
- Gong, Q.; Menon, L.; Ilina, T.; Miller, L.G.; Ahn, J.; Parniak, M.A.; Ishima, R. Interaction of HIV-1 reverse transcriptase ribonuclease H with an acylhydrazone inhibitor. Chem. Biol. Drug Des. 2011, 77, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Narang, R.; Narasimhan, B.; Sharma, S.; Sriram, D.; Yogeeswari, P.; Clercq, E.D.; Pannecouque, C.; Balzarini, J. Nicotinic acid benzylidene/phenyl-ethylidene hydrazides: synthesis, antimicrobial evaluation and QSAR studies. Lett. Drug Des. Discov. 2011, 8, 733–749. [Google Scholar] [CrossRef]
- Tributino, J.L.; Duarte, C.D.; Correa, R.S.; Doriguetto, A.C.; Ellena, J.; Romeiro, N.C.; Castro, N.G.; Miranda, A.L.; Barreiro, E.J.; Fraga, C.A. Novel 6-methanesulfonamide-3,4-methylenedioxyphenyl-N-acylhydrazones: Orally effective anti-inflammatory drug candidates. Bioorg. Med. Chem. 2009, 17, 1125–1131. [Google Scholar] [CrossRef] [PubMed]
- Tributino, J.L.; Santos, M.L.; Mesquita, C.M.; Lima, C.K.; Silva, L.L.; Maia, R.C.; Duarte, C.D.; Barreiro, E.J.; Fraga, C.A.; Castro, N.G.; et al. LASSBio-881: An N-acylhydrazone transient receptor potential vanilloid subfamily type 1 antagonist orally effective against the hypernociception induced by capsaicin or partial sciatic ligation. Br. J. Pharmacol. 2010, 159, 1716–1723. [Google Scholar] [CrossRef] [PubMed]
- Kummerle, A.E.; Raimundo, J.M.; Leal, C.M.; da Silva, G.S.; Balliano, T.L.; Pereira, M.A.; de Simone, C.A.; Sudo, R.T.; Zapata-Sudo, G.; Fraga, C.A.; et al. Studies towards the identification of putative bioactive conformation of potent vasodilator arylidene N-acylhydrazone derivatives. Eur. J. Med. Chem. 2009, 44, 4004–4009. [Google Scholar] [CrossRef] [PubMed]
- Gage, J.L.; Onrust, R.; Johnston, D.; Osnowski, A.; Macdonald, W.; Mitchell, L.; Urogdi, L.; Rohde, A.; Harbol, K.; Gragerov, S.; et al. N-Acylhydrazones as inhibitors of PDE10A. Bioorg. Med. Chem. Lett. 2011, 21, 4155–4159. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.G.; Zapata-Sudo, G.; Kummerle, A.E.; Fraga, C.A.; Barreiro, E.J.; Sudo, R.T. Synthesis and vasodilatory activity of new N-acylhydrazone derivatives, designed as LASSBio-294 analogues. Bioorg. Med. Chem. 2005, 13, 3431–3437. [Google Scholar] [CrossRef] [PubMed]
- Barreiro, E.J. Estratégia de simplificação molecular no planejamento racional de fármacos: A descoberta de novo agente cardioativo. Quim. Nova 2002, 25, 1172–1180. [Google Scholar] [CrossRef]
- Barreiro, E.J.; Fraga, C.A.M.; Miranda, A.L.P.; Rodrigues, C.R. A química medicinal de N-acilidrazonas: Novos compostos-protótipos de fármacos analgésicos, antiinflamatórios e anti-trombóticos. Quim. Nova 2002, 25, 129–148. [Google Scholar] [CrossRef]
- Gonzalez-Serratos, H.; Chang, R.; Pereira, E.F.; Castro, N.G.; Aracava, Y.; Melo, P.A.; Lima, P.C.; Fraga, C.A.; Barreiro, E.J.; Albuquerque, E.X. A novel thienylhydrazone, (2-thienylidene)3,4-methylenedioxybenzoylhydrazine, increases inotropism and decreases fatigue of skeletal muscle. J. Pharmacol. Exp. Ther. 2001, 299, 558–566. [Google Scholar] [PubMed]
- Lima, L.M.; Barreiro, E.J. Bioisosterism: A useful strategy for molecular modification and drug design. Curr. Med. Chem. 2005, 12, 23–49. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, E.X.; Barreiro, E.J.; Sudo, T.R. Thienylhydrazon with Digitalis-Like Properties (Positive Inotropic Effects). Patent CA2384525, C, 6 March 2007. [Google Scholar]
