Selective Reduction of Cr(VI) in Chromium, Copper and Arsenic (CCA) Mixed Waste Streams Using UV/TiO2 Photocatalysis
Abstract
:1. Introduction
2. Results and Discussion
2.1. The Reduction of Cr(VI)
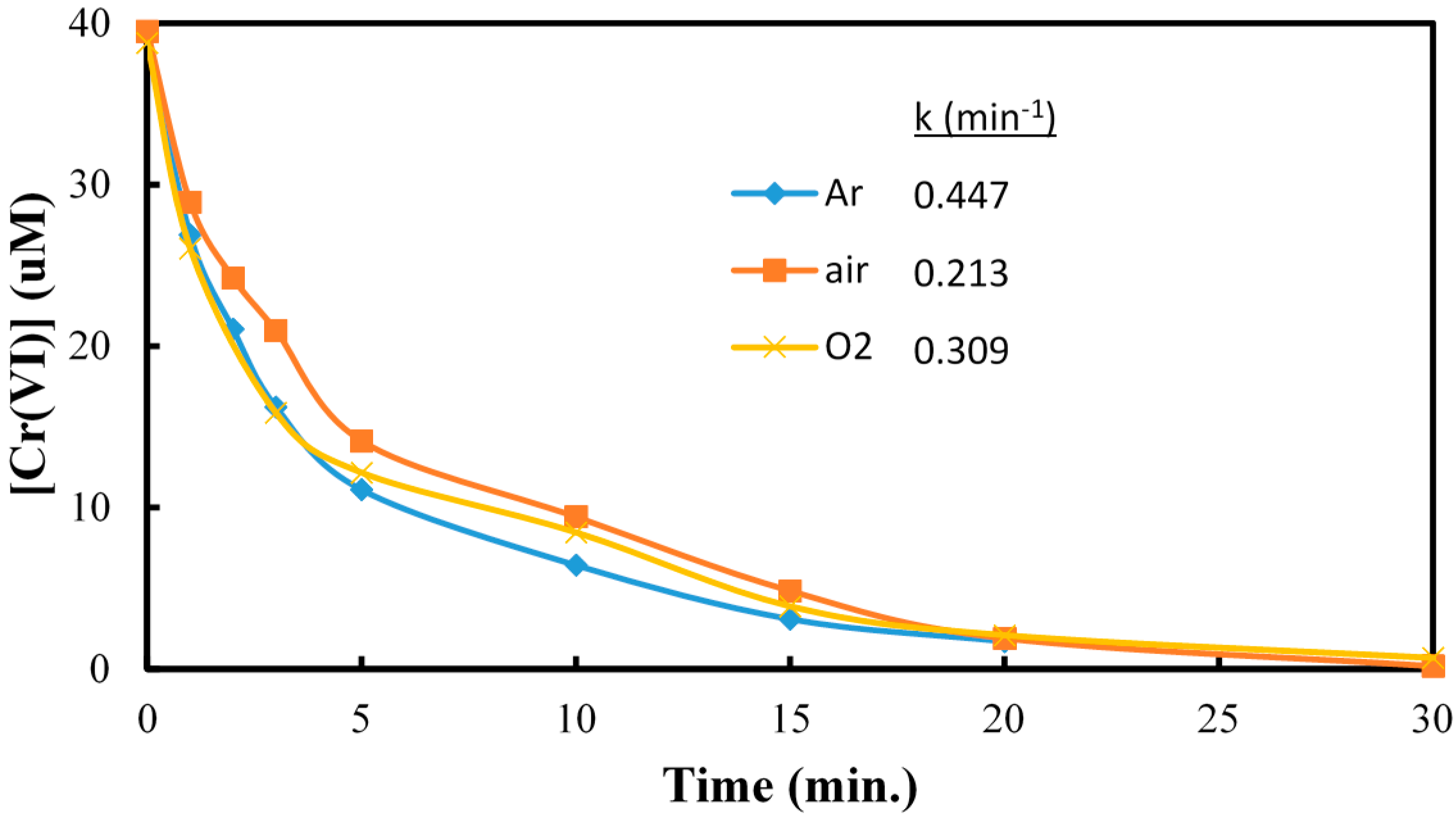
| Arsenic (As) | Chromium (Cr) | |
|---|---|---|
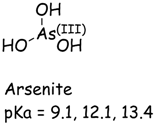 |  |  |
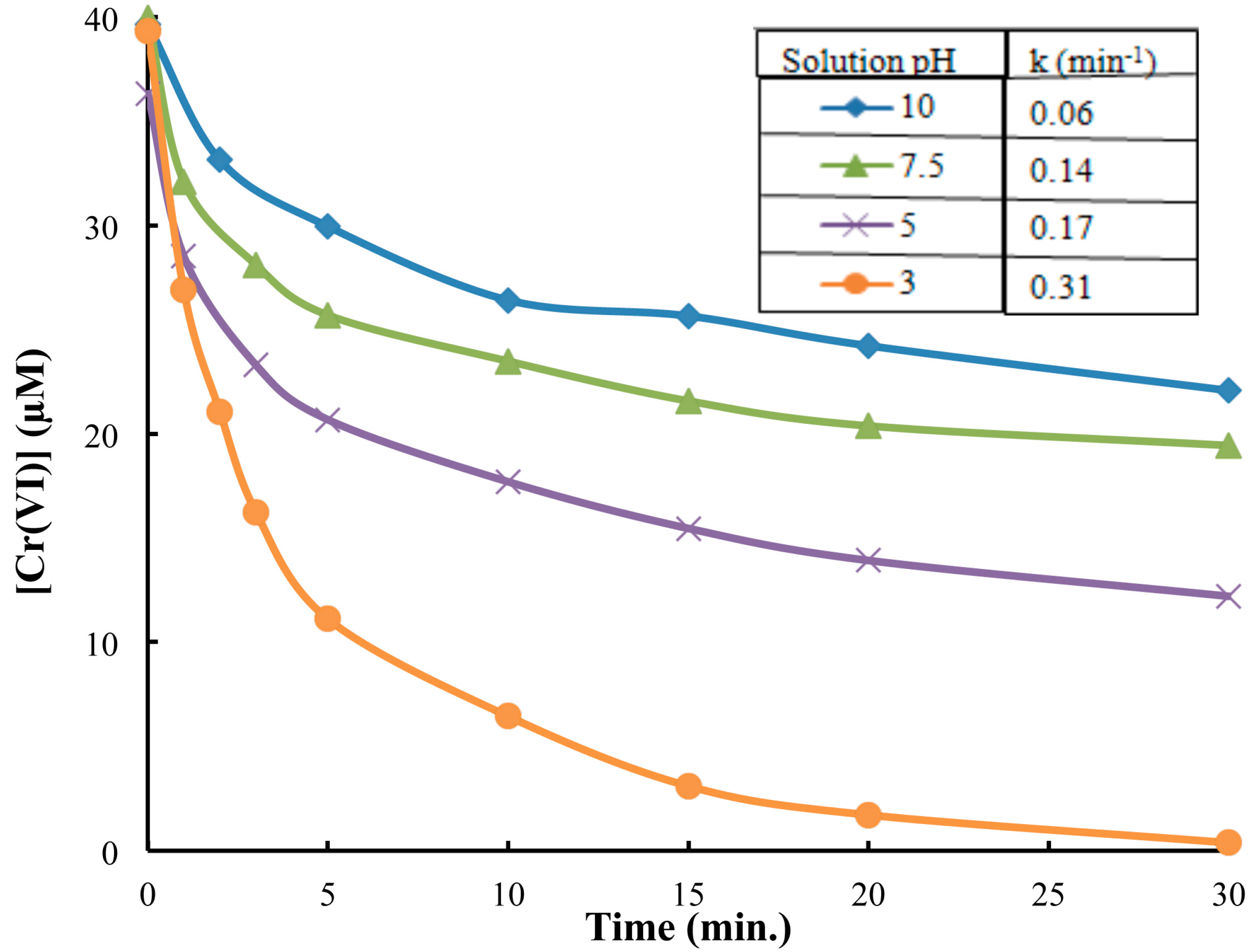
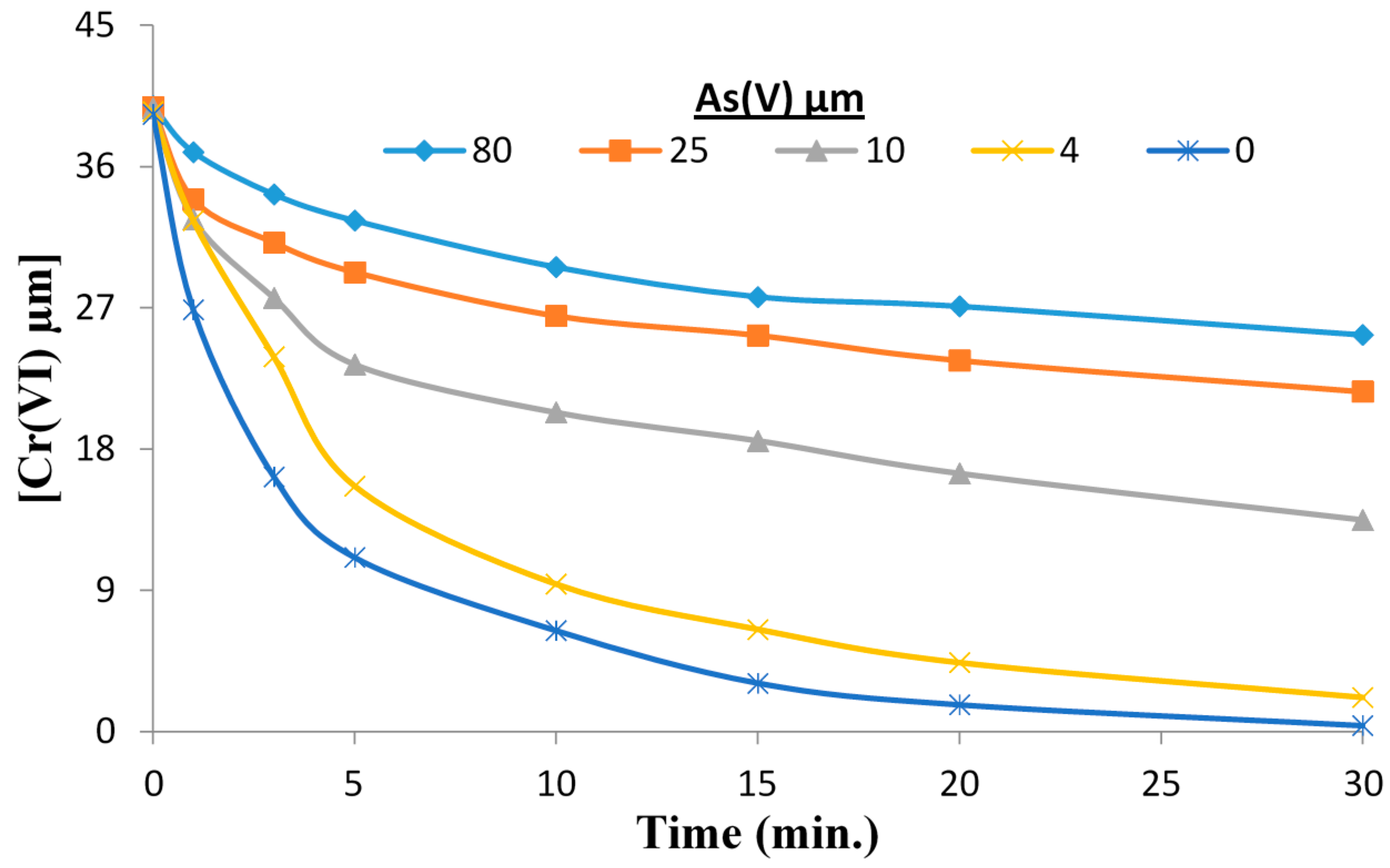