- Lima, P.C.; Lima, L.M.; da Silva, K.C.; Leda, P.H.; de Miranda, A.L.; Fraga, C.A.; Barreiro, E.J. Synthesis and analgesic activity of novel N-acylarylhydrazones and isosters, derived from natural safrole. Eur. J. Med. Chem. 2000, 35, 187–203. [Google Scholar] [CrossRef] [PubMed]
- Palla, G.P.G.; Domiano, P.; Vignali, C.; Turner, W. Conformational behaviour and E/Z isomerization of N-acyl and N-aroylhydrazones. Tetrahedron 1986, 42, 3649–3654. [Google Scholar] [CrossRef]
- Basso, E.A.; Oliveira, P.R.; Caetano, J.; Schuquel, T.A. Semiempirical and ab initio Calculations versus Dynamic NMR on Conformational Analysis of Cyclohexyl-N,N-dimethylcarbamate. J. Braz. Chem. Soc. 2001, 12, 215–222. [Google Scholar] [CrossRef]
- Palla, G.; Predieri, C.; Predieri, G.; Vignali, C. Conformational study on N-acylhydrazones of aromatic aldehydes by NMR spectroscopy. Gazz. Chim. Ital. 1982, 112, 339–341. [Google Scholar]
- Lopes, A.B.; Miguez, E.; Kummerle, A.E.; Rumjanek, V.M.; Fraga, C.A.; Barreiro, E.J. Characterization of amide bond conformers for a novel heterocyclic template of N-acylhydrazone derivatives. Molecules 2013, 18, 11683–11704. [Google Scholar] [CrossRef] [PubMed]
- Costa, F.N.; Braz, D.; Ferreira, F.F.; da Silva, T.F.; Barreiro, E.J.; Lima, L.M.; Colaço, M.V.; Kuplich, L.; Barroso, R.C. Synchrotron X-ray powder diffraction data of LASSBio-1515: A new N-acylhydrazone derivative compound. Radiat. Phys. Chem. 2014, 95, 292–294. [Google Scholar] [CrossRef]
- Hunskaar, S.; Fasmer, O.B.; Hole, K. Formalin test in mice, a useful technique for evaluating mild analgesics. J. Neurosci. Methods 1985, 14, 69–76. [Google Scholar] [CrossRef]
- Collier, H.O.; Dinneen, L.C.; Johnson, C.A.; Schneider, C. The abdominal constriction response and its suppression by analgesic drugs in the mouse. Br. J. Pharmacol. Chemother. 1968, 32, 295–310. [Google Scholar] [CrossRef] [PubMed]
- Ferrandiz, M.L.; Alcaraz, M.J. Anti-inflammatory activity and inhibition of arachidonic acid metabolism by flavonoids. Agents Actions 1991, 32, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Top, S.; Vessieres, A.; Leclercq, G.; Quivy, J.; Tang, J.; Vaissermann, J.; Huche, M.; Jaouen, G. Synthesis, biochemical properties and molecular modelling studies of organometallic specific estrogen receptor modulators (SERMs), the ferrocifens and hydroxyferrocifens: Evidence for an antiproliferative effect of hydroxyferrocifens on both hormone-dependent and hormone-independent breast cancer cell lines. Chemistry (Easton) 2003, 9, 5223–5236. [Google Scholar]
- Hunskaar, S.; Hole, K. The formalin test in mice: Dissociation between inflammatory and non-inflammatory pain. Pain 1987, 30, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Woolfe, G.; Mcdonald, A.L. The evaluation of the analgesic action of pethidine hydrochloride [Demerol]. J. Pharmacol. Exp. Ther. 1944, 80, 300–307. [Google Scholar]
- Leal, C.M.; Pereira, S.L.; Kummerle, A.E.; Leal, D.M.; Tesch, R.; de Sant’Anna, C.M.; Fraga, C.A.; Barreiro, E.J.; Sudo, R.T.; Zapata-Sudo, G. Antihypertensive profile of 2-thienyl-3,4-methylenedioxybenzoylhydrazone is mediated by activation of the A2A adenosine receptor. Eur. J. Med. Chem. 2012, 55, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Chung, J.M. An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain 1992, 50, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Z.Y.; Wen, Y.R.; Zhang, D.R.; Borsello, T.; Bonny, C.; Strichartz, G.R.; Decosterd, I.; Ji, R.R. A peptide c-Jun N-terminal kinase (JNK) inhibitor blocks mechanical allodynia after spinal nerve ligation: Respective roles of JNK activation in primary sensory neurons and spinal astrocytes for neuropathic pain development and maintenance. J. Neurosci. 2006, 26, 3551–3560. [Google Scholar] [CrossRef] [PubMed]