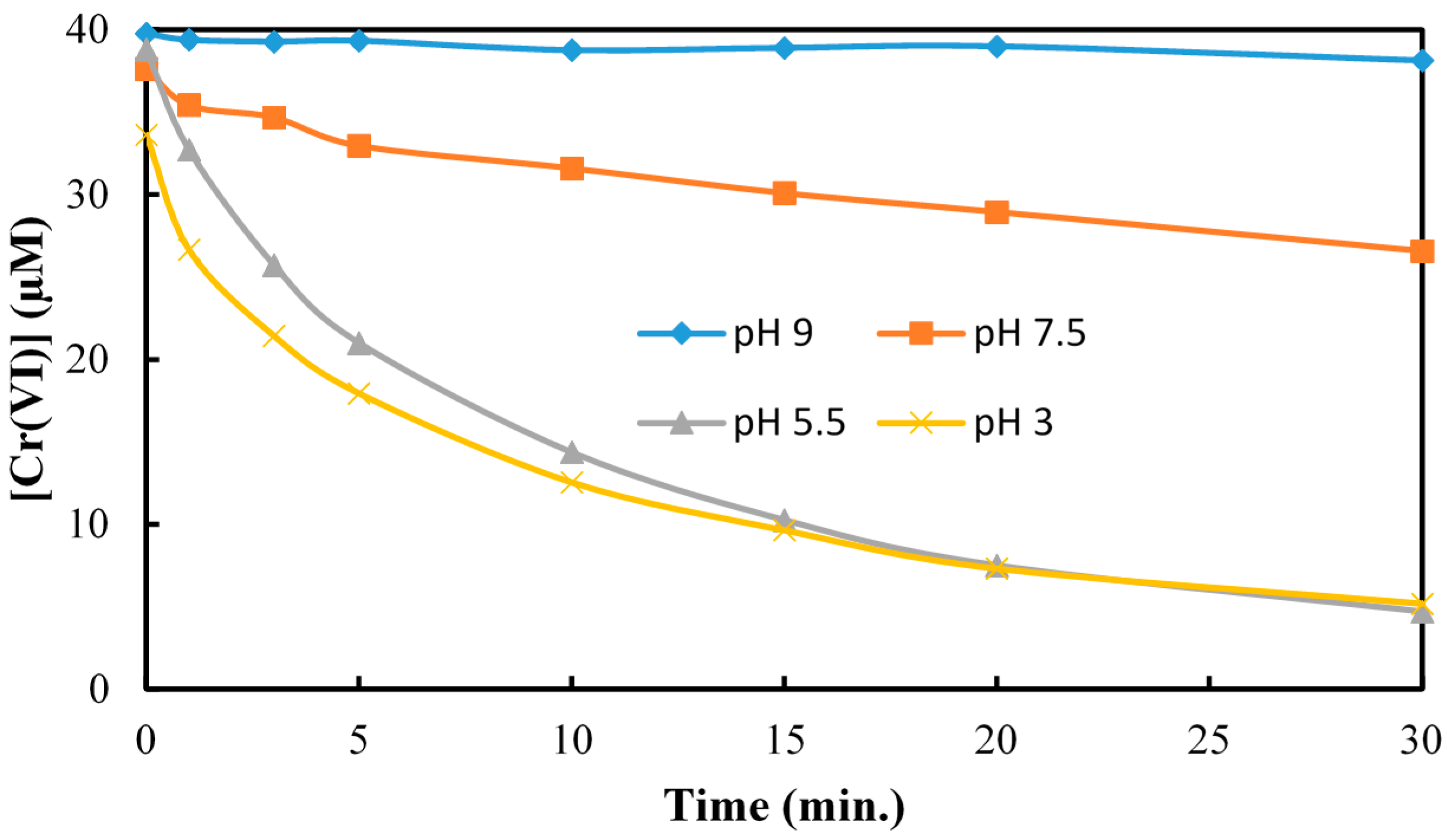
2.2. The Reduction of Cr(VI) in Presence of As(V) at pH 3.0
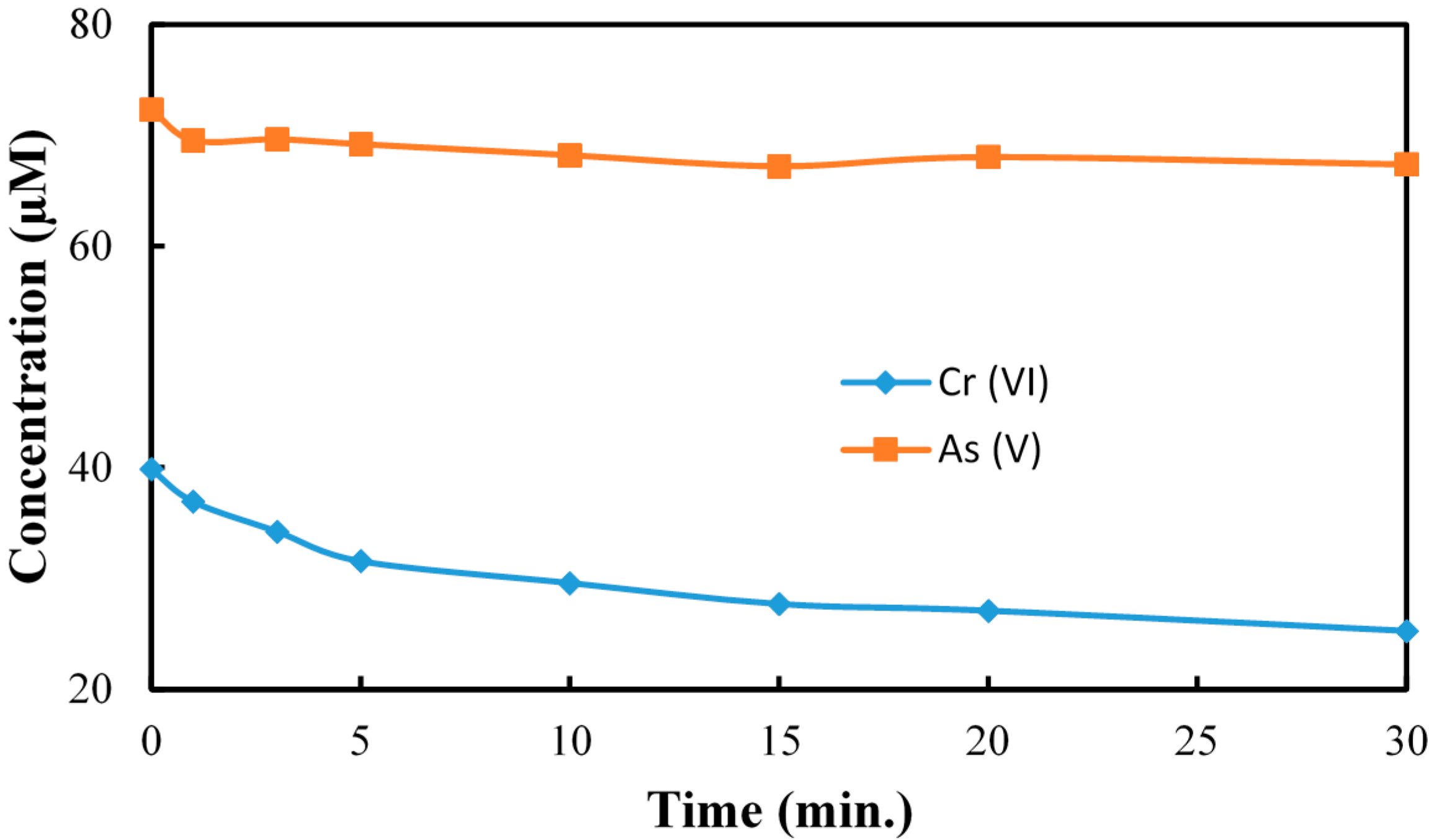
2.3. The Reduction of Cr(VI) in Presence of Cu(II)
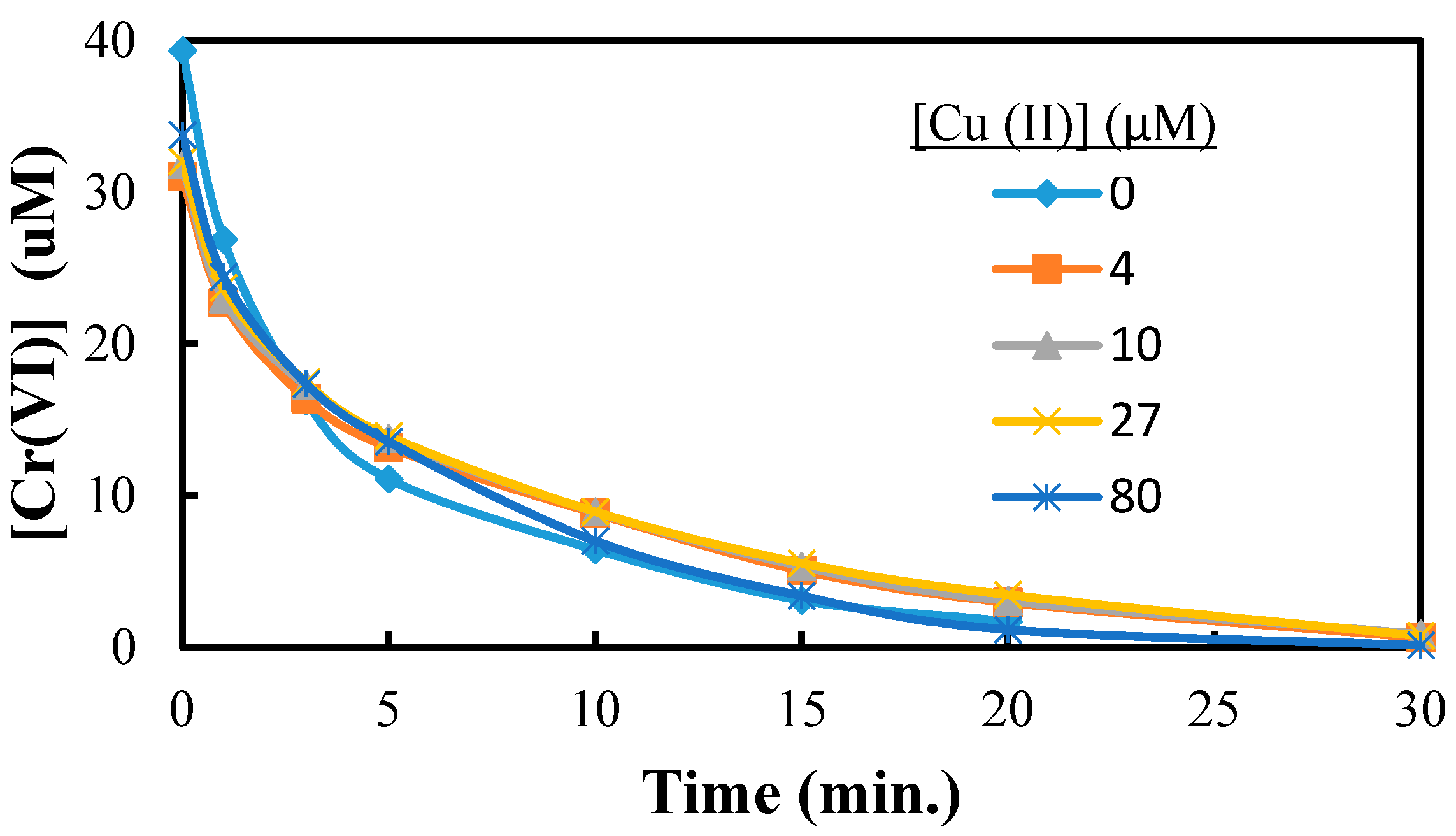
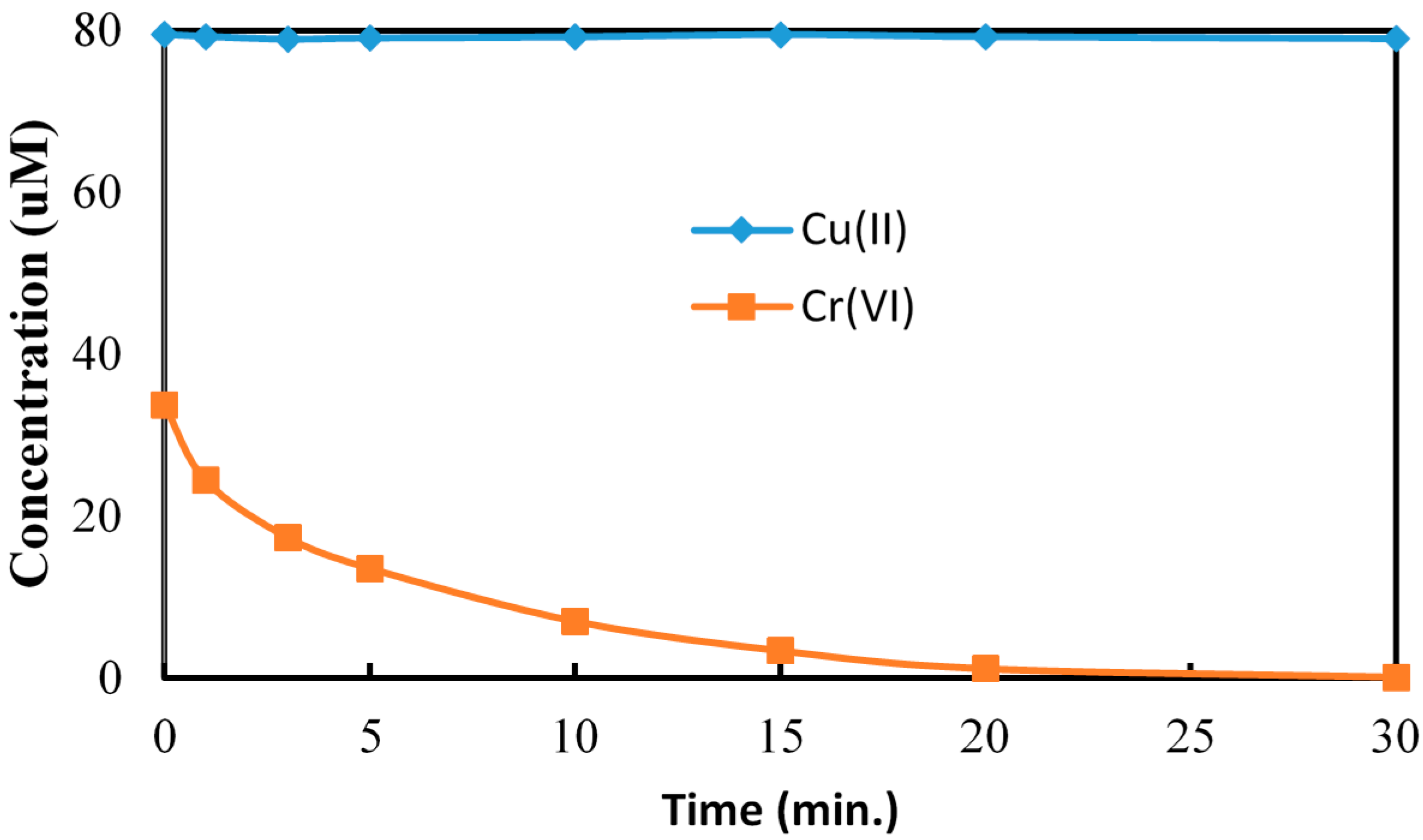
2.4. Cr(VI) Reduction in Presence of As(V) and Cu(II)

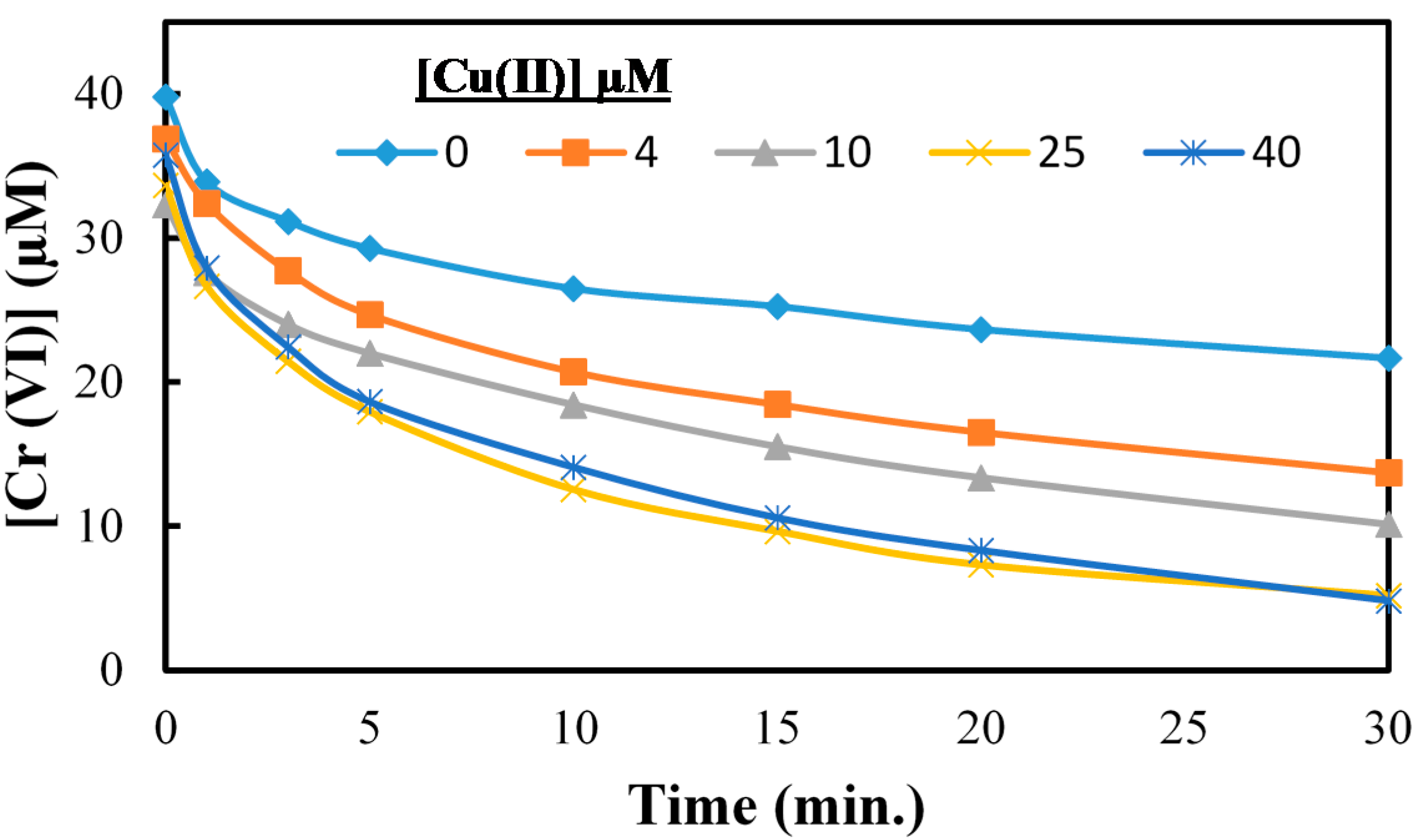
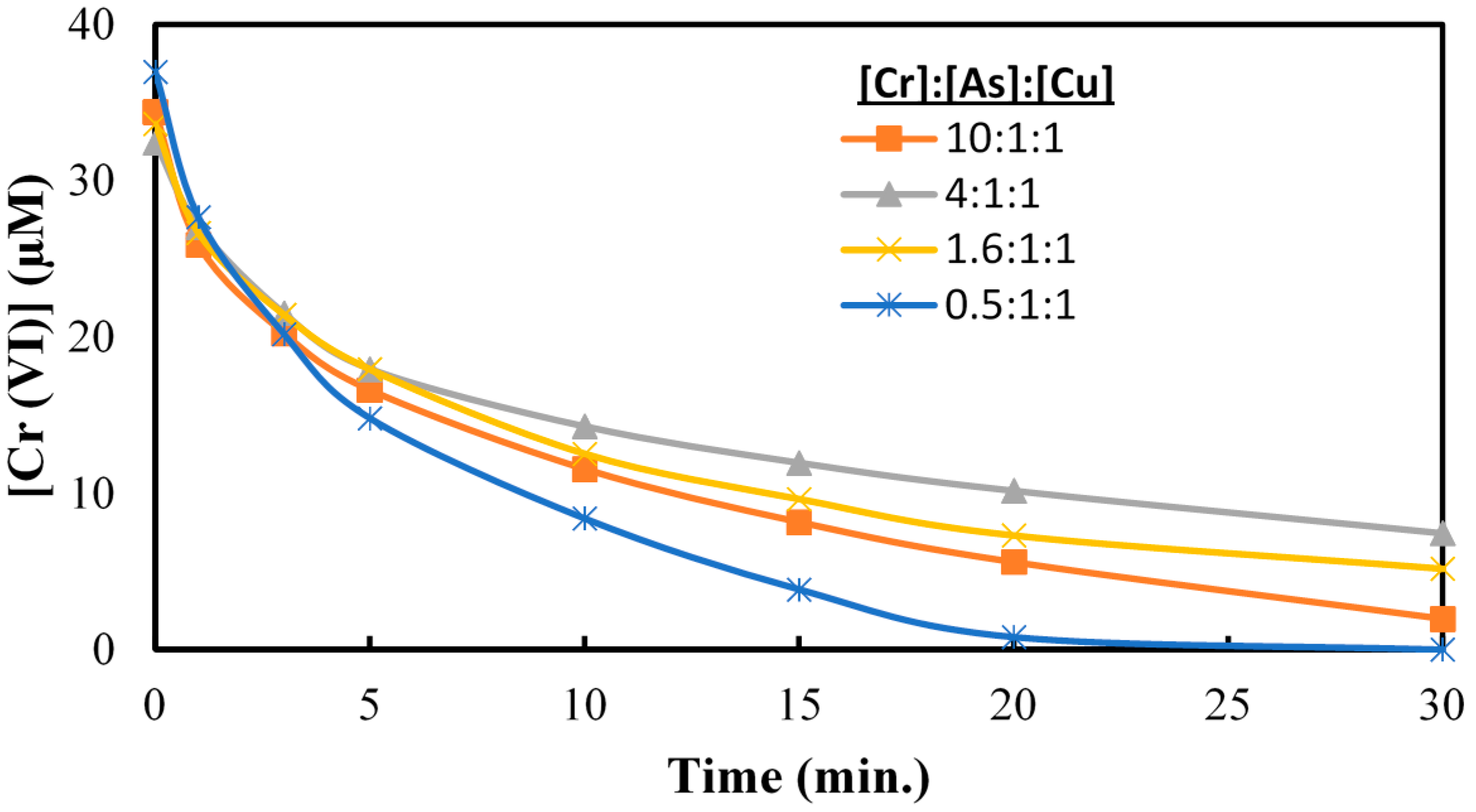
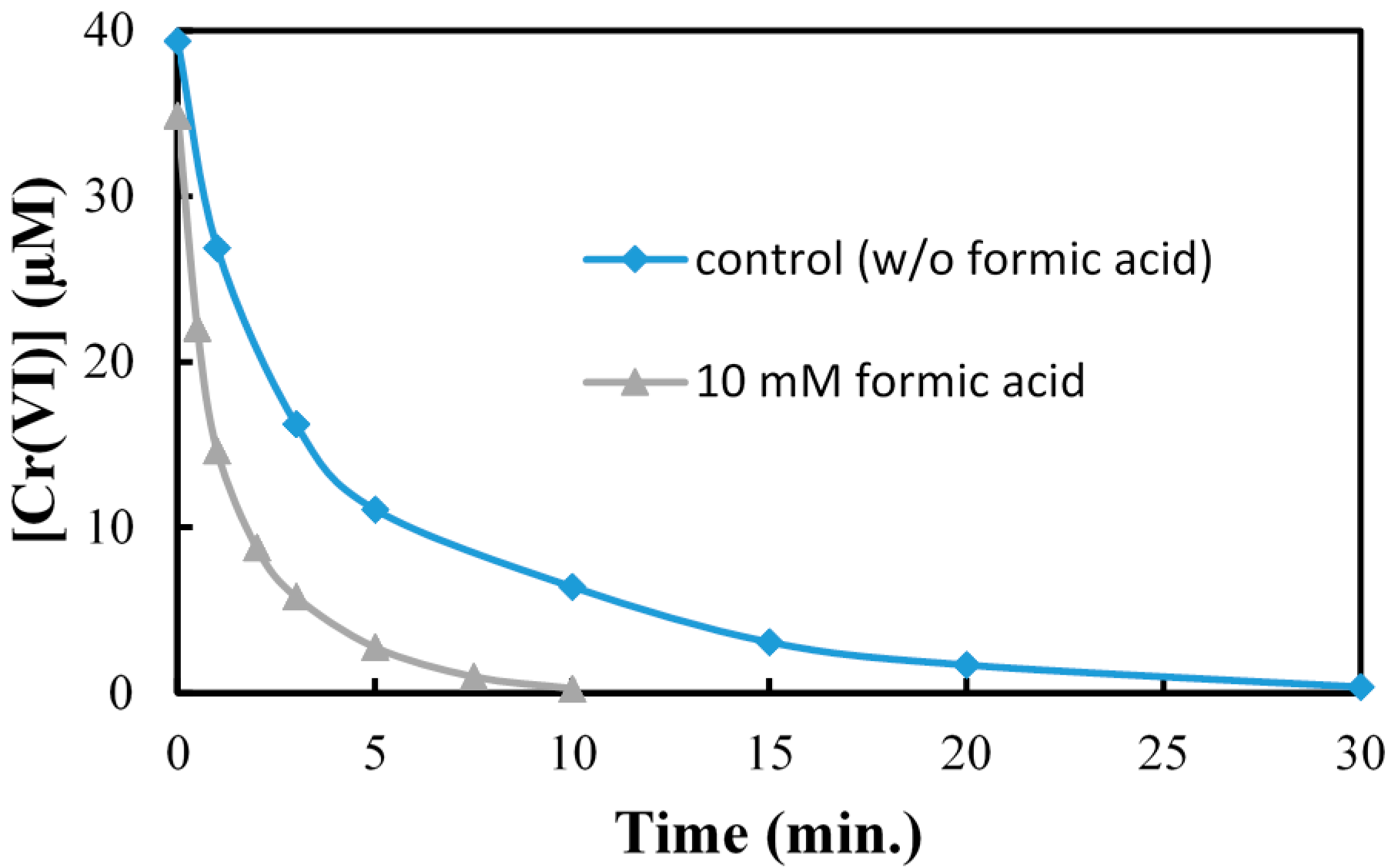
2.5. Effect of Hole Scavenger on Cr(VI) Reduction
3. Experimental
3.1. Materials
3.2. Sample Preparation and Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cooper, P.A. Leaching of CCA: Is it a problem? In Environmental Considerations in the Manufacturing, Use and Disposal of Preservative-Treated Wood; Forest Products Society: Madison, WI, USA, 1994. [Google Scholar]