- Rector, D.L.; Conder, G.A.; Folz, S.D. Anthelmintic acylhydrazones, method of use and compositions. Patent WO 1987006127, A1, 3 April 1987. [Google Scholar]
- Olsen, S.; Enkemeyer, E.-M. Notiz über Hexahydrobenzhydrazid. Chem. Ber. 1948, 81, 359–361. [Google Scholar] [CrossRef]
- David, W.I.F.; Shankland, K.; van de Streek, J.; Pidcock, E.; Motherwell, W.D.S. DASH: A program for crystal structure determination from powder diffraction data. J. Appl. Crystallogr. 2006, 39, 910–915. [Google Scholar] [CrossRef]
- Costa, F.N.; Ferreira, F.F.; da Silva, T.F.; Barreiro, E.J.; Lima, L.M.; Braz, D.; Barroso, R.C. Structure Re-determination of LASSBio-294-a cardioactive compound of the N-acylhydrazone class—Using X-ray powder diffraction data. Powder Diffr. 2013, 28, S491–S509. [Google Scholar] [CrossRef]
- Ferreira, F.F.; Antonio, S.G.; Rosa, P.C.; Paiva-Santos Cde, O. Crystal structure determination of mebendazole form A using high-resolution synchrotron x-ray powder diffraction data. J. Pharm. Sci. 2010, 99, 1734–1744. [Google Scholar] [PubMed]
- Coelho, A.A.; Evans, J.; Evans, I.; Kern, A.; Parsons, S. The TOPAS symboliccomputation system. Powder Diffr. 2011, 26, S22–S25. [Google Scholar] [CrossRef]
- Luthin, D.R.; Linden, J. Comparison of A4 and A2a binding sites in striatum and COS cells transfected with adenosine A2a receptors. J. Pharmacol. Exp. Ther. 1995, 272, 511–518. [Google Scholar] [PubMed]
- Kuraishi, Y.; Harada, Y.; Aratani, S.; Satoh, M.; Takagi, H. Separate involvement of the spinal noradrenergic and serotonergic systems in morphine analgesia: The differences in mechanical and thermal algesic tests. Brain Res. 1983, 273, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Hargreaves, K.; Dubner, R.; Brown, F.; Flores, C.; Joris, J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain 1988, 32, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Vivancos, G.G.; Verri, W.A., Jr.; Cunha, T.M.; Schivo, I.R.; Parada, C.A.; Cunha, F.Q.; Ferreira, S.H. An electronic pressure-meter nociception paw test for rats. Braz. J. Med. Biol. Res. 2004, 37, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds 8–26 are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, T.F.D.; Bispo Júnior, W.; Alexandre-Moreira, M.S.; Costa, F.N.; Monteiro, C.E.d.S.; Furlan Ferreira, F.; Barroso, R.C.R.; Noël, F.; Sudo, R.T.; Zapata-Sudo, G.; et al. Novel Orally Active Analgesic and Anti-Inflammatory Cyclohexyl-N-Acylhydrazone Derivatives. Molecules 2015, 20, 3067-3088. https://doi.org/10.3390/molecules20023067
Silva TFD, Bispo Júnior W, Alexandre-Moreira MS, Costa FN, Monteiro CEdS, Furlan Ferreira F, Barroso RCR, Noël F, Sudo RT, Zapata-Sudo G, et al. Novel Orally Active Analgesic and Anti-Inflammatory Cyclohexyl-N-Acylhydrazone Derivatives. Molecules. 2015; 20(2):3067-3088. https://doi.org/10.3390/molecules20023067
Chicago/Turabian StyleSilva, Tiago Fernandes Da, Walfrido Bispo Júnior, Magna Suzana Alexandre-Moreira, Fanny Nascimento Costa, Carlos Eduardo da Silva Monteiro, Fabio Furlan Ferreira, Regina Cely Rodrigues Barroso, François Noël, Roberto Takashi Sudo, Gisele Zapata-Sudo, and et al. 2015. "Novel Orally Active Analgesic and Anti-Inflammatory Cyclohexyl-N-Acylhydrazone Derivatives" Molecules 20, no. 2: 3067-3088. https://doi.org/10.3390/molecules20023067