- Joanna, S.W.; KongHwa, C. Extraction of chromated copper arsenate from wood wastes using green solvent supercritical carbon dioxide. J. Hazard. Mater. 2008, 158, 384–391. [Google Scholar] [CrossRef] [PubMed]
- Gordon, T.; Spanier, J.; Butala, J.H.; Li, P.; Rossman, T.G. In vitro bioavailability of heavy metals in pressure treated wood dust. J. Toxicol. Sci. 2002, 67, 32–37. [Google Scholar] [CrossRef]
- Stillwell, D.; Gorny, K. Contamination of soil with copper, chromium, and arsenic under decks built from pressure treated wood. Bull. Environ. Contam. Toxicol. 1997, 58, 22–29. [Google Scholar] [CrossRef] [PubMed]
- WHO. Arsenic in Drinking-Water, Background Document for Development of WHO Guidelines for Drinking-Water Quality; World Health Organization (WHO): Geneva, Switzerland, 2003. [Google Scholar]
- WHO. Chromium in Drinking-Water, Background Document for Development of WHO Guidelines for Drinking-Water Quality; Geneva, Switzerland, 2003. [Google Scholar]
- ATSDR. Toxicological Profile for Copper, Background and Environmental Exposures to Copper in the United States; Agency for Toxic Substances and Disease Registry (ATSDR): Atlanta, GA, USA, 2004. [Google Scholar]
- Mason, R.W.; Edwards, I.R. Acute toxicity of combinations of sodium dichromate, sodium arsenate and copper sulphate in the rat. Comp. Biochem. Physiol. Part C 1989, 93, 121–125. [Google Scholar] [CrossRef]
- Humphrey, D.G. The chemistry of chromated copper arsenate wood preservatives. Rev. Inorg. Chem. 2002, 22, 1–40. [Google Scholar] [CrossRef]
- Cooper, P.A. A review of issues and technical options for managing spent CCA treated wood. In Proceedings of the AWPA Annual Meeting, Boston, MA, USA, 27–29 April 2003.
- El-Fatah, S.M.; Goto, M.; Kodama, A.; Hirose, T. Supercritical fluid extraction of hazardous metals from CCA wood. J. Supercrit. Fluids 2004, 28, 21–27. [Google Scholar] [CrossRef]
- Gyu-Hyeok, K.; Jong-Bum, R.; li-Gon, K.; Yun-Sang, S. Optimization of hydrogen peroxide extraction conditions for CCA removal from treated wood by response surface methodology. For. Prod. J. 2004, 54, 141–144. [Google Scholar]
- Kartal, S.N. Removal of copper, chromium, and arsenic from CCA-C treated wood by EDTA extraction. Waste Manag. 2003, 23, 537–546. [Google Scholar] [CrossRef] [PubMed]
- Clausen, C.A.; Smith, R.L. Removal of CCA from treated wood by oxalic acid extraction, steam explosion, and bacterial fermentation. J. Ind. Microbiol. Biotechnol. 1998, 20, 251–257. [Google Scholar] [CrossRef]
- Janin, A.; Blais, J.F.; Mercier, G.; Drogui, P. Optimization of a chemical leaching process for decontamination of CCA-treated wood. J. Hazard. Mater. 2009, 169, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Khan, B.I.; Solo-Gabriele, H.M.; Townsend, T.G.; Cai, Y. Release of arsenic to the environment from CCA-treated wood.1.Leaching and speciation during service. Environ. Sci. Technol. 2006, 40, 988–993. [Google Scholar]
- Townsens, T.; Solo-Gabriele, H.; Tolaymat, T.; Stook, K.; Hosein, N. Chromium, Copper, and Arsenic Concentrations in Soil Underneath CCA-Treated Wood Structures. Soil Sediment Contam. 2003, 12, 779–798. [Google Scholar] [CrossRef]
- Janin, A.; Zaviska, F.; Drogui, P.; Blais, J.; Mercier, G. Selective recovery of metals in leachate from chromated copper arsenate treated wastes using electrochemical technology and chemical precipitation. Hydrometallurgy 2009, 96, 318–326. [Google Scholar] [CrossRef]
- Nygren, O.; Nilsson, C.A. Determination and speciation of chromium, copper and arsenic in wood and dust from CCA-impregnated timber. Analusis 1993, 21, 83–89. [Google Scholar]
- Jiang, W.; Cai, Q.; Xu, W.; Yang, M.; Cai, Y.; Dionysiou, D.D.; O’Shea, K.E. Cr(VI) Adsorption and Reduction by Humic Acid Coatedon Magnetite. Environ. Sci. Technol. 2014, 48, 8078–8085. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.K.; Agarwal, S.; Saleh, T.A. Chromium removal by combining the magnetic properties of iron oxide with adsorption properties of carbon nanotubes. Water Res. 2011, 45, 2207–2212. [Google Scholar] [CrossRef] [PubMed]
- Kongsricharoern, N.; Polprasert, C. Chromium removal by a bipolar electro-chemical precipitation process. Water Sci. Technol. 1996, 34, 109–116. [Google Scholar] [CrossRef]
- Aarthi, T.; Madras, G. Photocatalytic reduction of metals in presence of combustion synthesized nano-TiO2. Catal. Commun. 2008, 9, 630–634. [Google Scholar] [CrossRef]
- Chenthamarakshan, C.R.; Rajeshwar, K. Heterogeneous Photocatalytic Reduction of Cr(VI) in UV-Irradiated Titania Suspensions: Effect of Protons, Ammonium Ionsand, Other Interfacial Aspects. Langmuir 2000, 16, 2715–2721. [Google Scholar] [CrossRef]
- Lin, W.Y.; Wei, C.; Rajeshwar, K. Photocatalytic reduction and immobilization of hexavalent chromium at titanium dioxide in aqueous basic media. J. Electrochem. Soc. 1993, 140, 2477–2482. [Google Scholar] [CrossRef]
- Parida, K.; Mishra, K.G.; Dash, S.K. Adsorption of toxic metal ion Cr(VI) from aqueous state by TiO2-MCM-41: Equilibrium and kinetic studies. J. Hazard. Mater. 2012, 241–242, 395–403. [Google Scholar]
- Zhang, L.; Zhang, Y. Adsorption characteristics of hexavalent chromium on HCB/TiO2. Appl. Surf. Sci. 2006, 316, 649–656. [Google Scholar] [CrossRef]
- Zhang, Y.C.; Yang, M.; Zhang, G.; Dionysiou, D.D. HNO3-involved one-step low temperature solvothermal synthesis of N-doped TiO2 nanocrystals for efficient photocatalytic reduction of Cr(VI) in water. Appl. Catal. B 2013, 142–143, 249–258. [Google Scholar]
- Yoon, J.; Shim, E.; Joo, H. Photocatalytic reduction of hexavalent chromium (Cr(VI)) using rotating TiO2 mesh. Korean J. Chem. Eng. 2009, 26, 1296–1300. [Google Scholar] [CrossRef]
- Wang, Q.; Shang, Q.; Zhu, T.; Zhao, F. Efficient photoelectrocatalytic reduction of Cr(VI) usingTiO2 nanotube arrays as the photoanode and a large-area titanium mesh as the photocathode. J. Mol. Catal. A 2011, 335, 242–247. [Google Scholar] [CrossRef]
- Xu, X.R.; Li, H.B.; Gu, J.D. Simultaneous decontamination of hexavalent chromium and methyl tert-butyl ether by UV/TiO2 process. Chemosphere 2006, 63, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Ni, J.; Yin, X. Synergy of photocatalysis and adsorption for simultaneous removal of Cr(VI) and Cr(III) withTiO2 and titanate nanotubes. Water Res. 2014, 53, 12–25. [Google Scholar] [CrossRef]
- Prairie, M.R.; Evans, L.R.; Stange, B.; Martinez, S.L. An investigation of titanium dioxide photocatalysis for the treatment of water contaminated with metals and organic chemicals. Environ. Sci. Technol. 1993, 27, 1776–1782. [Google Scholar]
- Xu, T.; Cai, Y.; O’Shea, K.E. Adsorption and Photocatalyzed Oxidation of Methylated Arsenic Species in TiO2Suspensions. Environ. Sci. Technol. 2007, 41, 5471–5477. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Cai, Y.; O’Shea, K.E. TiO2photocatalytic degradation of phenylarsonic acid. J. Photochem. Photobiol. A 2010, 210, 61–68. [Google Scholar] [CrossRef]
- Zheng, S.; Jiang, W.; Cai, Y.; Dionysiou, D.D.; O’Shea, K.E. Adsorption and photocatalytic degradation of aromatic organoarsenic compounds in TiO2suspension. Catal. Today 2014, 224, 83–88. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are not available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, S.; Jiang, W.; Rashid, M.; Cai, Y.; Dionysiou, D.D.; O'Shea, K.E. Selective Reduction of Cr(VI) in Chromium, Copper and Arsenic (CCA) Mixed Waste Streams Using UV/TiO2 Photocatalysis. Molecules 2015, 20, 2622-2635. https://doi.org/10.3390/molecules20022622
Zheng S, Jiang W, Rashid M, Cai Y, Dionysiou DD, O'Shea KE. Selective Reduction of Cr(VI) in Chromium, Copper and Arsenic (CCA) Mixed Waste Streams Using UV/TiO2 Photocatalysis. Molecules. 2015; 20(2):2622-2635. https://doi.org/10.3390/molecules20022622
Chicago/Turabian StyleZheng, Shan, Wenjun Jiang, Mamun Rashid, Yong Cai, Dionysios D. Dionysiou, and Kevin E. O'Shea. 2015. "Selective Reduction of Cr(VI) in Chromium, Copper and Arsenic (CCA) Mixed Waste Streams Using UV/TiO2 Photocatalysis" Molecules 20, no. 2: 2622-2635. https://doi.org/10.3390/molecules20022622




_Dionysiou.jpg)

